p-Carborane-based androgen antagonists active in LNCaP cells with a mutated androgen receptor†
Shinya
Fujii
a,
Ayumi
Yamada
a,
Keiko
Tomita
b,
Mao
Nagano
b,
Tokuhito
Goto
c,
Kiminori
Ohta
c,
Takashi
Harayama
b,
Yasuyuki
Endo
c and
Hiroyuki
Kagechika
*a
aGraduate School of Biomedical Science, Institute of Biomaterials and Bioengineering, Tokyo Medical and Dental University, 2-3-10, Kanda-Surugadai, Chiyoda-ku, Tokyo, 101-0062, Japan. E-mail: kage.omc@tmd.ac.jp; Fax: +81-3-5280-8127; Tel: +81-3-5280-8032
bFaculty of Pharmaceutical Sciences at Kagawa Campus, Tokushima Bunri University, 1314-1,Shido, Sanuki, Kagawa 769-2193, Japan
cFaculty of Pharmaceutical Sciences, Tohoku Pharmaceutical University, 4-4-1, Komatsushima, Aoba-ku, Sendai, 981-8558, Japan
First published on 21st July 2011
Abstract
Development of antagonists for a mutated androgen receptor (AR) is important for treatment of anti-androgen-refractory prostate cancers. We describe here application of the p-carborane cage as a hydrophobic core structure for novel anti-androgens active toward LNCaP human prostate cancer cells with mutated AR. These compounds are expected to be versatile lead compounds not only for development of AR pan-antagonists, but also for discovery of mutant-selective anti-androgens.
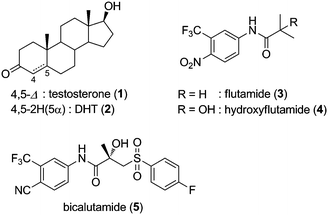
The androgen receptor (AR) is a member of the nuclear receptor superfamily of ligand-regulated transcriptional factors,1 and plays critical roles in numerous physiological processes, such as development and regulation of the male reproductive system, and maintenance of muscle and bone mass.2 AR is closely related to prostate cancer, and transcriptional activation by AR agonist exacerbates the disease in many cases.3 Prostate cancer is the most common type of cancer and the second leading cause of cancer deaths in males.4 Since endogenous androgens, testosterone (1) and its more active metabolite 5α-dihydrotestosterone (5α-DHT: 2, Fig. 1), are involved in aggravation of prostate cancer by activation of AR, synthetic androgen antagonists, so-called anti-androgens, are used in the treatment of prostate cancer. However, anti-androgen therapy often leads to hormone-refractory prostate cancer.5 In some cases, the androgen antagonists, such as flutamide (3) and hydroxyflutamide (4), function as agonists toward the hormone-refractory cancer cells, and exacerbate the disease. This is known as anti-androgen withdrawal syndrome, since withdrawal of androgen antagonist results in clinical improvement.6 Although the mechanism is not fully understood, mutation in AR is thought to be a major cause of hormone-refractory prostate cancer.7,8 Therefore, development of androgen antagonists effective for mutated AR is required for the therapy of prostate cancer.
 | ||
| Fig. 1 Structures of endogenous and synthetic AR ligands. | ||
Several mutated ARs have been found in the course of anti-androgen therapy of prostate cancer. The T877A mutation is the most common AR mutation observed clinically in prostate cancers.7 Anti-androgens 3 and 4 act as agonists toward T877A AR. Bicalutamide (5), the most widely used anti-androgen, acts as an antagonist for T877A AR, but exhibits significant androgenic activity toward W741L or W741C point-mutated AR; this is known as bicalutamide withdrawal syndrome.9 Other mutations of AR in prostate cancers are also well-documented, and several mutant ARs, such as T877S, H874Y, V715M, and L701H/T877A, have a broadened spectrum of ligand responsiveness.10 It is therefore of interest to develop a new generation of anti-androgens that would function as antagonists for mutant ARs.
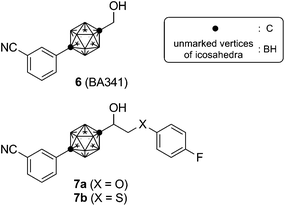
We previously reported development of potent androgen antagonists by using the carborane cage as a novel hydrophobic core structure.11,12 Carboranes, more specifically dicarba-closo-dodecaboranes, members of the class of carbon-containing boron clusters, have characteristic properties including spherical geometry, remarkable thermal and chemical stability, and a hydrophobic molecular surface.13 We have reported the development of a range of bioactive molecules, including specific ligands for estrogen receptor14 and retinoid receptors15 that contain a carborane cage as a hydrophobic pharmacophore, and we have obtained several promising drug candidates. The most potent carborane-containing molecule with androgen antagonist activity so far synthesized is 3-(12-hydroxymethyl-1,12-dicarba-closo-dodecaborane-1-yl)benzonitrile (6: BA341, Fig. 2),11 which exhibits remarkable anti-androgenic activity toward androgen-dependent Shionogi carcinoma cells SC-3 bearing wild-type AR.16 However, compound 6 increased the proliferation of human prostate cancer cell line LNCaP bearing T877A AR, with an EC50 value of 6.3 × 10−7 M.
 | ||
| Fig. 2 Structures of carborane derivatives with AR ligand activity. | ||
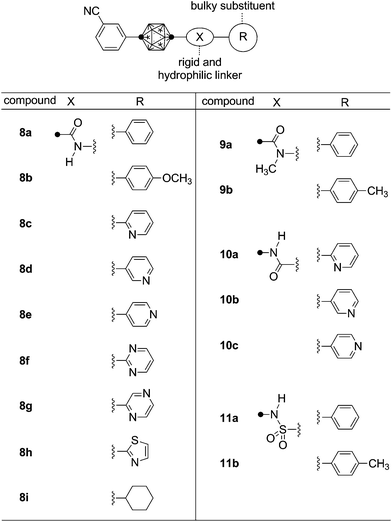
Recently, we have reported novel AR antagonists, such as compound 7, based on the structural features of both 5 and 6. These hybrid-type compounds acted as anti-androgens for LNCaP cells.17 These results suggest that introduction of a bulky substituent near the hydroxyl group of 6 is an effective strategy to develop anti-androgens for mutated ARs. The bulky substituent bound to the carborane core via an ether or a thioether linking group is assumed to inhibit proper folding of helix-12 of mutated AR, which is essential to transcriptional activation of nuclear receptors. According to this hypothesis, the rigidity of the linking group and the direction of the substituent would be important determinants of antagonistic potency. The T877A mutation in AR of LNCaP cells is thought to be one of the reasons for the change of 6 from antagonist to agonist. Docking simulation suggested that the T877 residue of wild-type AR interacts with the hydroxyl group of 6, as it does with the 17-hydroxyl group of 2.18 However, considering the antagonist activity of 6, the interaction of 6 with AR would induce a receptor conformation with slightly different folding of helix12 from that in the active form. In the case of T877A AR, both 2 and 6 will induce essentially the same active conformation of AR, because of the lack of hydrogen bonding with the T877A residue. If this is correct, we anticipated that introduction of a bulky substituent in place of the hydroxyl group would inhibit active folding of the mutated AR and so exert antagonistic activity. On the other hand, the hydroxyl group of 6 would also interact with N705, hence a polar functionality is needed at this position of the ligand structure to form hydrogen-bonding to N705 of both wild-type and T877A ARs. On the basis of these considerations, we designed carboranyl amide compounds 8–11, which have the required rigidity, hydrogen-bonding hydrophilic moiety and bulky folding-blocker, as candidate compounds with potent anti-androgenic activity toward LNCaP cells (Fig. 3).
 | ||
| Fig. 3 Structure of designed AR full antagonist candidates toward mutated AR. | ||
Scheme 1 illustrates the synthesis of the designed amide compounds. Compounds with the carboranecarboxamide moiety, 8 and 9, were synthesized from p-carborane (12) as a starting material. Ullmann-type coupling using the C-copper(I) derivative of p-carborane19 and 3-iodobenzonitrile gave phenylcarborane 13. Then a carboxyl moiety was introduced into the contralateral C-vertex of the carborane cage of 13 by using LDA and carbon dioxide to afford carboxylic acid 14. Condensation of 14 and various amines via acid chloride using oxalyl chloride gave the desired amide compounds 8a–i and 9a,b. Acyl- or sulfonylaminocarborane derivatives 10 and 11 were also synthesized from carboxylic acid 14. Primary amide 15 was prepared from 14 by amide condensation with ammonium hydroxide. Hofmann rearrangement of 15 using [bis(trifluoroacetoxy)iodo]benzene20 in tert-butyl alcohol gave the N-Boc-protected aminocarborane derivative 16 together with the free amine 17 as a minor product. The Boc group of 16 was cleaved with TFA to afford 17. Amide condensation of 17 with various acyl chlorides or sulfonyl chloride gave the desired molecules 10a–c and 11a,b, respectively.
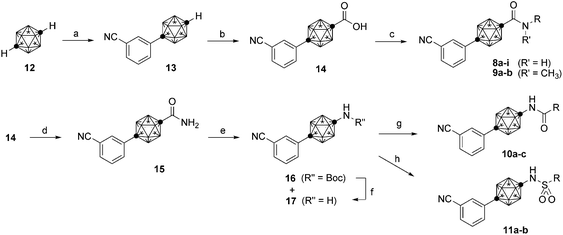 | ||
| Scheme 1 Synthesis of carborane derivatives 8–11. Reagents and conditions: (a) n-BuLi, CuCl, pyridine, DME, rt, then 3-iodobenzonitrile, reflux, 75%. (b) LDA, CO2, −78 °C, 83%. (c) (COCl)2, DMF, CH2Cl2, rt, then amine, pyridine, THF, rt. (d) (COCl)2, DMF, CH2Cl2, rt, then NH4OH, THF, rt, quant. (e) PhI(OCOCF3)2, tert-BuOH, 80 °C, 60% (16) and 13% (17). (f) TFA, CH2Cl2, rt, 87%. (g) Acyl chloride, pyridine, rt. (h) Sulfonyl chloride, pyridine, rt. | ||
The effects of the synthesized compounds on LNCaP cells were investigated in terms of growth-promoting or inhibitory activity.17 None of the synthesized compounds promoted cell growth in the absence of any other AR agonist, while some of them inhibited LNCaP cell growth promoted by 1 nM testosterone (Table 1). The anilide 8a potently inhibited cell growth with a submicromolar IC50 value. Heterocyclic amide derivatives also showed LNCaP cell growth-inhibitory activity with similar potency to that of anilide 8a. Compounds with both electron-deficient heterocycles (pyridine, pyrimidine and pyrazine) and an electron-rich heterocycle (thiazole) exhibited potent activity. Among the synthesized amide compounds, 2-pyridiyl amide 8c exhibited the most potent anti-androgenic activity toward LNCaP cells in this assay system. Anti-androgenic activity of cyclohexyl amide 8i was less potent as compared with aromatic amides, and N-methylation of anilide caused loss of inhibitory activity toward LNCaP cell proliferation (compounds 9a and 9b). These results suggested that a secondary N-heteroaromatic carboranecarboxamide structure is promising for anti-androgenic activity toward LNCaP cells. Compounds 10a–c bearing an acylaminocarborane moiety, i.e. retro-amide derivatives of compound 8, also exhibited anti-androgenic activity. Alteration of direction of the amide structure did not result in a significant change of anti-androgenic activity toward LNCaP cells. It was interesting that sulfonamides 11a and 11b exhibited no inhibitory activity toward LNCaP cell proliferation, whereas the benzenesulfonyl group is an important fragment of 5. This result can be partly explained by conformational differences. Secondary carboxamide compounds usually take linear trans conformation, whereas benzenesulfonamides take a bent gauche-like structure. N-Methylation of anilide also causes a structural change to folded cis conformation.21 These considerations suggest that linear secondary amide structure is important for novel carborane-based anti-androgens to exert anti-androgenic activity toward LNCaP cells.
| Compound | IC50a/M | Compound | IC50a/M |
|---|---|---|---|
| a LNCaP cells were incubated with test compounds in the presence of testosterone (1 nM) for 5 days. All assays were performed in triplicate (n = 3). b Compound 6 promoted LNCaP cell proliferation with an EC50 value of 6.3 × 10−7 M (see text). | |||
| 8a | 2.0 × 10−7 | 9a | >10−6 |
| 8b | 5.7 × 10−7 | 9b | >10−6 |
| 8c | 9.4 × 10−8 | 10a | 3.5 × 10−7 |
| 8d | 3.0 × 10−7 | 10b | 3.0 × 10−7 |
| 8e | 3.2 × 10−7 | 10c | 1.1 × 10−7 |
| 8f | 1.6 × 10−7 | 11a | >10−6 |
| 8g | 1.2 × 10−7 | 11b | >10−6 |
| 8h | 1.4 × 10−7 | 5 | 5.7 × 10−7 |
| 8i | 5.9 × 10−7 | 6 | Agonistb |
The effects of these compounds, which have potent anti-androgenic activity toward LNCaP cells, on wild-type AR were also evaluated in terms of growth-promoting or inhibitory activity toward androgen-dependent SC-3 cells and binding affinity to wild-type human AR ligand-binding domain (hAR-LBD).22 None of the tested compounds exhibited SC-3 cell growth-promoting activity. Table 2 summarizes the results of SC-3 cell growth-inhibitory activity and competitive binding assay to hAR-LBD. N-Heterocyclic amides (8c–h) showed SC-3 cell proliferation-inhibitory activity with a submicromolar IC50 value, and therefore these compounds were also anti-androgens for androgen-dependent SC-3 cells with wild-type AR. Among the tested compounds, 2-pyrimidinyl amide 8f and 2-thiazolyl amide 8h exhibited the most potent antagonistic activity toward SC-3 cells, and their potencies were comparable to that of the lead compound 6. The high binding affinity of 8f and 8h to hAR-LBD is consistent with these remarkable androgen-antagonistic activities. Compound 8c, the most potent anti-androgen for LNCaP cells, also exhibited considerable inhibitory potency toward DHT-promoted SC-3 cell growth. This result suggests that these N-heterocyclic carboranecarboxamides are full antagonists for both wild-type and T877A-mutated AR. Development of AR pan-antagonists which act as full antagonists for wild-type and any mutated AR is highly desirable for overcoming hormone-refractory prostate cancers, and these compounds should be versatile leads for such AR pan-antagonists. Regarding the acylaminocarborane derivatives, interestingly, isonicotinamide 10c did not exhibit significant SC-3 cell growth-inhibitory potency or binding affinity toward hAR-LBD. In the structure–anti-androgen activity relationship study for LNCaP cells, the direction of the amide structure was not critical for the activity, and both 10c and its retro-amide derivative 8e exhibited considerable anti-androgenic activity toward LNCaP cells. These results suggest that differences in the direction and substituent of the amide moiety are critical for receptor affinity and antagonistic activity toward wild-type AR, but are not important for anti-androgenic activity toward LNCaP cells. Therefore, it may be possible to discover selective antagonists for mutated AR by focusing on the differences in structure–activity relationships between wild-type AR and mutated AR. Mutant-selective AR modulators would have potential for various medicinal applications, and compound 10c is a potential lead compound for development of mutant-selective androgen receptor modulators.
| Compound | SC-3 proliferation IC50a/M | Inhibition of [3H]DHT binding to hARb |
|---|---|---|
| a SC-3 cells were incubated with test compounds in the presence of testosterone (1 nM) for 3 days. All assays were performed in triplicate (n = 3). b Values are percentage displacement of [3H]DHT (4 nM) specific binding to hAR-LBD by each compound at 10 μM. c Significant binding was not observed at the concentration of 10 μM. | ||
| 8c | 4.1 × 10−7 | 23 |
| 8d | 2.9 × 10−7 | 37 |
| 8e | 3.6 × 10−7 | 56 |
| 8f | 3.3 × 10−8 | 73 |
| 8g | 2.9 × 10−7 | 14 |
| 8h | 2.6 × 10−8 | 77 |
| 10a | 1.6 × 10−7 | 42 |
| 10b | 1.4 × 10−7 | 18 |
| 10c | >10−6 | NB |
| 6 | 1.5 × 10−8 | 95 |
In conclusion, we have developed novel anti-androgens active toward LNCaP cells, based on a carboranyl amide structure that is expected to inhibit the concise folding of helix-12. N-Heterocyclic amide 8c–h exhibited potent anti-androgenic activity toward both LNCaP cells with T877A-mutated AR and SC-3 cells with wild-type AR, whereas isonicotinamide 10c exhibited anti-androgenic activity toward only LNCaP cells. These compounds are expected to be versatile lead compounds not only for development of AR pan-antagonists, but also for discovery of mutant selective anti-androgens.
References
- (a) R. M. Evans, Science, 1988, 240, 889–894 CAS; (b) H. E. MacLean, G. L. Warne and J. D. Zajac, J. Steroid Biochem. Mol. Biol., 1997, 62, 233–242 CrossRef CAS; (c) I. J. McEwan, Biochem. Soc. Trans., 2000, 28, 369–373 CrossRef CAS; (d) M. E. Taplin, Expert Rev. Anti-Infect. Ther., 2008, 8, 1495–1508 CrossRef CAS.
- (a) A. D. Mooradian, J. E. Morley and S. G. Korenman, Endocr. Rev., 1987, 8, 1–28 CrossRef CAS; (b) C. J. Bagatell and W. J. Bremner, N. Engl. J. Med., 1996, 334, 707–714 CrossRef CAS; (c) J. M. Kaufman and A. Vermeulen, Endocr. Rev., 2005, 26, 833–876 CrossRef CAS.
- R. K. Ross, M. C. Pike, G. A. Coetzee, J. K. Reichardt, M. C. Yu, H. Feigelson, F. Z. Stanczyk, L. N. Kolonel and B. E. Henderson, Cancer Res., 1998, 58, 4497–4504 CAS.
- (a) J. L. Mohler, C. W. Gregory, O. H. Ford, III, D. Kim, C. M. Weaver, P. Petrusz, E. M. Wilson and F. S. French, Clin. Cancer Res., 2004, 10, 440–448 CrossRef CAS; (b) A. Jemal, H. Thomas, T. Murray and M. Thun, Ca-Cancer J. Clin., 2002, 52, 23–47 CrossRef; (c) T. Visakorpi, E. Hyytinen, P. Koivisto, M. Tanner, R. Keinanen, C. Palmberg, A. Palotie, T. Tammela, J. Isola and O. P. Kallioniemi, Nat. Genet., 1995, 9, 401–406 CrossRef CAS; (d) M. E. Taplin, G. J. Bubley, T. D. Shuster, M. E. Frantz, A. E. Spooner, G. K. Ogata, H. N. Keer and S. P. Balk, N. Engl. J. Med., 1995, 332, 1393–1398 CrossRef CAS.
- H. I. Scher, G. Steineck and W. K. Kelly, Urology, 1995, 46, 142–148 CrossRef CAS.
- (a) H. Suzuki, K. Akakura, A. Komiya, S. Aida, S. Akimoto and J. Shimazaki, Prostate (N. Y., NY, U. S.), 1996, 29, 153–158 CAS; (b) M. E. Taplin, B. Rajeshkumar, S. Halabi, C. P. Werner, B. A. Woda, J. Picus, W. Stadler, D. F. Hayes, P. W. Kantoff, N. J. Vogelzang and E. J. Small, J. Clin. Oncol., 2003, 21, 2673–2678 CrossRef CAS; (c) H. Miyamoto, M. M. Rahman and C. Chang, J. Cell. Biochem., 2003, 91, 3–12 CrossRef.
- (a) J. Veldscholte, C. A. Berrevoets, A. O. Brinkmann, J. A. Grootegoed and E. Mulder, Biochemistry, 1992, 31, 2393–2399 CrossRef CAS; (b) J. Zhou, B. Liu, G. Geng and J. H. Wu, Proteins: Struct., Funct., Bioinf., 2010, 78, 623–637 CAS.
- (a) M. A. Fenton, T. D. Shuster, A. M. Fertig, M. E. Taplin, G. Kolvenbag, G. J. Bubley and S. P. Balk, Clin. Cancer Res., 1997, 3, 1383–1388 CAS; (b) X. Y. Zhao, P. J. Malloy, A. V. Krishnan, S. Swami, N. M. Navone, D. M. Peehl and D. Feldman, Nat. Med. (N. Y.), 2000, 6, 703–706 CAS.
- (a) T. Hara, J. Miyazaki, H. Araki, M. Yamaoka, N. Kanzaki, M. Kusaka and M. Miyamoto, Cancer Res., 2003, 63, 149–153 CAS; (b) T. Yoshida, H. Kinoshita, T. Segawa, E. Nakamura, T. Inoue, Y. Shimizu, T. Kamoto and T. Ogawa, Cancer Res., 2005, 65, 9611–9616 CrossRef CAS.
- (a) Z. Culig, A. Hobisch, M. V. Cronauer, A. C. B. Cato, A. Hittmair, C. Radmayr, J. Eberle, G. Bartsch and H. Klocker, Mol. Endocrinol., 1993, 7, 1541–1550 CrossRef CAS; (b) M. Marcelli, M. Ittmann, S. Mariani, R. Sutherland, R. Nigam, L. Murthy, Y. Zhao, D. DiConcini, E. Puxeddu, A. Esen, J. Eastham, N. L. Weigel and D. J. Lamb, Cancer Res., 2000, 90, 944–949 Search PubMed.
- S. Fujii, T. Goto, K. Ohta, Y. Hashimoto, T. Suzuki, S. Ohta and Y. Endo, J. Med. Chem., 2005, 48, 4654–4662 CrossRef CAS.
- (a) S. Fujii, Y. Hashimoto, T. Suzuki, S. Ohta and Y. Endo, Bioorg. Med. Chem. Lett., 2005, 15, 227–230 CrossRef CAS; (b) T. Goto, K. Ohta, T. Suzuki, S. Ohta and Y. Endo, Bioorg. Med. Chem., 2005, 13, 6414–6424 CrossRef CAS; (c) K. Ohta, T. Goto, S. Fujii, T. Suzuki, S. Ohta and Y. Endo, Bioorg. Med. Chem., 2008, 16, 8022–8028 CrossRef CAS; (d) S. Fujii, K. Ohta, T. Goto, H. Kagechika and Y. Endo, Bioorg. Med. Chem., 2009, 17, 344–350 CrossRef CAS.
- (a) V. I. Bregadze, Chem. Rev., 1992, 92, 209–223 CrossRef CAS; (b) M. F. Hawthorne, Angew. Chem., Int. Ed. Engl., 1993, 32, 950–984 CrossRef; (c) A. H. Soloway, W. Tjarks and J. G. Barnum, Chem. Rev., 1998, 98, 1515–1562 CrossRef CAS.
- (a) Y. Endo, T. Iijima, Y. Yamakoshi, M. Yamaguchi, H. Fukasawa and K. Shudo, J. Med. Chem., 1999, 42, 1501–1504 CrossRef CAS; (b) Y. Endo, T. Iijima, Y. Yamakoshi, H. Fukasawa, C. Miyaura, M. Inada, A. Kubo and A. Itai, Chem. Biol., 2001, 8, 341–355 CrossRef CAS; (c) Y. Endo, T. Yoshimi, K. Ohta, T. Suzuki and S. Ohta, J. Med. Chem., 2005, 48, 3941–3944 CrossRef CAS; (d) M. Hirata, M. Inada, C. Matsumoto, M. Takita, T. Ogawa, Y. Endo and C. Miyaura, Biochem. Biophys. Res. Commun., 2009, 380, 218–222 CrossRef CAS; (e) K. Ohta, T. Ogawa, T. Suzuki, S. Ohta and Y. Endo, Bioorg. Med. Chem., 2009, 17, 7958–7963 CrossRef CAS.
- For retinoic acid receptor ligands: (a) T. Iijima, Y. Endo, M. Tsuji, E. Kawachi, H. Kagechika and K. Shudo, Chem. Pharm. Bull., 1999, 47, 398–404 CAS; (b) Y. Endo, K. Yaguchi, E. Kawachi and H. Kagechika, Bioorg. Med. Chem. Lett., 2000, 10, 1733–1736 CrossRef CAS; (c) Y. Endo, T. Iijima, K. Yaguchi, E. Kawachi, N. Inoue, H. Kagechika, A. Kubo and A. Itai, Bioorg. Med. Chem. Lett., 2001, 11, 1307–1311 CrossRef CAS For retinoid X receptor ligands: K. Ohta, T. Iijima, E. Kawachi, H. Kagechika and Y. Endo, Bioorg. Med. Chem. Lett., 2004, 14, 5913–5918 Search PubMed.
- D. Hiraoka, N. Nakamura, Y. Nishizawa, N. Uchida, S. Noguchi, K. Matsumoto and B. Sato, Cancer Res., 1987, 47, 6560–6564 CAS.
- T. Goto, K. Ohta, S. Fujii, S. Ohta and Y. Endo, J. Med. Chem., 2010, 53, 4917–4926 CrossRef CAS.
- K. Ohta, T. Goto, S. Fujii, M. Kawahata, A. Oda, S. Ohta, K. Yamaguchi, S. Hirono and Y. Endo, Bioorg. Med. Chem., 2011, 19, 3540–3548 CrossRef CAS.
- R. Coult, M. A. Fox, W. R. Gill, P. L. Herbertson, J. A. H. MacBride and K. Wade, J. Organomet. Chem., 1993, 462, 19–29 CrossRef CAS.
- A. S. Radhakrishna, M. E. Parham, R. M. Riggs and G. M. Loudon, J. Org. Chem., 1979, 44, 1746–1747 CrossRef CAS.
- (a) A. Itai, Y. Toriumi, N. Tomioka, H. Kagechika, I. Azumaya and K. Shudo, Tetrahedron Lett., 1989, 30, 6177–6180 CrossRef CAS; (b) H. Kagechika, E. Kawachi, Y. Hashimoto, K. Shudo and T. Himi, J. Med. Chem., 1988, 31, 2182–2192 CrossRef CAS.
- T. Ishioka, T. A. Kubo, Y. Koiso, K. Nagasawa, A. Itai and Y. Hashimoto, Bioorg. Med. Chem., 2002, 10, 1555–1566 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c1md00001b |
| This journal is © The Royal Society of Chemistry 2011 |
