Extended side chain analogues of 8-aminoquinolines: Synthesis and evaluation of antiprotozoal, antimicrobial, β-hematin inhibition, and cytotoxic activities
Kirandeep
Kaur
a,
Meenakshi
Jain
a,
Shabana I.
Khan
cd,
Melissa R.
Jacob
c,
Babu L.
Tekwani
ce,
Savita
Singh
b,
Prati Pal
Singh
b and
Rahul
Jain
*a
aDepartment of Medicinal Chemistry, National Institute of Pharmaceutical Education and Research, Sector 67, S. A. S. Nagar, Punjab, 160 062, India. E-mail: rahuljain@niper.ac.in; Fax: +91-172-221-4692; Tel: +91-172-229-2024
bDepartment of Pharmacology and Toxicology, National Institute of Pharmaceutical Education and Research, Sector 67, S. A. S. Nagar, Punjab, 160 062, India
cNational Center for Natural Products Research, School of Pharmacy, University of Mississippi, MS 38677, USA
dDepartment of Pharmacognosy, School of Pharmacy, University of Mississippi, MS 38677, USA
eDepartment of Pharmacology, School of Pharmacy, University of Mississippi, MS 38677, USA
First published on 28th February 2011
Abstract
We report the synthesis of double, triple and quadruple extended side chain analogues of the antimalarial drug primaquine and some other 8-aminoquinolines. The synthesized analogues have exhibited potent antimalarial activities in vitro against both the drug-sensitive D6 strain (IC50 = 0.19–0.92 μg mL−1) and the drug-resistant W2 strain (IC50 = 0.12–0.82 μg mL−1) of P. falciparum and in vivo against drug-sensitive P. berghei infected mice (100% curative at 25 mg kg−1 day−1, and resulted in either 4/6 or 5/6 cures at 10 mg kg−1 day−1) for the most promising structures. These analogues were also found to be free of cytotoxic effects at the highest test concentration of 23.8 μg mL−1 in a panel consisting of six cell lines. The promising 8-aminoquinolines inhibited β-hematin (IC50 = 9.6–20.8 μM) in vitro underlining the disruption of the heme catabolism pathway in the malaria parasite as their potential biochemical pathway for antimalarial action. The analogues also displayed potent antileishmanial activities in vitro against L. donovani promastigotes (IC50 = 1.6–32 μg mL−1; IC90 = 4–40 μg mL−1) and moderate in vitro antimicrobial activities against a panel of bacteria and fungi.
Introduction
Primaquine (PQ, 1, Fig. 1), is the only drug active against both the latent liver forms of the relapsing malaria caused by the Plasmodium vivax and P. ovale and the gametocytes from all species of the parasite causing human malaria.1 However, primaquine has a short plasma half-life of approximately 4–6 h,2 presumably due to its rapid metabolism, including oxidative deamination of the parent side chain leading to the formation of carboxyprimaquine (2, Fig. 1).3–5 The use of PQ is often associated with serious adverse effects as a consequence of its toxic metabolites generated through cytochrome P450 mediated reactions, which have been considered to be directly responsible for complications, such as hemolytic anemia.6PQ toxicity is further aggravated in people with a genetic deficiency of glucose-6-phosphate dehydrogenase. Other side effects include mild anemia, cyanosis and methemoglobinemia. The adverse effects are further amplified by the fact that PQ must be repeatedly administered at high doses, owing to its limited oral bioavailability.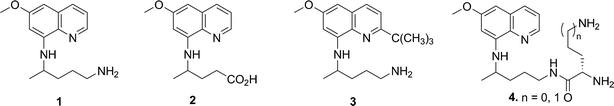 | ||
| Fig. 1 8-Aminoquinolines. | ||
Peptide and amino acid derivatives of 1 have been prepared to reduce the toxicity of the parent drug as well as to suppress the metabolic pathway leading to 2,7–10 but many of these derivatives are rapidly hydrolyzed to primaquine by aminopeptidases and endopeptidases.8,10 It is well known that 4-aminoquinolines accumulate in the food vacuole through pH trapping and the presence of the basic aminoalkyl side chain plays an essential role in β-hematin inhibition.11 However, the role of the basic aminoalkyl side chain of 8-aminoquinolines (8-AQ) in β-hematin inhibition is still an unexplored area of interest. Previous work in our laboratory has shown that attachment of basic amino acids to the side chain of 8-AQ led to an overall improvement in the therapeutic index due to the protection of the parent side chain functionality.12 Therefore, it is safe to assume that the conversion of the primary amino group in the parent aminoalkyl side chain of 8-AQ to a secondary amino group in the designed analogues may not allow its oxidative deamination, resulting in increased antimalarial efficacy. Based on these considerations, we proposed to synthesize 8-aminoquinolines bearing an extended basic aminoalkyl chain.
Recently, we have demonstrated that 2-tert-butylprimaquine (BPQ, 3, Fig. 1), an 8-AQ analogue devoid of methemoglobin toxicity, inhibits β-hematin formation in vitro (IC50 = 2.1–2.9 μM). The observation that 3 binds with heme indicated that its antimalarial mechanism may arise from the inhibition of heme crystallization through the formation of a complex with heme, resulting in the increase of the toxicity of free heme against the malaria parasite.13 Previously, a small number of other 8-AQ analogues, including tafenoquine, have also been shown to inhibit β-hematin formation in vitro.14 We proposed that increasing the length of side chain in 8-AQ would increase their overall basicity resulting in greater accumulation in the malaria parasite’s food vacuole (the site for β-hematin formation).15 Encouraged by the activity results of previously reported Lys and Orn conjugates of 8-AQ (4, Fig. 1),12 we selected N4-alkylpentane-1,4-diamine as the basic moiety to be attached to the aminoalkyl side chain of 8-aminoquinolines, thereby providing compounds analogous to Lys or Orn conjugates of 8-AQ with increased stability to aminopeptidases and endopeptidases. We also expect these extended side chain analogues to be more efficacious than parent 8-AQ especially against chloroquine-resistant (CQR) strains of P. falciparum, because some 4-aminoquinolines like tert-butylisoquine and ferroquine having lipophilic moieties in the side chain have retained activity against CQR malaria parasites.16–18 Similarly, the attachment of one to three N4-alkylpentane-1,4-diamine chains to the parent side chain of 8-AQ will increase the lipophilicity, while increasing or retaining their basicity.
Results and discussion
The extended side chain modified analogues 13–17, 22–25 and 30–33 of 8-AQ were prepared according to the synthetic methodology shown in Scheme 1. The reaction of PQ (1) or its analogues 3 or 5–7 with 2-(4-bromopentyl)-1,3-isoindolinedione in the presence of triethylamine (Et3N) easily provided diones 8–12 (Scheme 1). The compounds 8–12 upon hydrazinolysis using hydrazine hydrate in 95% EtOH gave the desired 1,4-diamines, 13–17, in excellent yields except for 17, which was obtained in 54% yield due to the degradation of product during column chromatography. The latter compounds 13–16, upon repeat condensation reaction with 2-(4-bromopentyl)-1,3-isoindolinedione in Et3N gave diones 18–21. The hydrazinolysis reaction of compounds 18–21, as described earlier, easily afforded 1,4-diamines 22–25. The second repeat of the condensation and side-chain deprotection reactions afforded 1,4-diamines 30–33 (Scheme 1).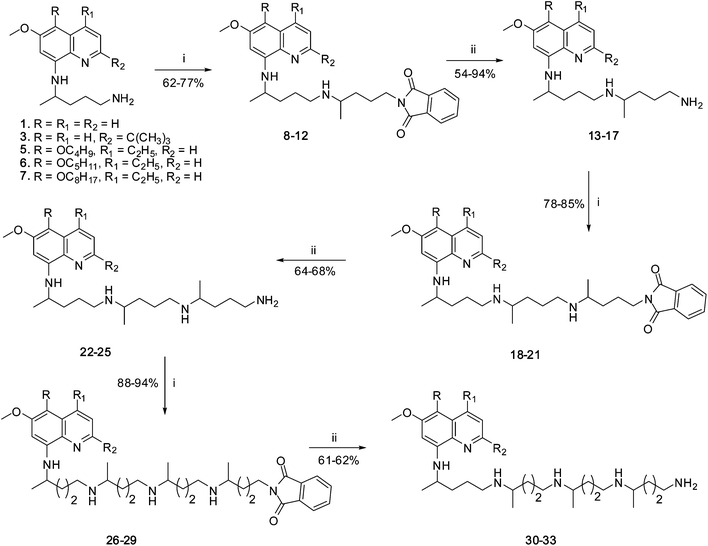 | ||
| Scheme 1 Reagents and conditions: (i) 2-(4-bromopentyl)-1,3-isoindolinedione, Et3N, rt, 8 h; (ii) NH2NH2·H2O, EtOH, reflux, 1–6 h. | ||
In vitro antimalarial activity of the synthesized compounds was determined on the basis of plasmodial lactate dehydrogenase (LDH) activity,19 and expressed as IC50 values versuschloroquine-sensitive (D6) and chloroquine-resistant (W2) strains of P. falciparum. The analogues were also evaluated for in vivo blood-schizontocidal antimalarial activity against P. berghei (sensitive strain) in a rodent malaria model.20 The in vitro cytotoxicity of analogues was determined against four human cancer cell lines (SK-MEL, KB, BT-549, and SK-OV-3) and two noncancerous cell lines (VERO and LLC-PK1) (obtained from ATCC, American Type Culture Collection) by neutral red assay.21,22 In line with the earlier observation that BPQ (3) acts via the inhibition of β-hematin, the synthesized analogues were also evaluated for β-hematin inhibitory activity in vitro.13 In view of the use of 8-aminoquinolines as potential antileishmanial drugs,1 antileishmanial activity of the compounds was tested in vitro against a culture of L. donovani promastigotes by Alamar Blue assay.23,24 The antibacterial activities of the synthesized compounds were evaluated in vitro against Staphylococcus aureus, methicillin-resistant S. aureus (MRSA), Mycobacterium intracellulare, Escherichia coli, and Pseudomonas aeruginosa. The susceptibility of S. aureus and MRSA to test compounds was determined according to the procedure as described by the NCCLS.25–28 The susceptibility of M. intracellulare was done using the modified Alamar Blue procedure of Franzblau et al.29 The antifungal activities of the target compounds against pathogenic fungi associated with opportunistic infections (Candida albicans, C. glabrata, C. krusei, Cryptococcus neoformans, and Aspergillus fumigatus) were determined according to NCCLS methods.25–28
The in vitroantimalarial activity (P. falciparum, D6 and W2 clones), cytotoxicity, β-hematin (BH) inhibition and in vitro antileishmanial activity results of the tested compounds are summarized in Table 1. The extended side chain analogues in general produced high antimalarial activity. The most promising analogue 14 [R = R1 = H, R2 = C(CH3)3] displayed IC50 values of 0.19 and 0.12 μg mL−1 against D6 and W2 strains, respectively. Analogue 16 (R = OC5H11, R1 = C2H5, R2 = H), possessed IC50 values of 0.52 and 0.18 μg mL−1 against D6 and W2 strains, respectively. While analogue 32 (R = OC4H9, R1 = C2H5, R2 = H) exhibited IC50 values of 0.49 μg mL−1 for D6 clone and 0.35 μg mL−1 for W2 clone. Most interestingly and importantly, the extended side chain analogues displayed superior activities against the drug-resistant W2 strain with high selectivity indices indicating the promise of this class in the treatment of drug-resistant malaria. None of the analogues showed cytotoxicity up to highest test concentration of 23.8 μg mL−1 providing high selective indices in the range between 9.1 and 198.
| Compd. No. | R | R1 | R2 | P. falciparum (D6) | P. falciparum (W2) | Cytotoxicity (Vero) | BH Inhibition | L. donovani | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IC50 (μg mL−1) | SI b | IC50 (μg mL−1) | SI b | IC50 (μg mL−1) | IC50 (μM) | IC50 (μg mL−1) | IC90 (μg mL−1) | ||||
| a IC50 and IC90 are the sample concentration that kills 50% and 90% cells compared to vehicle control. NC, not cytotoxic up to (23.8 μg mL−1). NA, Not active. “—”, Not tested. Chloroquine: IC50 = 0.014 μg mL−1, SI = 1700 (D6 clone); IC50 = 0.1 μg mL−1, SI = 238 (W2 clone). Artemisinin: IC50 = 0.015 μg mL−1, SI = 1565 (D6 clone); IC50 = 0.009 μg mL−1, SI = 2644 (W2 clone). b Selectivity index (SI) is the ratio of IC50 in Vero cells to IC50 in P. falciparum (D6 or W2). BH inhibition activity: Chloroquine: IC50 = 80 μM, BPQ: IC50 = 2.9 μM, PQ: IC50 > 1000 μM. Antileishmanial activity: Pentamidine: IC50 = 1 μg mL−1, IC90 = 3.8 μg mL−1. Amphotericin B: IC50 = 0.19 μg mL−1, IC90 = 0.35 μg mL−1. | |||||||||||
| 13 | H | H | H | 0.57 | >41.7 | 0.80 | >29.7 | NC | 18.5 | 32 | NA |
| 14 | H | H | C(CH3)3 | 0.19 | >125 | 0.12 | >198 | NC | 7.5 | 2.8 | 7.2 |
| 15 | OC4H9 | C2H5 | H | 0.60 | >39.6 | 0.37 | >64.2 | NC | 10.2 | 3.5 | 7 |
| 16 | OC5H11 | C2H5 | H | 0.52 | >45.7 | 0.18 | >132 | NC | 9.6 | 3.7 | 7 |
| 17 | OC8H17 | C2H5 | H | 0.92 | >25.8 | 0.82 | >29.0 | NC | 15.7 | 17 | 32 |
| 22 | H | H | H | 2.6 | >9.1 | 1.5 | >15.8 | NC | 75 | 4.2 | 40 |
| 23 | H | H | C(CH3)3 | NA | — | NA | — | NC | >1000 | 1.6 | 4 |
| 24 | OC4H9 | C2H5 | H | 0.54 | >44.0 | 0.58 | >41.0 | NC | 19.7 | 14 | 32 |
| 25 | OC5H11 | C2H5 | H | 0.57 | >41.7 | 0.48 | >49.5 | NC | 20.8 | 3.5 | 7 |
| 30 | H | H | H | 2.3 | >10.3 | 1.3 | >18.3 | NC | 75 | 5 | 40 |
| 31 | H | H | C(CH3)3 | 0.70 | >34 | 0.56 | >42.5 | NC | 10.2 | 1.6 | 7.3 |
| 32 | OC4H9 | C2H5 | H | 0.49 | >48.5 | 0.35 | >68 | NC | 10.7 | 3.2 | 7 |
| 33 | OC5H11 | C2H5 | H | 0.44 | >54.0 | 0.42 | >56.6 | NC | 11.2 | 3.1 | 7 |
| 1 (PQ) | 2.0 | >11.9 | 2.8 | >8.5 | NC | >1000 | 19.9 | NA | |||
Most of the analogues displayed high inhibition of β-hematin formation in vitro with IC50 values in the range of 7.5 to 20.8 μM (except compound 22, and 30) indicating it as a potential biochemical pathway of antimalarial action in this class. The synthesized analogues also showed promising antileishmanial activity. The most potent analogues 23 [R = R1 = H, R2 = C(CH3)3] and 31 [R = R1 = H, R2 = C(CH3)3] displayed an IC50 value of 1.6 μg mL−1, and an IC90 in the range of 4–7 μg mL−1, which are comparable to the standard antileishmanial drug pentamidine (IC50 = 1 μg mL−1, IC90 = 3.8 μg mL−1). The IC50 values for remaining analogues were in the range between 2.8 and 32 μg mL−1.
The analogues with extended side chain (13, 14, 22, 23, 30, 31) were 100% curative at doses of 100, 50 and 25 mg kg−1 day−1 for 4 days (6/6 cures) and suppressive at the lowest test dose of 10 mg kg−1 day−1 for 4 days (resulting in either 4/6 or 5/6 cures) in vivo in a P. berghei murine malaria model (Table 2). It can be proposed that the attachment of basic groups at the side chain leads to the protection of the primary amino function against metabolism to inactive or toxic metabolites, resulting in increased antimalarial activity. However, to our surprise, all extended side chain analogues of 4,5-disubstituted primaquine (15, 16, 24, 25, 32, and 33) were devoid of antimalarial activity in vivo with all mice dying by D + 14 at the primary test dose of 100 mg kg−1.
| Compd. No. | P. berghei | |||
|---|---|---|---|---|
| (10 mg kg−1 day−1 × 4, oral) | (25 mg kg−1 day−1 × 4, oral) | (50 mg kg−1 day−1 × 4, oral) | (100 mg kg−1 day−1 × 4, oral) | |
| a The term ‘curative’ indicates complete elimination of malaria parasites from the body, and animals survive up to day D + 60. The term ‘suppressive’ indicates that all of the treated animals show negative parasitemia up to D + 7. However, by D + 60, some mice die, and some survive with complete elimination of parasitemia as indicated by numbers given in parentheses. The term ‘inactive’ indicates that the treated animals show positive parasitemia either on D + 4 or D + 7 and usually die by D + 14. b “—”, Not tested. | ||||
| 13 | (5/6) Suppressive | (6/6) Curative | (6/6) Curative | (6/6) Curative |
| 14 | (5/6) Suppressive | (6/6) Curative | (6/6) Curative | (6/6) Curative |
| 15 | — | — | — | (0/6) Inactive |
| 16 | — | — | — | (0/6) Inactive |
| 17 | — | — | — | (0/6) Inactive |
| 22 | (4/6) Suppressive | (6/6) Curative | (6/6) Curative | (6/6) Curative |
| 23 | (5/6) Suppressive | (6/6) Curative | (6/6) Curative | (6/6) Curative |
| 24 | — | — | — | (0/6) Inactive |
| 25 | — | — | — | (0/6) Inactive |
| 30 | (5/6) Suppressive | (6/6) Curative | (6/6) Curative | (6/6) Curative |
| 31 | (4/6) Suppressive | (6/6) Curative | (6/6) Curative | (6/6) Curative |
| 32 | — | — | — | (0/6) Inactive |
| 33 | — | — | — | (0/6) Inactive |
The antibacterial activities of potential 8-AQ are reported in Table 3. None of the analogues were active against P. aeruginosa and E. coli (data not shown). The analogues 15–17, 24, 25, 32 and 33 possessed moderate activity against S. aureus exhibiting IC50 values in the range of 8.6–13.6 μg mL−1, and MIC values of 20 μg mL−1. Except for analogue 17, all were bactericidal at 20 μg mL−1. The compounds were also active against MRSA with IC50 values ranging between 6.5–13.4 μg mL−1, MIC and MBC values of 10–20 μg mL−1. The analogues 14, 25, 32 and 33 showed moderate activity against M. intracellulare with IC50 values of 9–17.8 μg mL−1, and MIC and MBC of 20 μg mL−1 for some analogues.
| Compd. No. | S. aureus | MRSA | M. intracellulare | C. neoformans | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IC50 (μg mL−1) | MIC (μg mL−1) | MBC (μg mL−1) | IC50 (μg mL−1) | MIC (μg mL−1) | MBC (μg mL−1) | IC50 (μg mL−1) | MIC (μg mL−1) | MBC (μg mL−1) | IC50 (μg mL−1) | MIC (μg mL−1) | MFC (μg mL−1) | |
| a IC50 = the concentration (μg mL−1) that affords 50% growth inhibition. MIC, minimum inhibitory concentration (the lowest concentration in μg mL−1 that allows no detectable growth). MBC, minimum bactericidal concentration (the lowest concentration in μg mL−1 that kills the organism). NA, no activity at the highest test concentration of 20 μg mL−1. “— ”, not tested; Ciprofloxacin: IC50 = 0.12 μg mL−1, MIC = 0.50 μg mL−1, MBC = 50 μg mL−1 (Sa); IC50 = 0.09 μg mL−1, MIC = 0.31 μg mL−1, MBC = 2.5 μg mL−1 (MRSA); IC50 = 0.3 μg mL−1, MIC = 0.63 μg mL−1, MBC = 2.5 μg mL−1 (Mi), IC50 = 0.003 μg mL−1, MIC = 0.016 μg mL−1, MBC = 0.016 μg mL−1 (Ec). Amphotericin B: IC50 = 0.25 μg mL−1, MIC = 0.63 μg mL−1, MFC = 1.25 μg mL−1 (Ca); IC50 = 0.07 μg mL−1, MIC = 0.31 μg mL−1, MFC = 0.625 μg mL−1 (Cg); IC50 = 0.6 μg mL−1, MIC = 1.25 μg mL−1, MFC = 1.25 μg mL−1 (Ck); IC50 = 0.75 μg mL−1, MIC = 1.25 μg mL−1, MFC = 1.5 μg mL−1 (Cn). | ||||||||||||
| 13 | — | — | — | NA | NA | NA | 10 | NA | NA | NA | NA | NA |
| 14 | — | — | — | 6.5 | 10 | 20 | NA | — | — | 10 | 20 | 20 |
| 15 | 13.7 | 20 | 20 | 12.5 | 20 | 20 | >20 | NA | NA | 7.9 | 20 | NA |
| 16 | 13.6 | 20 | 20 | 13.4 | 20 | 20 | >20 | NA | NA | 5.8 | 10 | 10 |
| 17 | 14.5 | NA | NA | 10.4 | 20 | 20 | >20 | NA | NA | 8.5 | 20 | 20 |
| 22 | — | — | — | NA | — | — | NA | — | — | NA | — | — |
| 23 | — | — | — | NA | NA | NA | 9 | 20 | 20 | 7.5 | 20 | 20 |
| 24 | 12. | 20 | 20 | >20 | NA | NA | >20 | NA | NA | 12.1 | 20 | 20 |
| 25 | 8.6 | 20 | 20 | 9.5 | 20 | 20 | 16.6 | 20 | NA | 5.5 | 10 | 10 |
| 30 | — | — | — | NA | — | — | NA | — | — | NA | — | — |
| 31 | — | — | — | 10 | 20 | 20 | 15 | 20 | 20 | 10 | 20 | 20 |
| 32 | 13.6 | 20 | 20 | 13.2 | 20 | NA | 15 | NA | NA | 8.0 | 20 | 20 |
| 33 | 9.8 | 20 | 20 | 11 | 20 | 20 | 17.8 | 20 | NA | 5.6 | 10 | 10 |
The in vitro antifungal activities of 8-aminoquinolines against C. neoformans are summarized in Table 3. None of the analogues were active against C. albicans, C. glabrata, C. krusei, and A. fumigatus (data not shown). Most of the extended side chain 8-aminoquinolines 13–17, 22–25 and 30–33 were active against C. neoformans with IC50 values ranging between 5.5 and 12.1 μg mL−1. The analogues 16, 25, and 33 showed promising activities with IC50 values in the range of 5.5–5.8 μg mL−1, and MIC of 10 μg mL−1. These analogues were also fungicidal at 10 μg mL−1.
To conclude, the in vitroantimalarial activity data of the 8-aminoquinolines reported herein clearly indicates a preference for increased inhibition of the drug-resistant strain. This observation indicates the therapeutic potential of the reported 8-aminoquinolines in the treatment of drug-resistant malaria infections. None of the analogues showed cytotoxicity up to the highest tested concentration providing evidence of their safety profile. We have also observed potent in vivo blood-schizontocidal activities for these compounds in a drug-sensitive P. berghei murine malaria model underlining their potential as candidates for further studies. Several analogues also displayed promising antileishmanial and moderate antimicrobial activities providing proof of the untapped potential of this class of compounds in the chemotherapy of diseases other than malaria. It can be safely concluded that newly synthesized 8-aminoquinolines exhibit a broad spectrum of activities against several pathogenic protozoal and microbial infections, which will be further explored to provide additional promising compounds with improved biological activities.
Experimental
Melting points were recorded on a capillary melting point apparatus and are uncorrected. The synthesized compounds were routinely checked for their purity on pre-coated silica gel G254TLC plates (Merck) and the spots were visualized under UV spectrophotometer and then by exposing them to iodine vapors. Column chromatographic purification was carried out on Merck silica gel (100–200 mesh). IR spectra (λmax in cm−1) were recorded on a Nicolet FT-IR Impact 410 instrument either using KBr pellets or in CH2Cl2. 1H and 13C NMR spectra were recorded on a 300 MHz Bruker FT-NMR (Avance DPX 300) spectrometer using tetramethylsilane as internal standard and the chemical shifts are reported in δ units. Mass spectra were recorded on either HRMS (Finnigan Mat LCQ spectrometer) (APCI/ESI) or Ultraflex Tof/Tof Bruker instrument (MALDI). Elemental analyses were recorded on Elementar Vario EL spectrometer. The elemental analyses of all final compounds were within ±0.4% of the expected values, unless otherwise stated. All reagents were purchased from Aldrich Chemicals Ltd.General method for the synthesis of 2-[4-({4-[(6-methoxy-2/4,5-substitutedquinolin-8-yl)amino]pentyl}amino)pentyl]-1H-isoindole-1,3(2H)-diones (8–12), 2-(4-{[4-({4-[(6-methoxy-2/4,5-substitutedquinolin-8-yl)amino]pentyl}amino)pentyl]amino}pentyl)-1H-isoindole-1,3(2H)-diones (18–21), and 2-{4-[(4-{[4-({4-[(6-methoxy-2/4,5-substitutedquinolin-8-yl)amino]pentyl}amino)pentyl]amino}pentyl)amino]pentyl}-1H-isoindole-1,3(2H)-diones (26–29)
A mixture of 8-aminoquinoline (1 or 3, or 5–7, or 13–17 or 22–25, 1 mmol), 2-(4-bromopentyl)-1,3-isoindolinedione (4.40 mmol) and Et3N (4.40 mmol) was stirred at ambient temperature for 8 h. EtOAc (20 mL) was added to the thick reaction mass, and the separated salt was filtered. The filtrate was concentrated and residue was purified by column chromatography on silica gel (100–200 mesh) using a mixture of CH3OH in CH2Cl2 to produce 8–12 or 18–21 or 26–29 as viscous oil.General method for the synthesis of 1,4-diamines 13–17, 22–25, and 30–33
To a solution of 8–12 or 18–21 or 26–29 (0.26 mmol) in 95% ethanol (15 mL), was added NH2NH2·H2O (6.60 mmol) and the reaction mixture was stirred with refluxing for 6 h. The solvent was removed under reduced pressure. The residue was diluted with water (20 mL) and extracted with CH2Cl2 (3 × 20 mL). The organic layer washed with brine solution (10 mL), dried over Na2SO4 and concentrated to yield 13–17, 22–25, and 30–33 as oil, which upon treatment with 2 N ethereal HCl solution provided their dihydrochloride salts.Acknowledgements
Kirandeep Kaur thanks the Council of Scientific and Industrial Research (CSIR), New Delhi for the award of Senior Research Fellowship. Antimicrobial testing was supported by the NIH, NIAID, Division of AIDS, Grant No. AI 27094. Support from the USDA Agricultural Research Service Specific Cooperative Agreement No. 58-6408-2-0009 is also acknowledged in the in vitro screening of antimicrobial, antiprotozoal, and cytotoxic activity.References
- B. L. Tekwani and L. A. Walker, Curr. Opin. Infect. Dis., 2006, 19, 623–631 CrossRef CAS.
- G. W. Mihaly, S. A. Ward, G. Edwards, M. L. E. Orme and A. M. Breckenridge, Br. J. Clin. Pharmacol, 1984, 17, 441–446 CAS.
- J. K. Baker, J. A. Bedford, A. M. Clark and J. D. McChesney, Pharm. Res., 1984, 1, 98–100.
- J. K. Baker, R. H. Yarber, N. P. D. Nanayakkara, J. D. Mc Chesney, F. Homo and I. Landau, Pharm. Res., 1990, 7, 91–95 CAS.
- L. Constantino, P. Paixão, R. Moreira, M. J. Portela, V. E. J. Rosário and J. Iley, Exp. Toxicol. Pathol, 1999, 51, 299–303 CAS.
- S. Ganesan, B. L. Tekwani, R. Sahu, L. M. Tripathi and L. A. Walker, Toxicol. Appl. Pharmacol., 2009, 241, 14–22 CrossRef CAS.
- A. Philip, J. A. Kepler, B. H. Johnson and F. Y. Carroll, J. Med. Chem., 1988, 31, 870–874 CrossRef CAS.
- R. Borissova, P. Stjarnkvist, M. O. Karlsson and I. Sjoholm, J. Pharm. Sci., 1995, 84, 256–262 CrossRef CAS.
- M. C. Chung, M. F. Gonçalves, W. Colli, E. I. Ferreira and M. T. M. Miranda, J. Pharm. Sci., 1997, 86, 1127–1132 CrossRef CAS.
- M. J. Portela, R. Moreira, E. Valente, L. Constantino, J. Iley, J. Pinto, R. Rosa, P. Cravo and V. E. Rosário, Pharm. Res., 1999, 16, 949–955 CrossRef CAS.
- T. J. Egan, R. Hunter, C. H. Kaschula, H. M. Marques, A. Misplon and J. Walden, J. Med. Chem., 2000, 43, 283–291 CrossRef CAS.
- S. Vangapandu, S. Sachdeva, M. Jain, S. Singh, P. P. Singh, C. L. Kaul and R. Jain, Bioorg. Med. Chem., 2004, 12, 239–247 CrossRef CAS.
- N. T. Huy, K. Mizunuma, K. Kaur, N. T. T. Nhien, M. Jain, D. H. Uyen, S. Harada, R. Jain and K. Kamei, Antimicrob. Agents Chemother., 2007, 51, 2842–2847 CrossRef CAS.
- J. L. Vennerstrom, E. O. Nuzum, R. E. Miller, A. Dorn, L. Gerena, P. A. Dande, W. Y. Ellis, R. G. Ridley and W. K. Milhous, Antimicrob. Agents Chemother., 1999, 43, 598–602 CAS.
- B. L. Tekwani and L. A. Walker, Comb. Chem. High Throughput Screening, 2005, 8, 63–79 CrossRef CAS.
- P. M. O'Neill, A. Mukhtar, P. A. Stocks, L. E. Randle, S. Hindley, S. A. Ward, R. C. Storr, J. F. Bickley, I. A. O'Neil, J. L. Maggs, R. H. Hughes, P. A. Winstanley, P. G. Bray and B. K. Park, J. Med. Chem., 2003, 46, 4933–4945 CrossRef CAS.
- P. M. O'Neill, A. E. Shone, D. Stanford, G. Nixon, E. Asadollahy, B. K. Park, J. L. Maggs, P. Roberts, P. A. Stocks, G. Biagini, P. G. Bray, J. Davies, N. Berry, C. Hall, K. Rimmer, P. A. Winstanley, S. Hindley, R. B. Bambal, C. B. Davis, M. Bates, S. L. Gresham, R. A. Brigandi, F. M. Gomez-de-las-Heras, D. V. Gargallo, S. Parapini, L. Vivas, H. Lander, D. Taramelli and S. A. Ward, J. Med. Chem., 2009, 52, 1828–1844 CrossRef CAS.
- M. A. L. Blackie, P. Beagley, S. L. Croft, H. Kendrick, J. R. Mossa and K. Chibale, Bioorg. Med. Chem., 2007, 15, 6510–6516 CrossRef CAS.
- M. T. Makler and D. J. Hinrichs, Am. J. Trop. Med. Hyg., 1993, 48, 205–210 Search PubMed.
- M. Jain, S. Vangapandu, S. Sachdeva, S. Singh, P. P. Singh, G. B. Gena, K. Tikoo, P. Ramarao, C. L. Kaul and R. Jain, J. Med. Chem., 2004, 47, 285–287 CrossRef CAS.
- E. Borenfreund, H. Babich and N. Martin-Alguacil, In Vitro Cell. Dev. Biol., 1990, 26, 1030–1034 CAS.
- J. Mustafa, S. I. Khan, G. Ma, L. A. Walker and I. A. Khan, Lipids, 2004, 39, 167–172 CAS.
- J. Mikus and D. Steverding, Parasitol. Int., 2000, 48, 265–269 CrossRef CAS.
- G. Ma, S. I. Khan, M. R. Jacob, B. L. Tekwani, Z. Li, D. S. Pasco, L. A. Walker and I. A. Khan, Antimicrob. Agents Chemother., 2004, 48, 4450–4452 CrossRef CAS.
- NCCLS, Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically M7-A5. National Committee on Clinical Laboratory Standards, 2000, 20 Search PubMed.
- NCCLS, Reference method for broth dilution antifungal susceptibility testing of yeasts approved standard M27-A2. National Committee on Clinical Laboratory Standards, 2002, 22 Search PubMed.
- NCCLS, Reference method for broth dilution antifungal susceptibility testing of filamentous fungi approved standard, M38-A. National Committee on Clinical Laboratory Standards, 2002, 22 Search PubMed.
- NCCLS, Susceptibility testing of Mycobacteria, Nocardia, and other aerobic Actinomycetes Tentative Standard-Second Edition, M24-T2. National Committee on Clinical Laboratory Standards, 2000, 20 Search PubMed.
- S. G. Franzblau, R. S. Witzig, J. C. McLaughlin, P. Torres, G. Madico, A. Hernandez, M. T. Degnan, M. B. Cook, V. K. Quenzer, R. M. Ferguson and R. H. Gilman, J. Clin. Microbiol., 1998, 36, 362–366 CAS.
| This journal is © The Royal Society of Chemistry 2011 |
