Increase in plasma ceramide levels via secretory sphingomyelinase activity in streptozotocin-induced diabetic rats†
Keiko
Kobayashi
,
Ikuyo
Ichi
*,
Tomoka
Nakagawa
,
Chiaki
Kamikawa
,
Yuko
Kitamura
,
Eriko
Koga
,
Yukiko
Washino
,
Yukiko
Hoshinaga
and
Shosuke
Kojo
*
Department of Food Science and Nutrition, Nara Women's University, Nara, 630-8506, Japan. E-mail: ichi.ikuyo@ocha.ac.jp; kojo@ouj.ac.jp; Fax: +81 43 298 4691; Tel: +81 43 298 4691
First published on 21st April 2011
Abstract
Ceramide mediates apoptosis and is upregulated by oxidative stress. To reveal the causative agent of diabetes-induced complications, we examined the changes in ceramide metabolism during diabetes. Two and 8 weeks after intraperitoneal injection of streptozotocin (STZ: 40 mg kg−1 body weight) to rats, tissue ceramide levels were analyzed by liquid chromatography-electrospray tandem mass spectrometry (LC-MS/MS). Blood glucose was significantly increased 2 weeks after STZ administration. Alanine aminotransferase (ALT), aspartate aminotransferase (AST), and blood urea nitrogen (BUN) levels were also increased in the diabetic rats, suggesting that hepatic and renal damage was induced by STZ administration. Vitamin C, an indicator of oxidative stress, was significantly decreased in the plasma, liver, and kidney of rats 2 weeks after STZ administration. Although no differences in hepatic ceramide levels were observed between the control and diabetic rats, plasma and renal ceramide levels were significantly increased 8 weeks after STZ administration. In the liver and kidney, acid and neutral sphingomyelinase (SMase) activities were not increased, while secretory sphingomyelinase (sSMase) activity was increased in the plasma of diabetic rats after STZ administration. These data indicated that STZ administration induced the increase in plasma ceramide levels via the increase in sSMase activity. It was suggested that increased plasma ceramide levels were involved in the renal damage induced by STZ in diabetic rats accompanied with the enhancement of oxidative stress.
Introduction
Diabetes mellitus and its complications (such as nephropathy, atherosclerosis, retinopathy, neuropathy, and so on) are serious worldwide concerns in public health. Diabetes mellitus is a metabolic disease characterized by hyperglycaemia, and chronic hyperglycaemia and reactive oxygen species (ROS) are increased through non-enzymatic glycosylation and glucose autoxidation.1 Oxidative stress has been implicated in the development and progression of various diabetes-induced complications2 and antioxidants decrease diabetes-induced complications by ameliorating the damage by free radicals.3Treatment of rats with the beta-cell toxin streptozotocin (STZ) results in a diabetic state characterized by insulin deficiency and is used as a model of type 1 diabetes.4 Generation of ROS is involved in cytotoxic actions in STZ-induced diabetic rats,5,6 and STZ administration causes diabetes-induced complications in various tissues including the liver and kidney.7,8 It is possible that toxic materials are increased by the enhanced oxidative stress during diabetic conditions, thereby damaging the liver and kidney.
Ceramide has been implicated in regulating cell cycle arrest, apoptosis, and cellular senescence,9 and serves as an intracellular second messenger in these processes.10 Oxidative stress such as UV light, antineoplastic drugs and radiation induces ceramide accumulation in cells.11–14Ceramide is generated from sphingomyelin (SM) by sphingomyelinase (SMase), which is classified based on pH optimum, subcellular localization, and cation dependency.13,15 Neutral SMase (nSMase) is localized in the plasma membrane and exhibits an optimal pH of around 7.5 and Mg2+ dependence. Acid SMase (aSMase), with an optimal pH of 4.8, operates in the endosomal–lysosomal compartments or plasma membrane. Lysosomal and secretory SMase (sSMase) is derived from aSMase by differential protein trafficking of a common protein precursor,16 and sSMase is secreted by the vascular endothelium and macrophages and is the only enzyme responsible for sphingolytic activity in the plasma.17
We previously reported that the ceramide level in the plasma, liver, and kidney was increased via nSMase activation when oxidative stress was induced by CCl4 intoxication in rats.18,19 In addition, other studies have demonstrated that SMase activation and the increase in ceramide levels were important participants in atherosclerosis.20,21 Hyperglycaemia causes increased production of ROS in all tissues and plays a role in the development of complications in diabetes mellitus.22,23 A recent study demonstrated that the ceramide level was elevated in the plasma of patients with type 2 diabetes.24 Therefore, in the STZ-induced diabetic rats, ceramide may also be a putative mediator of lipotoxicity in diabetes-induced complications. In order to find a target enzyme to prevent diabetic complications, we investigated whether ceramide metabolism was altered in damaged tissues by STZ administration, and whether ceramide accumulation is correlated with the pathogenesis of diabetes-induced complications.
Results
Changes in blood glucose levels, ALT and AST activities, and blood urea nitrogen levels
This study was approved by the Animal Care Committee of Nara Women's University. The blood glucose level in the diabetic group after STZ administration was significantly higher than that in the control group (Fig. 1a). Within the control and diabetic groups, no differences in blood glucose levels at 2 and 8 weeks were observed. To examine the effect of STZ administration on hepatic and renal injury, we analyzed alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities and blood urea nitrogen (BUN) levels. Plasma AST and ALT levels were significantly increased by STZ administration (Fig. 1b and c). These levels at 8 weeks in the diabetic rats were further increased. At 2 weeks, the BUN levels in the control group were almost equal to those in the diabetic group. However, these levels in the diabetic rats at 8 weeks were significantly higher than those in the other groups (Fig. 1d).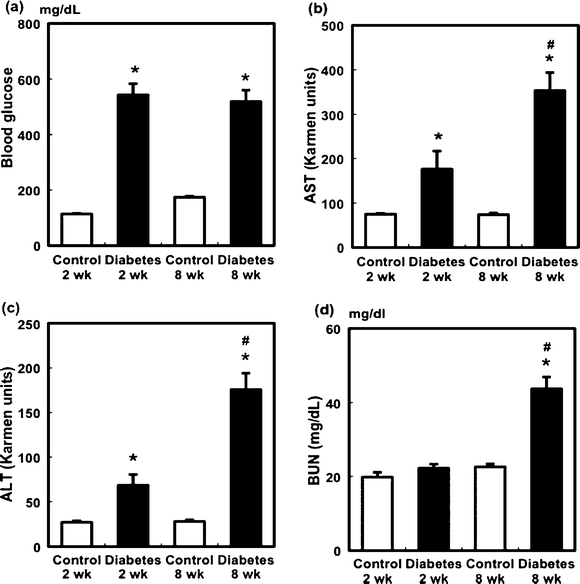 | ||
| Fig. 1 Changes in blood glucose levels (a), plasma AST (b) and ALT (c) activities, and BUN levels (d) in STZ-induced diabetic rats. Values are mean ± SEM (n = 5 or 6 in each group). *Significant difference between the control and diabetic groups (p < 0.05). #Significant difference between the 2- and 8-week groups (p < 0.05). | ||
Effect of STZ administration on vitamin C level in plasma, liver, and kidney
In the liver and kidney, the vitamin C level in the diabetic rats was significantly decreased 2 and 8 weeks after STZ administration. In the diabetic rats, the hepatic and renal vitamin C levels at 8 weeks were significantly lower than those at 2 weeks (Fig. 2a and b). Although no significant differences were observed in plasma vitamin C levels between the control and diabetic groups at 2 weeks, the diabetic rats exhibited a significant decrease in plasma vitamin C compared with the control group at 8 weeks (Fig. 2c).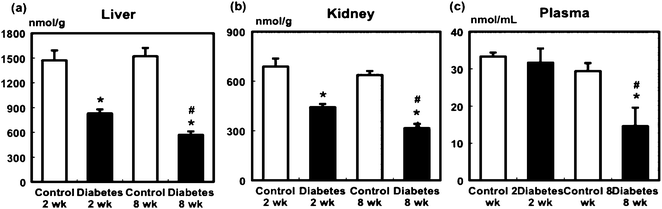 | ||
| Fig. 2 Effect of STZ administration on the level of vitamin C in the liver (a), kidney (b), and plasma (c) of the control and diabetic rats. Values are mean ± SEM (n = 5 or 6 in each group). *Significant difference between the control and diabetic groups (p < 0.05). #Significant difference between 2- and 8-week groups (p < 0.05). | ||
Effect of STZ administration on ceramide level in the liver, plasma, and kidney
Tissue ceramide concentration was determined using LC-MS/MS as described previously.14,18 In the liver, the major ceramides were C24:0 and C24:1 (Fig. 3a). STZ administration did not increase hepatic ceramide levels except for that of C16:0 at 8 weeks. However, in the diabetic rats, C24:1 and C24:2 levels were significantly increased at 8 weeks compared with those at 2 weeks.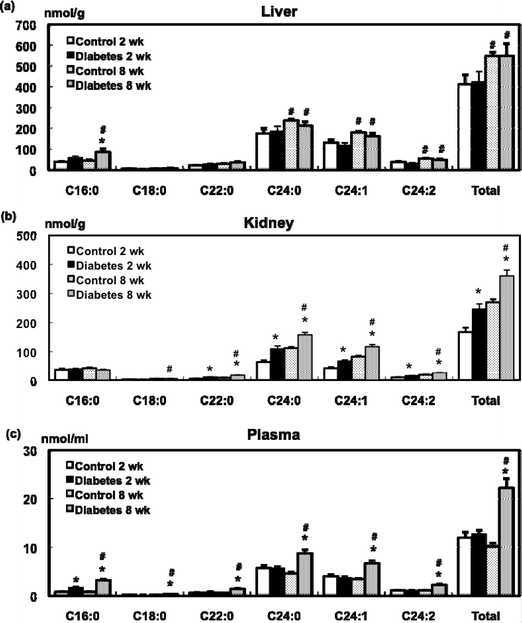 | ||
| Fig. 3 Effect of STZ administration on the level of ceramide in the liver (a), kidney (b), and plasma (c) of the control and diabetic rats. Values are mean ± SEM (n = 5 or 6 in each group). *Significant difference between the control and diabetic groups (p < 0.05). #Significant difference between 2- and 8-week groups (p < 0.05). | ||
C24:0 and C24:1 were also found to be major ceramides in the kidney (Fig. 3b). Levels of long-chain fatty acid ceramides such as C22:0, C24:0, C24:1, and C24:2 and total ceramides in the kidney in the diabetic rats were higher than those in the control rats at 2 weeks. The levels of these ceramides were further increased and a significant difference was also observed between the control and diabetic groups 8 weeks after STZ administration.
The major ceramides in the plasma were C24:0 and C24:1 in the control and diabetic rats (Fig. 3c). Plasma C16:0 levels in the diabetic rats at 2 weeks were increased compared to the control rats, and total plasma ceramide levels in the diabetic rats were higher than those in the control rats at 8 weeks. In the diabetic groups, the total plasma ceramide levels at 8 weeks were significantly higher than those at 2 weeks.
Changes in the SMase activity in STZ-induced diabetic rats
SMase, which directly generates ceramide, is a key regulatory step in the ceramide-signalling cascade.9 In the liver, STZ administration did not affect nSMase activity at 2 or 8 weeks (Fig. 4a). After 2 weeks, hepatic aSMase activity in the diabetic group was significantly lower than that in the control group (Fig. 4b). Although renal nSMase activity in the diabetic rats was higher than that in the control rats at 2 weeks (Fig. 4c), aSMase activity in the diabetic group at 2 and 8 weeks was lower compared to that in the control group (Fig. 4d). Because the plasma ceramide level was increased in STZ-induced diabetic rats, sSMase activity was measured. Plasma sSMase activity was found to be significantly increased 2 and 8 weeks after STZ administration compared to that in the control group (Fig. 4e).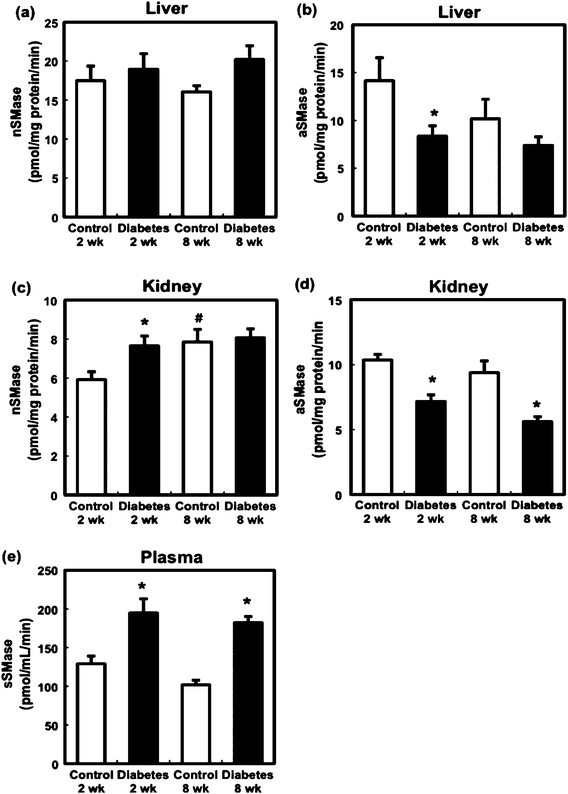 | ||
| Fig. 4 Effect of STZ administration on nSMase activity in the liver (a) and kidney (c), aSMase activity of liver (b) and kidney (d), and sSMase activity (e) in the control and diabetic rats. Values are mean ± SEM (n = 5 or 6 in each group). *Significant difference between the control and diabetic groups (p < 0.05). #Significant difference between 2- and 8-week groups (p < 0.05). | ||
Discussion
Ceramide has been implicated in various diseases such as atherosclerosis,25,26 which accompanies enhanced oxidative stress. STZ injection in animals produces various kinds of ROS such as superoxide, hydroxyl radical, and lipid hydroperoxides.5–8 In this study, we examined whether STZ administration caused tissue damage and affected ceramide metabolism at 2 weeks (early) and 8 weeks (advanced stage) during the progression of oxidative stress.Chronic hyperglycaemia occurring in uncontrolled diabetes leads to significant long-term damage and failure of various organs. STZ-induced diabetic rats also exhibit many complications observed in human diabetic patients.27 Our results demonstrated that plasma ALT and AST levels were significantly augmented 2 weeks after STZ injection and further increased after 8 weeks, showing signs of liver injury as early as 2 weeks after STZ administration. A major complication of diabetes is renal disease such as nephropathy.2 In this study, we also demonstrated that the plasma BUN level 8 weeks after STZ administration was significantly increased in the diabetic group compared to the control group, showing that STZ injection induced diabetes-induced complications in the rat kidney. Similar increases in BUN and ceramide levels in the plasma and kidney have been reported under severe oxidative stress leading to renal failure during fulminant hepatic failure induced by CCl4 intoxication.18
Diabetic mellitus induces a requirement for antioxidants such as vitamin C28 and enhancement of oxidative stress induces the development of diabetes. Our previous studies have showed that the concentrations of vitamin C, which is an outstanding hydrophilic antioxidant in tissues and is consumed primarily under conditions of enhanced oxidative stress, most sensitively reflects oxidative stress in the rat liver during chemical intoxication.29 In this study, the liver vitamin C level was significantly decreased 2 weeks after STZ injection, showing that oxidative stress was enhanced in the liver. Therefore, the decrease in the level of antioxidants such as vitamin C may induce the liver injury in STZ-induced diabetic rats similar to chemically induced hepatitis18,30,31 where hepatic vitamin C levels are decreased by a similar extent.
We have previously demonstrated that hepatic ceramide levels and nSMase activity were increased by CCl4 administration, which enhances oxidative stress.18,19 However, in this study, using STZ-induced diabetic rats, the hepatic ceramide level was almost identical to that in the control group, and hepatic nSMase and aSMase activities were not increased in diabetic rats compared with control rats. Although the hepatic ceramide level was increased in a rat model of type 2 diabetes,24,32 our results indicated that the hepatic damage in type 1 diabetic rats was not due to ceramide accumulation. These results suggested that the enhancement of oxidative stress via hyperglycaemia led to liver dysfunction, and that the ceramide accumulation was not associated with the pathogenesis of liver injury in the STZ diabetic rats at least 8 weeks after STZ administration.
Hyperglycaemia augments oxidative stress in the plasma due to the overproduction of free radicals and decreased efficiency of the antioxidant defense system. In this study, the plasma vitamin C level was decreased as early as 2 weeks after STZ administration and its level remained at this low level 8 weeks after STZ administration. Two weeks after STZ administration, only plasma C16:0 ceramide was significantly increased and this increase may correspond to the elevation of plasma sSMase activity. However increase in other ceramides was not observed. This result suggests that catabolism system of ceramide for example in the kidney still effectively operated at the early phase of diabetes. At 8 weeks the levels of all species of plasma ceramide were elevated, and the activity of plasma sSMase, the only sphingolytic enzyme in plasma, was significantly increased following STZ administration. Therefore, the increase in plasma ceramide level by STZ injection may be ascribed to the elevated sSMase activity. STZ-induced-β-cell destruction mediates the release of cytokines such as TNF-α,33 and that sSMase secretion from endothelial cells is stimulated by a variety of proinflammatory mediators such as TNF-α.34 Therefore, the increase in plasma sSMase activity might be stimulated by increased cytokine production.
Renal damage was confirmed based on BUN levels 8 weeks after STZ administration when low concentration of renal vitamin C was maintained. Kashiba et al.35 reported the impaired regeneration of renal vitamin C and urinary excretion of vitamin C were increased following STZ injection. Consistent with this study, which demonstrated that STZ administration induced renal deterioration along with the decrease of vitamin C, antioxidant supplements were found to inhibit the increased ROS production and effectively prevent renal dysfunction in STZ-induced diabetic rats.36 Because the cellular uptake of vitamin C is promoted by insulin and inhibited in conditions such as hyperglycaemia,37 it is reasonable that vitamin C treatment significantly decreased BUN levels in diabetic rats.38 Therefore, the enhancement of oxidative stress is an important factor leading to the development of renal damage at an advanced stage.
In the kidney, all long-chain fatty acid-bound ceramides except C16:0 and C18:0 were increased in STZ-induced diabetic rats. Therefore, accumulation of these ceramides may also contribute to the renal damage. In this study, renal nSMase and aSMase activities did not increase in the diabetic rats at 8 weeks compared with those in control rats, suggesting that increased ceramide levels at the advanced stage of diabetes were not due to the production but the transport of ceramides from the plasma. These results imply that ceramide accumulation is a cause of renal dysfunction in STZ-induced diabetic rats.
Conclusions
Ceramide accumulation was induced in the plasma and kidney of STZ-induced in diabetic rats, and accompanied by a decrease in vitamin C levels. Furthermore, elevated sSMase activity was suggested to be involved in the increase in plasma ceramide levels. Thus, the increase in plasma ceramide levels may play an important role in the pathogenesis of diabetes-induced complications. Therefore sSMase is a possible target to develop an inhibitor for the prevention of diabetic complications.Abbreviations
| aSMase | acid sphingomyelinase |
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| BUN | blood urea nitrogen |
| LC-MS/MS | liquid chromatography-electrospray tandem mass spectrometry |
| NBD | nitrobenzofurazan |
| nSMase | neutral sphingomyelinase |
| ROS | reactive oxygen species |
| SM | sphingomyelin |
| SMase | sphingomyelinase |
| sSMase | secretory sphingomyelinase |
| STZ | streptozotocin |
Acknowledgements
This work was supported by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology, Japan.References and notes
- L. W. Oberley, Free Radical Biol. Med., 1988, 5, 113–124 CrossRef CAS.
- J. Baynes, Diabetes, 1991, 40, 405–412 CrossRef CAS.
- T. Peerapatdit, A. Likidlilid, N. Patchanans and A. Somkasetrin, J. Med. Assoc. Thailand, 2006, 89, S141–S146 Search PubMed.
- Y. Le Marchand-Brustel and P. Freychet, J. Clin. Invest., 1979, 64, 1505–1515 CrossRef CAS.
- T. Szkudelski, Physiol. Res., 2001, 50, 536–546 Search PubMed.
- F. Sun, K. Iwaguchi, R. Shudo, Y. Nagaki, K. Tanaka, K. Ikeda, S. Tokumaru and S. Kojo, Clin. Sci., 1999, 96, 185–190 Search PubMed.
- G. Andican and G. Burcak, Clin. Exp. Pharmacol. Physiol., 2005, 32, 663–666 CrossRef CAS.
- A. R. Kraynak, R. D. Storer, R. D. Jensen, M. W. Kloss, K. A. Soper, J. H. Clair, J. G. Deluca, W. W. Nichols and R. S. Eydelloth, Toxicol. Appl. Pharmacol., 1995, 135, 279–286 CrossRef CAS.
- Y. A. Hannun and L. M. Obeid, J. Biol. Chem., 2002, 277, 25847–25850 CrossRef CAS.
- R. Kolesnick, Trends Cell Biol., 1992, 2, 232–236 CrossRef CAS.
- A. M. Farrell, Y. Uchida, M. M. Nagiec, I. R. Harris, R. C. Dickson, P. M. Elias and W. M. Holleran, J. Lipid Res., 1998, 39, 2031–2038 CAS.
- P. Santana, L. A. Pena, A. Haimovitz-Friedman, S. Martin, D. Green, D. M. McLoughlin, C. Cordon-Cardo, E. H. Schuchman, Z. Fuks and R. Kolesnick, Cell, 1996, 86, 189–199 CrossRef CAS.
- A. H. Merrill and D. D. Jones, Biochim. Biophys. Acta, 1990, 1044, 1–12 CAS.
- Y. Yamada, K. Kajiwara, M. Yano, E. Kishida, Y. Masuzawa and S. Kojo, Biochim. Biophys. Acta, 2001, 1532, 115–120 CAS.
- F. M. Goni and A. Alonso, FEBS Lett., 2002, 531, 38–46 CrossRef CAS.
- S. L. Schissel, G. A. Keesler, E. H. Schuchman, K. J. Williams and I. Tabas, J. Biol. Chem., 1998, 273, 18250–18259 CrossRef CAS.
- I. Tabas, Chem. Phys. Lipids, 1999, 102, 123–130 CrossRef CAS.
- I. Ichi, C. Kamikawa, T. Nakagawa, K. Kobayashi, R. Kataoka, E. Nagata, Y. Kitamura, C. Nakazaki, T. Matsura and S. Kojo, Toxicology, 2009, 261, 33–40 CrossRef CAS.
- I. Ichi, K. Nakahara, K. Fujii, C. Iida, Y. Miyashita and S. Kojo, J. Nutr. Sci. Vitaminol., 2007, 53, 53–56 Search PubMed.
- S. Marathe, G. Kuriakose, K. J. Williams and I. Tabas, Arterioscler., Thromb., Vasc. Biol., 1999, 19, 2648–2658 CAS.
- N. Auge, A. Negre-Salvayre, R. Salvayre and T. Levade, Prog. Lipid Res., 2001, 39, 207–229.
- N. Arun and N. Nalini, Plant Foods Hum. Nutr., 2002, 57, 41–52 CrossRef CAS.
- D. A. Allen, S. Harwood, M. Varagunam, M. J. Raftery and M. M. Yaqoob, FASEB J., 2003, 17, 908–910 CAS.
- J. M. Haus, S. R. Kashyap, T. Kasumov, R. Zhang, K. R. Kelly, R. A. DeFronzo and J. P. Kirwan, Diabetes, 2009, 58, 337–343 CAS.
- T. S. Park, W. Rosebury, E. K. Kindt, M. C. Kowala and R. L. Panek, Pharmacol. Res., 2008, 58, 45–51 CrossRef CAS.
- I. Ichi, Y. Takashima, N. Adachi, K. Nakahara, C. Kamikawa, M. Harada-Shiba and S. Kojo, Lipids, 2007, 42, 893–900 CrossRef CAS.
- N. Rakieten, M. L. Rakieten and M. V. Nadkarni, Cancer Chemother. Rep., 1963, 29, 91–98 Search PubMed.
- S. R. Maxwell, H. Thomason and D. Sandler, Eur. J. Clin. Invest., 1997, 27, 484–490 CrossRef CAS.
- S. Kojo, Curr. Med. Chem., 2004, 11, 1041–1064 CrossRef CAS.
- F. Sun, E. Hamagawa, C. Tsutsui, N. Sakaguchi, Y. Kakuta, S. Tokumaru and S. Kojo, Biochem. Pharmacol., 2003, 65, 101–107 CrossRef CAS.
- F. Sun, S. Hayami, Y. Ogiri, S. Haruna, K. Tanaka, Y. Yamada, S. Tokumaru and S. Kojo, Biochim. Biophys. Acta, 2000, 1500, 181–185 CAS.
- J. Turinsky, D. M. O'Sullivan and B. P. Bayly, J. Biol. Chem., 1990, 265, 16880–16885 CAS.
- K. C. Herold, V. Vezys, Q. Sun, D. Viktora, E. Seung, S. Reinr and D. R. Brown, J. Immunol., 1996, 156, 3521–3527 CAS.
- S. Marathe, S. L. Schissel, M. J. Yellin, N. Beatini, R. Mintzer, K. J. Williams and I. Tabas, J. Biol. Chem., 1998, 273, 4081–4088 CrossRef CAS.
- K. Kashiba, J. Oka, R. Ichikawa, E. Kasahara, T. Inayama, A. Kageyama, H. Kageyama, T. Osaka, K. Umegaki, A. Matsumoto, T. Ishikawa, M. Nishikimi, M. Inoue and S. Inoue, Free Radical Biol. Med., 2002, 33, 1221–1230 CrossRef.
- P. Manna, M. Sinha and P. C. Sil, Chem.-Biol. Interact., 2009, 181, 297–308 CrossRef CAS.
- J. J. Cunningham, J. Am. Coll. Nutr., 1998, 17, 105–108 CAS.
- M. Al-Shamsi, A. Amin and E. Adeghate, Ann. N. Y. Acad. Sci., 2006, 1084, 371–390 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c0md00154f |
| This journal is © The Royal Society of Chemistry 2011 |
