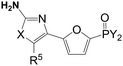
Oxazole phosphonic acids as fructose 1,6-bisphosphatase inhibitors with potent glucose-lowering activity
Qun
Dang†
*,
Srinivas Rao
Kasibthatla‡
,
Tao
Jiang§
,
Frank
Taplin
,
Tony
Gibson¶
,
Scott C.
Potter
,
Paul D.
van Poelje||
and
Mark D.
Erion†
Departments of Chemistry and Biochemistry, Metabasis Therapeutics, Inc., 11119 North Torrey Pines Road, La Jolla, CA 92037, USA. E-mail: qun_dang@merck.com
First published on 21st February 2011
Abstract
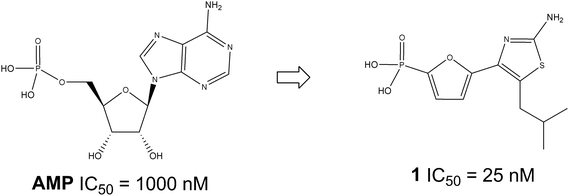
To discover an alternative heterocyclic scaffold to the thiazole series of FBPase inhibitors such as thiazole1, oxazoles were investigated to see if replacing the sulfur of the thiazole scaffold with an oxygen is tolerated. Numerous oxazoles with a phosphonic group linked by both 2,5-furandiyl and –COOCH2– groups were prepared and several oxazoles were discovered to have similar potency as thiazole1. For example, oxazole3.10 has an IC50 of 70 nM and lowered blood glucose in normal fasted rats by 61%.
Fructose 1,6-bisphosphatase (FBPase) has long been recognized as a drug discovery target for lowering glucose in type 2 diabetes mellitus (T2DM).1,2 Recent reports of oral FBPase inhibitors provided proof-of-concept in both T2DM animal models3–7 and patients,8 although past research in this field led to the discovery of various FBPase inhibitors without in vivo efficacy.9–16
The discovery of phosphonic acids as novel AMP mimics produced several series of potent and selective FBPase inhibitors.5,17–20 For example, MB06322 (CS-917), a phosphonic diamide prodrug of the thiazole FBPase inhibitor1 (Fig. 1), demonstrated significant glucose lowering in T2DM patients thus established inhibition of FBPase as a proven approach to lower glucose levels in vivo.8 To expand the SAR of the thiazole series of FBPase inhibitors, various oxazole phosphonic acids were also explored as potential FBPase inhibitors. Establishment of oxazoles as FBPase inhibitors will not only expand intellectually property coverage, but also provide an alternative scaffold to the thiazoles. Given the potential differences between oxazoles and thiazoles with regards to polarity and metabolic stability, having oxazoles FBPase inhibitors will enable fine-tuning of pharmacokinetic properties and solubility. Herein we report the synthesis and the SAR of oxazole phosphonic acids as FBPase inhibitors.
Synthesis
The 2-amino-oxazoles were prepared via thermal cyclization reactions of α-bromoketones with urea, as depicted in Scheme 1. Thus, α-bromination of ketones220 using copper(II) bromide followed by treatment with excess urea in tert-butanol, and final TMSBr-mediated removal of the phosphonate diethyl ester produced oxazole phosphonic acids 3.1–3.20.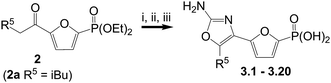 | ||
| Scheme 1 Reagents and conditions: i. CuBr2, EtOAc–CHCl3; ii. Urea, tBuOH, 80 °C; iii. TMSBr, CH2Cl2. | ||
C2 oxazole analogs were prepared using cyclization reactions of bromoketones with substituted ureas and amides, as outlined in Scheme 2. Thus, α-bromination of ketones2a using copper(II) bromide and cyclization of the resulting bromide with excess R2CONH2 in tert-butanol followed by TMSBr-mediated removal of the diethyl ester gave oxazoles4.1–4.5.
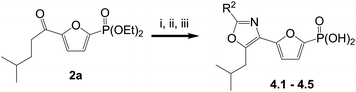 | ||
| Scheme 2 Reagents and conditions: i. CuBr2, EtOAc–CHCl3; ii. Urea, tBuOH, 80 °C; iii. TMSBr, CH2Cl2. | ||
Oxazoles with ester-linked phosphonic acids were prepared from pyruvate esters5, as shown in Scheme 3. Thus, esters5, which were prepared via Fisher esterification as previously reported,20 were brominated using copper(II) bromide and subsequent cyclization with urea followed by TMSBr-mediated deprotection of phosphonate diester gave oxazoles6.1–6.5.
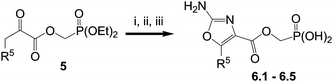 | ||
| Scheme 3 Reagents and conditions: i. CuBr2, EtOAc–CHCl3; ii. Urea, tBuOH, 80 °C; iii. TMSBr, CH2Cl2. | ||
The phosphonic diamide prodrugs of the oxazole phosphonate FBPase inhibitor3.10 were prepared using the dichloridate method21 as depicted in Scheme 4.
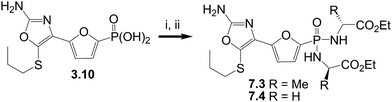 | ||
| Scheme 4 Reagents and conditions: i. SO2Cl2; ii. amine, Hunig's base, CH2Cl2. | ||
Results and discussion
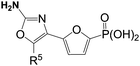
The 2-amino-oxazole analogs (3.1–3.20) with various C5-groups were evaluated as FBPase inhibitors and results are summarized in Table 1.22|
|
||||
|---|---|---|---|---|
| Compds | R5 | HLF IC50/μMa | RLF IC50/μMa | Glucose lowering (%) |
| a HLF, human liver FBPase; RLF, rat liver FBPase; IC50 is an average of 3 runs; glucose lowering screening assay was carried out using 3 rats per group, after i.v. dosing of 10 mg kg−1 compounds. b ND, not determined. | ||||
| 3.1 | iBu | 0.12 | 2 | 51 |
| 3.2 | H | 10 | NDb | ND |
| 3.3 | Allyl | 0.14 | 3 | 19 |
| 3.4 | nBu | 0.17 | 2.3 | 10 |
| 3.5 | nPentyl | 0.21 | 2.7 | 7 |
| 3.6 | -CH2-cyclohexyl | 0.32 | 5.5 | ND |
| 3.7 | Ph | 0.16 | 2.5 | 8 |
| 3.8 | Bn | 0.9 | 10 | ND |
| 3.9 | -CH2-(2-thienyl | 0.26 | 3.75 | ND |
| 3.10 | nPrS | 0.07 | 0.8 | 61 |
| 3.11 | iPrS | 0.11 | 0.9 | 61 |
| 3.12 | tBuS | 0.12 | 1.7 | ND |
| 3.13 | PhS | 4 | ND | ND |
| 3.14 | -CO2Me | 0.067 | 3.9 | 0 |
| 3.15 | -CO2Et | 0.038 | 2.9 | 18 |
| 3.16 | -CO2Pr-i | 0.04 | ND | 7 |
| 3.17 | -CO2Bn | 0.085 | 2.25 | 0 |
| 3.18 | -COSEt | 0.03 | 2.3 | 51 |
| 3.19 | -CONHMe | 1 | ND | 0 |
| 3.20 | -COBu-t | 0.855 | ND | ND |
Oxazole 3.1, which is the corresponding analog of thiazole1, is a potent inhibitor of human liver FBPase with an IC50 of 120 nM, albeit it is about 5-fold weaker than thiazole1. The C5-H oxazole 3.2 is more than 80-fold weaker than 3.1, indicating that a suitable C5-group is essential for FBPase inhibitory activity. Oxazoles3.3–3.9 with other C5-groups such as allyl, alkyl, phenyl and benzyl are not more potent than 3.1, on the other hand the C5-propylsulfide oxazole 3.10 is about 2-fold more potent than 3.1. Moreover, oxazole3.10 elicited potent glucose lowering effects after i.v. administration in the normal fasted rat assay, 61% reduction of blood glucose levels compared to vehicle-treated animals. Oxazoles3.11 and 3.12, which have larger alkyl-sulfides and are 2-fold less potent than 3.10, while the C5-phenylthio analog 3.13 lost FBPase inhibitory activity significantly (>57-fold) compared to 3.10. Esters and thioesters are also tolerated at the C5-position of the oxazole scaffold, leading to analogs that are more potent than 3.1 (IC50 30–85 nM). It is noteworthy that oxazole3.18 exhibited biological activities comparable to thiazole1 (IC50 against human liver FBPase and i.v. glucose-lowering in rats). The C5-amido (3.19) and C5-keto (3.20) oxazoles are weaker than 3.1, suggesting that amido and keto groups are not tolerated as well as esters. Next, the C2-SAR was investigated using isobutyl as the C5-group, and results are summarized in Table 2.
|
|
||
|---|---|---|
| Compds | R2 | HLF, IC50/μMa |
| a HLF, human liver FBPase; IC50 is an average of 3 runs. b This is the regio-isomer of 4.2: 2-methyl-4-isobutyl-5-[2-(5-phosphono)-furanyl]oxazole. | ||
| 3.1 | H2N- | 0.12 |
| 4.1 | Meb | 6 |
| 4.2 | Me | 0.6 |
| 4.3 | HO | 10 |
| 4.4 | H | 1.9 |
| 4.5 | Me2N- | 2.1 |
| 4.6 | iPr- | 2.2 |
| 4.7 | MeHN- | 4.3 |
| 4.8 | Et | 0.95 |
| 4.9 | EtHN- | 10 |
| 4.10 | Vinyl | 6.7 |
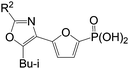
Two C2-methyl oxazoles with the phosphonofuranyl group linked to either the 4- (4.2) or 5-positions (4.1) were prepared. Oxazole4.2 is 10-fold more potent then 4.1: assuming oxazoles4.1 and 4.2 are binding to the AMP site of FBPase in a similar manner as thiazole1,5 this result suggests that N is a better hydrogen-bond acceptor than O for binding to FBPase. Conversely, oxazole4.2 is 5-fold weaker than 3.1, indicating that the 2-amino group is preferred and consistent with reported modeling studies.23 Other groups such as hydroxyl, H, alkyl, vinyl and substituted-amino groups (4.3–4.10) at the C2-position all led to loss of inhibitory potency against human liver FBPase. The ester linker group was reported for the thiazole scaffold leading to analogs with comparable potency against human liver FBPase to the furan linker. Therefore, various ester-linked oxazoles (6.1–6.9) were evaluated and results are summarized in Table 3.
|
|
|||||
|---|---|---|---|---|---|
| Compds | R2 | R5 | HLF IC50/μMa | RLF IC50/μMa | Glucose lowering (%) |
| a HLF, human liver FBPase; RLF, rat liver FBPase; IC50 is an average of 3 runs; glucose lowering was measured after i.v. dosing of 10 mg kg−1 compounds to 3 rats. b ND, not determined. | |||||
| 6.1 | H2N- | H | 7 | NDb | ND |
| 6.2 | H2N- | Me | 0.42 | ND | ND |
| 6.3 | H2N- | Et | 0.38 | ND | ND |
| 6.4 | H2N- | nPr | 0.28 | ND | ND |
| 6.5 | H2N- | iPr | 0.58 | ND | ND |
| 6.6 | H2N- | nButyl | 0.25 | 1.7 | 22 |
| 6.7 | H2N- | nPentyl | 0.35 | ND | ND |
| 6.8 | Me | CF3- | 10 | ND | ND |
| 6.9 | H | Ph | 10 | ND | ND |
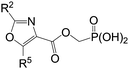
The SAR presented in Table 3 indicates that similar to the furan-oxazole scaffold the ester-oxazole scaffold also prefers an amino group at C2-position and an alkyl group at C5-position for inhibition of human liver FBPase. The most potent ester-linked oxazole6.6 is 2-fold weaker than 3.1, but maintained inhibitory potency against rat liver FBPase. Thus, oxazole6.6 was evaluated in the normal fasted rat assay, but no significant glucose-lowering was achieved after i.v. administration.
The oxazole-phosphonate FBPase inhibitors are not expected to have good oral bioavailability (OBAV) due to its highly charged nature under physiological conditions. To achieve oral efficacy, phosphonic diamideprodrugs were prepared for oxazole3.10 and results are summarized in Table 4.
|
|
||||||
|---|---|---|---|---|---|---|
| Compds | X | R5 | Y | MW | OBAV (%) | cLogP |
| a OBAV, determined by measuring urinary excretion of 3.10 following oral administration of 7.3 and 7.4vs. i.v. administration of 3.10; cLoP was calculated using ADME Boxes version 3.5 (Pharma Algorithm, Toronto, Canada). | ||||||
| MB06322 | S | iBu | Ala-OEt | 500.6 | 22 | 2.75 |
| 7.2 | S | nPrS | Ala-OEt | 518.6 | 22 | 2.84 |
| 7.3 | O | nPrS | Ala-OEt | 502.5 | 12 | 1.99 |
| 7.4 | O | nPrS | Gly-OEt | 474.5 | 11 | 1.42 |
The oxazole diamides 7.3 and 7.4 showed OBAV of 12 and 11%, respectively, which are 2-fold lower than thiazole diamides MB06322 and 7.2. Molecular weight is not the cause for the differences between the oxazole and thiazole scaffolds since all four diamides have similar MW. On the other hand, the oxazole scaffold appears to be more polar than the thiazole scaffold, as suggested by the lower cLogP values, which could be a contributing factor to the lower OBAV observed for oxazoles.
To evaluate in vivoglucose activity, oxazole3.1 was selected for a detailed study in the normal fasted rat assay, and results are shown in Fig. 2 and Fig. 3.
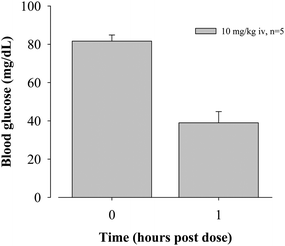 | ||
| Fig. 2 Glucose-lowering activity of oxazole3.1. | ||
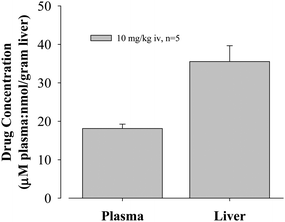 | ||
| Fig. 3 Liver and plasma levels of oxazole3.1. | ||
After intravenous administration of oxazole3.1 at 10 mg kg−1 dose, blood glucose levels were lowered by 51% compared to vehicle-treated animals.
Drug levels for oxazole3.1 are about 2-fold higher in the liver compared to plasma. Liver drug levels (measured using wet liver samples) correlated well with glucose-lowering activity.
In summary, oxazoles were explored as an alternative heterocyclic scaffold to the thiazole series of FBPase inhibitors such as thiazole1. Replacement of the sulfur in the thiazole scaffold with an oxygen is permitted, leading potent inhibitors of FBPase. Numerous oxazoles with a phosphonic acid group linked via either 2,5-furandiyl or –COOCH2– groups were prepared. Several oxazoles were discovered to have similar potency as thiazole1. For example, oxazole3.10 has an IC50 of 70 nM and lowered blood glucose in normal fasted rats by 61%; oxazole3.18 has an IC50 of 30 nM and lowered blood glucose in normal fasted rats by 51%. Two phosphonic diamideprodrugs of oxazole3.10 were prepared, compounds 7.3 and 7.4, which gave OBAV of 11–12%.
References
- P. D. Van Poelje, Q. Dang and M. D. Erion, Drug Discovery Today: Ther. Strategies, 2007, 4, 103 Search PubMed.
- M. L. Mohler, Y. He, Z. Wu, D. J. Hwang and D. D. Miller, Med. Res. Rev., 2009, 29, 125 CrossRef CAS.
- M. D. Erion, P. D. van Poelje, Q. Dang, S. R. Kasibhatla, S. C. Potter, M. R. Reddy, K. R. Reddy, T. Jiang and W. N. Lipscomb, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 7970 CrossRef CAS.
- P. D. van Poelje, S. C. Potter, V. C. Chandramouli, B. R. Landau, Q. Dang and M. D. Erion, Diabetes, 2006, 55, 1747 CrossRef CAS.
- Q. Dang, S. R. Kasibhatla, K. R. Reddy, T. Jiang, M. R. Reddy, S. C. Potter, J. M. Fujitaki, P. D. van Poelje, J. Huang, W. N. Lipscomb and M. D. Erion, J. Am. Chem. Soc., 2007, 129, 15491 CrossRef CAS.
- T. Yoshida, A. Okuno, M. Izumi, K. Takahashi, Y. Hagisawa, J. Ohsumi and T. Fujiwara, Eur. J. Pharmacol., 2008, 601, 192 CAS.
- T. Tsukada, O. Kanno, T. Yamane, J. Tanaka, T. Yoshida, A. Okuno, T. Shiiki, M. Takahashi and T. Nishi, Bioorg. Med. Chem., 2010, 18, 5346 CrossRef CAS.
- J. Triscari, J. Walker, K. Feins, B. Tao, S. R. Bruce. Multiple Ascending Doese of CS-917, a Novel Fructose 1,6-bisphosphatase (FBPase) Inhibitor, in Subjects with Type 2 Diabetes Treated for 14 Days, ADA, 2006 Search PubMed.
- B. E. Maryanoff, A. B. Reitz, G. F. Tutwiler, S. J. Benkovic, P. A. Benkovic and S. J. Pilkis, J. Am. Chem. Soc., 1984, 106, 7851 CrossRef CAS.
- S. W. Wright, D. L. Hageman, L. D. McClure, A. A. Carlo, J. L. Treadway, A. M. Mathiowetz, J. M. Withka and P. H. Bauer, Bioorg. Med. Chem. Lett., 2001, 11, 17 CrossRef CAS.
- S. W. Wright, A. A. Carlo, M. D. Carty, D. E. Danley, D. L. Hageman, G. A. Karam, C. B. Levy, M. N. Mansour, A. M. Mathiowetz, L. D. McClure, N. B. Nestor, R. K. McPherson, J. Pandit, L. R. Pustilnik, G. K. Schulte, W. C. Soeller, J. L. Treadway, I. K. Wang and P. H. Bauer, J. Med. Chem., 2002, 45, 3865 CrossRef CAS.
- S. W. Wright, A. A. Carlo, D. E. Danley, D. L. Hageman, G. A. Karam, M. N. Mansour, L. D. McClure, J. Pandit, G. K. Schulte, J. L. Treadway, I. K. Wang and P. H. Bauer, Bioorg. Med. Chem. Lett., 2003, 13, 2055 CrossRef CAS.
- C. Lai, R. J. Gum, M. Daly, E. H. Fry, C. Hutchins, C. Abad-Zapatero and T. W. von Geldern, Bioorg. Med. Chem. Lett., 2006, 16, 1807 CrossRef CAS.
- T. W. von Geldern, C. Lai, R. J. Gum, M. Daly, C. Sun, E. H. Fry and C. Abad-Zapatero, Bioorg. Med. Chem. Lett., 2006, 16, 1811 CrossRef CAS.
- M. S. Shaikh, A. Mittal and P. V. Bharatam, J. Mol. Graphics Modell., 2008, 26, 900 CrossRef CAS.
- A. Rudnitskaya, K. Huynh, B. Torok and K. Stieglitz, J. Med. Chem., 2009, 52, 878 CrossRef CAS.
- M. D. Erion, Q. Dang, M. R. Reddy, S. R. Kasibhatla, J. Huang, W. N. Lipscomb and P. D. van Poelje, J. Am. Chem. Soc., 2007, 129, 15480 CrossRef CAS.
- Q. Dang, B. S. Brown, Y. Liu, R. M. Rydzewski, E. D. Robinson, P. D. van Poelje, R. M. Reddy and M. D. Erion, J. Med. Chem., 2009, 52, 2880 CrossRef CAS.
- Q. Dang, S. R. Kasibhatla, W. Xiao, Y. Liu, J. DaRe, F. Taplin, K. R. Reddy, G. R. Scarlato, T. Gibson, P. D. van Poelje, S. C. Potter and M. D. Erion, J. Med. Chem., 2010, 53, 441 CrossRef CAS.
- Q. Dang, Y. Liu, D. K. Cashion, S. R. Kasibhatla, T. Jiang, F. Taplin, J. Jacintho, H. Li, Z. Sun, K. Fan, J. DaRe, F. Tian, W. Li, T. Gibson, R. Lemus, P. D. van Poelje, S. C. Potter and M. D. Erion, J. Med. Chem., 2011, 54, 153 CrossRef CAS.
- Q. Dang, S. R. Kasibhatla, T. Jiang, K. Fan, Y. Liu, F. Taplin, W. Schulz, D. K. Cashion, K. R. Reddy, P. D. van Poelje, J. M. Fujitaki, S. C. Potter and M. D. Erion, J. Med. Chem., 2008, 51, 4331 CrossRef CAS.
- Biological methods: human and rat liver FBPase assays,3,5 normal fasted rat glucose-lowering assay,18 and oral bioavailability screening assay21 were carried out as previously reported.
- M. R. Reddy and M. D. Erion, J. Am. Chem. Soc., 2001, 123, 6246 CrossRef CAS.
- General synthetic procedures: 2-Amino-5-isobutyl-4-[2-(5-phosphono)furanyl]oxazole (3.1). A solution of ketone2a (786 mg, 2.6 mmol) in anhydrous ethanol (10 mL) was treated with copper(II) bromide (1.28 g, 5.72 mmol) heated to reflux under nitrogen for 1 h. The cooled reaction mixture was filtered and the solid washed with ethanol (2 × 10 mL). The combined filtrate and washing was concentrated under reduced pressure to give the crude α-bromoketone as an oil. The crude bromoketone was then dissolved in tert-butanol (50 mL), treated with urea (313 mg, 5.2 mmol) and heated at reflux. After 24 h, the cooled reaction solution was concentrated under reduced pressure, and the crude material was purified by flash chromatography (SiO2, 1, 3, 5% MeOH-CH2Cl2, gradient elution) to give 2-amino-5-isobutyl-4-[2-(5-diethylphosphono)furanyl]oxazole as a yellow solid (220 mg, 25%). A solution of 2-amino-5-isobutyl-4-[2-(5-diethylphosphono)furanyl]oxazole (100 mg, 0.29 mmol) in anhydrous dichloromethane (1 mL) was treated with TMSBr (442 mg, 2.9 mmol). After stirring at room temperature for 16 h, the reaction mixture was evaporated to dryness and the residue was suspended in water (2 mL). The resulting solid was collected viafiltration (washed with water, 2 × 5 mL) and dried under vacuum to give 3.1 as an white solid (70 mg, 84%). mp 250 °C (decomp). 1H NMR (DMSO- d6): δ 6.89 (m, 1H), 6.66 (bs, 2H), 6.45 (m, 1H), 2.69 (d, 2H, J = 6.8 Hz), 1.92 (m, 1H), 0.90 (d, 6H, J = 6.8 Hz). [MH]+ calcd for C11H15N2O5P: 287. Found: 287. Anal. calcd. for C11H15N2O5P: C: 46.16, H: 5.28, N: 9.79. Found, C: 45.83, H: 4.90, N: 9.66. Other compounds 3.2–3.20, 4.1–4.10, 6.1–6.9 were prepared in a similar manner as compound 3.1, while prodrugs7.3 and 7.4 were prepared using previously reported procedures21 and all final compounds have purity >95% determined viaelemental analysis (CHN).
Footnotes |
| † Present address: Merck Research Laboratories, Rahway, NJ, USA. |
| ‡ Present address: BMS Biocon Research Center, Bangalore, India. |
| § Present address: Genomics Institute of the Novartis Research Foundation, San Diego, CA, USA. |
| ¶ Present address: Takeda San Diego Inc., San Diego, CA, USA. |
| || Present Address: Pfizer, Inc., Groton, CT, USA. |
| This journal is © The Royal Society of Chemistry 2011 |