N-(5-substituted thiazol-2-yl)-2-aryl-3-(tetrahydro-2H-pyran-4-yl) propanamides as glucokinase activators†
Zhiqing
Liu‡
a,
Qingzhang
Zhu‡
b,
Fuying
Li§
a,
Lina
Zhang
b,
Ying
Leng
*b and
Ao
Zhang
*a
aSynthetic Organic & Medicinal Chemistry Laboratory (SOMCL), Shanghai Institute of Materia Medica (SIMM), Chinese Academy of Sciences, Shanghai, 201203, China. E-mail: aozhang@mail.shcnc.ac.cn; Fax: +86-21-50806035; Tel: +86-21-50806035
bState Key Laboratory of Drug Research, Shanghai Institute of Materia Medica (SIMM), Chinese Academy of Sciences, Shanghai, 201203, China. E-mail: yleng@mail.shcnc.ac.cn; Fax: +86-21-50806059; Tel: +86-21-50806059
First published on 14th April 2011
Abstract
A series of novel arylacetamides were designed to further explore the GK binding property at the aminothiazole C5 position. The C5-amide substituted aminothiazoles 7a–f generally displayed decreased potency, whereas most of the C5-triazole substituted aminothiazoles retained good GK potency. Triazole 15 with a hydroxyethyl side chain was the most potent among the current series possessing an EC50 value of 0.18 μM. Its R-enantiomerR-15 showed similar potency (0.22 μM) that deserves for further evaluation.
Introduction
Glucokinase (GK), a member of the four hexokinase isozymes,1 catalyzes the first step in glycolysis and phosphorylation of glucose to glucose 6-phosphate. It has a limited tissue distribution and highly expressed in metabolically active tissues, including brain, pancreas, liver and gut.2 In the pancreatic β-cells, GK acts as a glucose-sensor determining the rate of glucose-induced insulin secretion therefore maintaining plasma glucose homeostasis.3–5 In the liver, GK is a rate-determining factor regulating glucose metabolism.6–11 Therefore, small molecules targeting this enzyme (GKA) have been proposed to activate the allosteric binding sites of this protein and to achieve anti-hyperglycemic effects by enhancing glucose uptake in the liver as well as by potentiating insulin secretion from pancreatic β-cells.12–17 This approach has led to several glucokinase activators (GKAs) in the early phases of clinical testing.19–24We recently reported structure–activity relationship study25 (SAR) on a series of C4-mono substituted or C4,C5-disubstituted aminothiazole analogues derived from the phase I clinical GK activator R-1 (PSN-GK1),18,26,27 which was invented by Fyfe and his colleagues26 at OSI Pharmaceuticals (Fig. 1). Different from R-1 that was designed to block the aminothiazolo C5-metabolically sensitive site by introduction of a C5-F substituent, our strategy was to indirectly block the C5-metabolic liability by introducing substituents at C4- or both C4 and C5 in the aminothiazole component. In this regard, compound 2 was identified with GK enzymatic potency of 157 nM (EC50) and its R-enantiomer significantly increased both glucose uptake and glycogen synthesis in rat primary cultured hepatocytes.25a
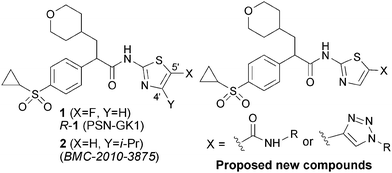 | ||
| Fig. 1 Representative GK activators and our newly proposed analogues. | ||
On the basis of the available co-crysral structures of GK and small molecule activator complex,12,28 it has been postulated that in GKAs like 1 and 2, the backbone hydrogen and carbonyl oxygen of Arg63 anchor the thiazole nitrogen and amide NH through H-bonding, respectively. The tetrahydro-2H-pyran-4-yl and the aryl are embedded in a lipophilic environment created by Met235, Tyr214, and Val62. Since the aminothiazole C-5 position is surrounded by a Glu67 residue, a hydrophilic substituent other than the previously explored alkyl or halogen moiety might be well tolerated.2,12,28 With this envision, we designed and synthesized a series of analogues of 1 or 2, by introducing an amide or its equivalent - triazole function at the aminothiazole C-5 position, and evaluated their GK enzymatic potency. Herein, we report the synthesis and pharmacology of these novel arylacetamide derivatives (Fig. 1).
Results and discussion
Chemistry
Similar to our previous study,25a all the newly proposed α-(tetrahydro-2H-pyran-4-yl)methyl-β-arylacetamides were synthesized as racemates (corresponding to the α–carbon in the arylacetamide skeleton) for preliminary evaluation. It has to be pointed out that the GK activation potency of racemic arylacetamides and corresponding R-enantiomers does not differentiate from each other significantly in vitro, however, only the R-enantiomer was active in vivo.12,26 Condensation of racemic 4-cyclopropylsulfonyl substituted phenylacetic acid (3)25,26 with corresponding aminothiazole was conducted following a standard literature procedure.25a,26 For the most potent compound, its R-configurated enantiomer was prepared by using R-configurated acid R-3, which was prepared in 95% ee, respectively by enantioselective hydrogenation of corresponding acryclic acid with Ir(I) complex of SpinPHOX (spiro[4,4]-1,6-nonadiene-based phosphine-oxazoline) following a procedure25b we reported recently.Preparation of compounds 7a–f containing an amino acid side chain was illustrated in Scheme 1. BOC-protected 2-aminothiazol-5-carboxylic acid 429 was condensed with naturally occurring amino acid esters H-Ala-OMe, H-Val-OMe, or H-Leu-OMe to give corresponding thiazoloamides 5, which were then de-BOC followed by condensation with acid 3 or its chloride yielding corresponding aminothiazol-5-yl-carbonylaminoacid esters 7a, 7c, 7e in 50–67% yields (final two steps). Hydrolysis30 of these methyl esters with LiOH afforded corresponding acids 7b, 7d, 7f in high yields.
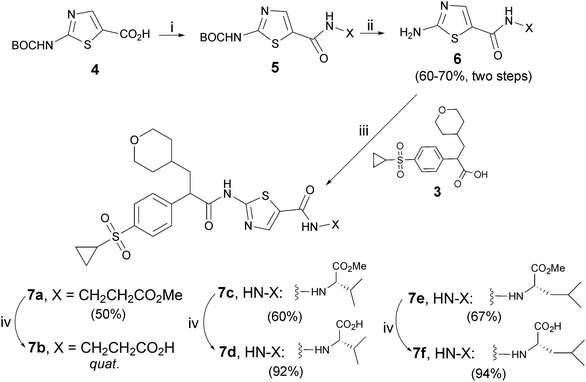 | ||
Scheme 1 Reagents and conditions: i) corresponding amino acid methylester, (COCl)2, cat.DMF, CH2Cl2, 0 °C to rt; ii) TFA, CH2Cl2, rt; iii) (COCl)2, DMF, CH2Cl2, -20 °C to rt; iv) LiOH·H2O, THF–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), rt. 1), rt. | ||
“Click” reaction was employed to introduce a triazole function as the C-5 appendage in aminothiazole component (Scheme 2). First, tert-butyl 5-ethynylthiazol-2-ylcarbamate 831 was reacted with an appropriate azido-ester under CuI/DIPEA and sodium L-ascorbate32 to give the corresponding triazolo-thiazole networks 9a–c in 93–95% yields. Final compounds 10a, 10b and 10d were obtained by de-protection of 9a–c followed by condensation with acid 3 in 29%, 68% and 48% yield, respectively. The lower yields were due to the incompletion of the condensation reaction. Acid 10c was obtained in 93% yield by hydrolysis30 of ester 10b. In addition, a series of amides 11a–g were prepared30 by hydrolysis of ester 9c followed by condensation with variant primary or secondary amines in 67–79% overall yields. A similar condensation of 11a–g with acid 3 under TBTU/DIPEA33 led to thiazole-triazoles 12a–g in 39–63% yields.
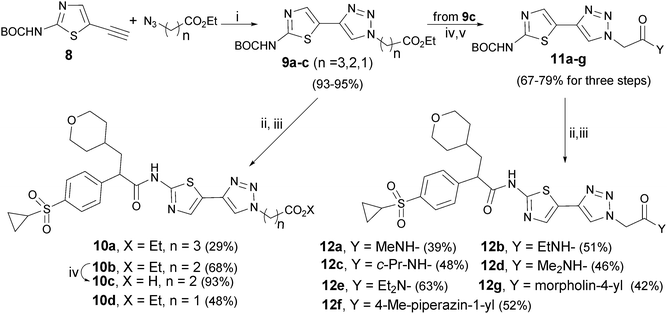 | ||
Scheme 2 Reagents and conditions: i) CuI, DIPEA, sodium L-ascorbate, THF, rt; ii) TFA, CH2Cl2, rt; iii) arylpropanoic acid 3, TBTU, DIPEA, CH2Cl2, rt; iv) LiOH·H2O, THF–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), rt; v) corresponding amine, TBTU, Et3N, CH2Cl2, rt. 1), rt; v) corresponding amine, TBTU, Et3N, CH2Cl2, rt. | ||
Benzyloxyethyltriazole 13 was prepared from thiazole 831 and benzyloxyethyl azide in two steps (30%), which was then treated with acid 3 to yield thiazole-triazole 14 in 58% yield. Removal34 of benzyl group in ether 14 with AlCl3 yielded hydroxyethyltriazole 15 in 51% yield (Scheme 3).
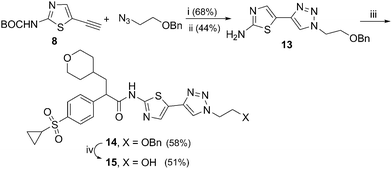 | ||
| Scheme 3 Reagents and conditions: i) CuI, DIPEA, sodium L-ascorbate, THF, rt; ii) TFA, CH2Cl2, rt; iii) arylpropanoic acid 3, TBTU, DIPEA, CH2Cl2, rt; iv) AlCl3, PhNMe2, CH2Cl2, rt, 5h. | ||
Glucokinase enzymatic assays
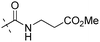
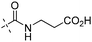
All the newly synthesized compounds were evaluated in an enzymatic glucokinase (GK) assay using purified recombinant human islet GK. GK activity was measured in a coupled reaction with glucose-6-phosphate dehydrogenase (G6PDH) through monitoring nicotinamide adenine dinucleotide phosphate (NADPH) production by the increase rate of absorbance at 340 nm in a Molecular Device SpectraMax 190 plate reader. The assay protocol is similar to that reported by us25a and others,26 and the GK activity of each compound was evaluated in six different concentrations and was reported as EC50 value and maximum activation fold (versus control level). Clinical compound R-1 (PSN-GK1),26,27 its racemic form 127 and compound 2 were also evaluated in our assays for comparison.As described in Table 1, introducing an amino acid methyl ester moiety (H-Ala-OMe, H-Val-OMe, and H-Leu-OMe) as the C-5 substituent in the 2-aminothiazole core led to compounds 7a, 7c, 7e displaying decreased GK potency. They showed EC50 values of 0.52, 1.44 and 1.52 μM, respectively, and were 5- to 17-fold less potent than compound 1. The less steric H-Ala-OMe analogue 7a has the highest potency among these three compounds. Hydrolysis of these methyl esters to their corresponding acids 7b,7d,7f remarkably decreased GK potency. This result indicated that an amide moiety with less steric effect is slightly tolerated at the C5 site of the aminothiazole component.
|
|
|||
|---|---|---|---|
| Cpd | R | EC50 (μM) | Activation Folda |
| a Maximum fold activation of GK over control level. NA = not active (EC50 > 20 μM). | |||
| R-1 | F | 0.062 (0.1325a) | 2.59 |
| 1 | F | 0.089 | — |
| 2 25a | — | 0.157 | 2.97 |
| 7a |
|
0.52 | 1.63 |
| 7b |
|
1.80 | 1.61 |
| 7c |
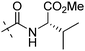
|
1.44 | 1.79 |
| 7d |
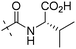
|
11.35 | 1.63 |
| 7e |
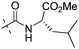
|
1.52 | 1.50 |
| 7f |
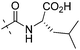
|
NA | 1.27 |
Triazole moiety can be viewed as a bioisosteric equivalent of the amide moiety. Therefore, C5-substituents containing this function were applied to evaluate the effect of H-bonding donor–acceptor property on the GK activity (Table 2). Triazole compounds 10a, 10b, 10d bearing an alkyl acid ester with different length as the tail group showed higher potency than the amides 7a–f. These compounds displayed almost identical EC50 values (0.45, 0.35, and 0.33 μM) indicating 1) the H-bonding acceptor property of triazole function is likely beneficial to the GK enzymatic activity, whereas the H-bonding donor property of the amide moiety in compounds 7a–f may hamper the ligand-enzyme interaction; 2) the length of the alkyl chain attached to the triazole function did not have impact to the interaction. However, the corresponding acid 10c showed a 4-fold decrease in potency, similar to acid 7b indicating that a carboxylic acid function has a negative contribution to GK activation.
|
|
|||
|---|---|---|---|
| Cpd | R | EC50 (μM) | Activation Folda |
| a Maximum fold activation of GK over control level. NA = not active (EC50 > 20 μM). | |||
| R-1 | F | 0.062 (0.1326) | 2.59 |
| 1 | F | 0.089 | - |
| 2 25a | — | 0.157 | 2.97 |
| 10a |
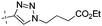
|
0.45 | 1.35 |
| 10b |
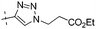
|
0.35 | 1.49 |
| 10c |
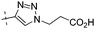
|
1.35 | 1.43 |
| 10d |
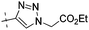
|
0.33 | 1.40 |
| 12a |
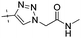
|
0.17 | 1.30 |
| 12b |
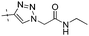
|
NA | 1.41 |
| 12c |
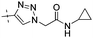
|
NA | 1.19 |
| 12d |
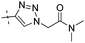
|
0.30 | 1.29 |
| 12e |
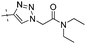
|
0.21 | 1.44 |
| 12f |
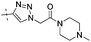
|
0.79 | 1.40 |
| 12g |
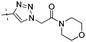
|
0.64 | 1.28 |
| 14 |
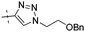
|
0.29 | 1.32 |
| 15 |
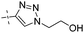
|
0.18 | 1.37 |
| R- 15 |
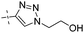
|
0.22 | 1.77 |
Conversion of the ester function in 10d to a series of amides led to compounds 12a–g showing a wide range of GK potency. Among the N-mono substituted amides 12a–c, only 12a with an N-Me substituent displayed good GK potency. It has an EC50 value of 0.17μM, while the larger N-Et (12b) and N-c-Pr (12c) substituents abolished GK potency completely. N,N-Disubstituted amides 12d–g all displayed high potency with similar EC50 values of 0.30, 0.21, 0.79, 0.64 μM, respectively, compatible to that of parent ester 10d. Interestingly, reduction of the ester 10d to alcohol 15 gave a 2-fold increase in potency with an EC50 value of 0.18 μM. Blocking the alcoholic OH to its benzyl ether (14) remained good GK potency.
From the results above, triazoles 12a and 15 stand out as the most potent GKAs among our present analogues, showing identical EC50 value (0.17 vs. 0.18 μM). However, better solubility was observed for compound 15 during the structural characterization. Therefore, the R-isomer of triazole 15, R-15, was prepared from the chiral acid precursor 3 (95% ee).25b However, partial racemerization was determined during the condensation reaction yielding compound R-15 with 82% ee. Similar to the clinical compound R-1, R-15 also displayed similar GK potency (EC50, 0.22 vs. 0.18 μM) as its racemate 15.
Discussion
The capacity of GK activators in augmentation of the insulin release from pancreatic β-cells as well as in suppression of the hepatic glucose production has hold GK as a promising anti-diabetic target. Although the structure model of GK allosteric activation and the co-crystal structures of GK with a few small molecules have been reported making the rational design of GKA possible, the currently reported GKAs (e.g. PSN-GK1, R-1) were dominantly derived from high-throughput-screening (HTS) campaign. As a continuation of SAR on the clinical compound R-1, we developed a series of novel compounds bearing a hydrophilic amide or triazole function at the C5 of the aminothiazole component. These compounds were designed to explore the H-bonding donor–acceptor property at the aminothiazole C5 binding site of the GK enzyme. The amide series 7a–f generally showed decreased potency, whereas most compounds in the triazole series retained good GK activity, indicating H-bonding acceptor property in the triazole function rather than the H-bonding donor property in the amide function is compatible to the enzyme-ligand interaction mode at the C5 site. The alkyl esteric side chain attaching to the triazole core is also important for the GK potency, since hydrolysis of the terminal ester moiety in compound 16b to acid 16c led to significant drop of potency (0.35 vs. 1.35 μM). This result indicated that a remote H-bonding acceptor (ester function) around C-5 is tolerated, whereas a H-bonding donor (acid function) is not. Further, conversion of the ester function to a small size amide (–CONHMe, 12a) increased the GK potency. In view of the statistically identical EC50 values of amides 12a, 12d and 12e, the H-bonding donor property of the amido NH in compound 12a seems not a key contributor to the GK interaction. This is also the case for compound 15 which contains a free alcoholic OH group displaying similar GK potency with the corresponding benzyl ether 14. Similar to the clinical compound R-1, the R-enantiomer of 15 (R-15) displayed nearly identical GK potency. Our next study will be focused on in vivo evaluation of this compound and it R-enantiomer.Conclusion
In summary, a series of novel arylacetamides were designed to further explore the GK binding property at the aminothiazole C5 position. The C5-amide substituted aminothiazoles 7a–f generally displayed low potency, whereas most of the C5-triazole substituted aminothiazoles retained good GK potency. Triazole 15 with a hydroxyethyl side chain was the most potent among the current series possessing an EC50 of 0.18 μM. Its R-enantiomerR-15 showed similar high potency (0.22 μM) that deserves for further evaluation.Acknowledgements
This work was supported by grants from Chinese National Science Foundation (81072528), National Basic Research Program of China (973 Program, 2009CB522300), National Science & Technology Major Project on “Key New Drug Creation and Manufacturing Program”, (2009ZX09301-001, 2009ZX09103-062), and Shanghai Commission of Science and Technology (10410702600,10JC1417100, 10dz1910104).References
- M. L. Cárdenas ( 1995) Glucokinase: its regulation and role in liver metabolism, R.G. Landes Co, Austin Search PubMed.
- A. M. Efanov, D. G. Barrett, M. B. Brenner, S. L. Briggs, A. Delaunois and J. D. Durbin, et al, Endocrinology, 2005, 146, 3696–3701 CrossRef CAS.
- F. M. Matschinsky, Diabetes, 1996, 45, 223–241 CrossRef CAS.
- F. M. Matschinsky and J. E. Ellerman, J. Biol. Chem., 1968, 243, 2730–2736 CAS.
- A. L. Gloyn, S. Odili, C. Buettger, P. R. Njolstad, C. Chiyo, M. A. Magnuson, F. M. Matschinsky, In Glucokinase and Glycemic Disease: From Basics to Novel Therapeutics;Matschinksy, F. M.; Magnuson, M. A.; ed.; Karger: Basel, 2004; 92–109 Search PubMed.
- E. Van Schaftingen, Diabetologia, 1994, 37(Suppl. 2), S43–47 CrossRef CAS.
- D. Farrelly, K. S. Brown, A. Tieman, J. Ren, S. A. Lira, D. Hagan, R. Gregg, K. A. Mookhtiar and N. Hariharan, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 14511–14516 CrossRef CAS.
- S. A. Ferris, Metab., Clin. Exp., 1964, 13, 1478–1481 CrossRef CAS.
- A. Basu, R. Basu, P. Shah, A. Vella, C. M. Johnson, K. S. Nair, M. D. Jensen, W. F. Schwenk and R. A. Rizza, Diabetes, 2000, 49, 272–283 CrossRef CAS.
- M. Hawkins, I. Gabriely, R. Wozniak, C. Vilcu, H. Shamoon and L. Rossetti, Diabetes, 2002, 51, 606–14 CrossRef CAS.
- L. Agius, Biochem. J., 2008, 414, 1–18 CrossRef CAS.
- J. Grimsby, R. Sarabu, W. L. Corbett, N.-E. Haynes and F. T. Bizzarro, et al, Science, 2003, 301, 370–373 CrossRef CAS.
- A. M. Efanov, D. G. Barrett, M. B. Brenner and S. L. Briggs, et al, Endocrinology, 2005, 146, 3696–3701 CrossRef CAS.
- R. Sarabu and J. Tilley, Annu. Rep. Med. Chem., 2005, 40, 167–181 Search PubMed.
- R. Sarabu and J. Grimsby, Curr. Opin. Drug Discovery Dev., 2005, 8, 631–637 CAS.
- T. O. Johnson and P. S. Humphries, Annu. Rep. Med. Chem., 2006, 41, 141–154 Search PubMed.
- K. R. Guertin and J. Grimsby, Curr. Med. Chem., 2006, 13, 1839–1843 CrossRef CAS.
- M. C. T. Fyfe, J. White, A. Taylor, R. Chatfield and E. Wargent, et al, Diabetologia, 2007, 50, 1277–1287 CrossRef CAS.
- M. Coghlan and B. Leighton, Expert Opin. Invest. Drugs, 2008, 17, 145–167 CrossRef CAS.
- J. Grimsby, S. J. Berthel and R. Sarabu, Curr. Top. Med. Chem., 2008, 8, 1524–1532 CrossRef CAS.
- R. Sarabu, S. J. Berthel, R. F. K. Kester and J. W. T. Tilley, Expert Opin. Ther. Pat., 2008, 18, 759–768 Search PubMed.
- F. M. Matschinsky, Nat. Rev. Drug Discovery, 2009, 8, 399–416 CrossRef CAS.
- M. Pal, Curr. Med. Chem., 2009, 16, 3858–3874 CrossRef CAS.
- M. Pal, Drug Discovery Today, 2009, 14, 784–792 CrossRef CAS.
- (a) F. Li, Q. Zhu, Y. Zhang, Y. Feng, Y. Leng and A. Zhang, Bioorg. Med. Chem., 2010, 18, 3875–3884 CrossRef CAS; (b) Y. Zhang, Z. Han, F. Li, K.- L. Ding and A. Zhang, Chem. Commun., 2010, 46, 156–158 RSC.
- L. S. Bertram, D. Black, P. H. Briner, R. Chatfield, A. Cooke and M. C. Fyfe, et al, J. Med. Chem., 2008, 51, 4340–4345 CrossRef CAS.
- P. H. Briner, M. C. T. Fyfe, J. P. Madeley, P. J. Murray, M. J. Procter, F. Spindler, World Patent Application No. WO 2006/016178.
- K. Kamata, M. Mitsuya, T. Nishimura, J. Eiki and Y. Nagata, Structure, 2004, 12, 429–438 CrossRef CAS.
- J. Das, P. Chen, D. Norris, R. Padmanabha, J. Lin and R. V. Moquin, et al, J. Med. Chem., 2006, 49, 6819–6832 CrossRef CAS.
- M. D. Chappell, S. E. Conner, A. E. Tripp, G. Zhu, World Patent Application No. WO2006086488.
- T. Sakamoto, H. Nagata, Y. Kondo, M. Shiraiwa and H. Yamanaka, Chem. Pharm. Bull., 1987, 35, 823–828 CAS.
- V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596 CrossRef CAS.
- P. D. Pierson, A. Fettes, C. Freichel, S. Gatti-McArthur and C. Hertel, et al, J. Med. Chem., 2009, 52, 3855–3868 CrossRef CAS.
- T. Akiyama, H. Hirofuji and S. Ozaki, Tetrahedron Lett., 1991, 32, 1321–1324 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c1md00002k |
| ‡ These two authors contributed equally to this work. |
| § Current Address: Drug Design & Synthesis Section, National institute on Drug Abuse and National Institute on Alcohol Abuse and Alcoholism, National Institute of Health, Rockville, MD, 20892-9415. |
| This journal is © The Royal Society of Chemistry 2011 |