DOI:
10.1039/C0MD00262C
(Concise Article)
Med. Chem. Commun., 2011,
2, 743-751
Received
15th December 2010
, Accepted 6th May 2011
First published on 17th June 2011
Abstract
Novel N-protected derivatives of substituted isatins have been synthesized and evaluated for their potency in inhibiting TNF-α-induced ICAM-1 activity on human endothelial cells as a marker for anti-inflammatory activity. Compound 3p was found to be most potent in inhibiting the ICAM-1 expression in a concentration- and time-dependent manner. The structure–activity relationship of these compounds in inhibiting ICAM-1 expression activity is elucidated in the present study.
Introduction
Leukocytes are the key players in the pathogenesis of multiple inflammatory disorders including asthma, COPD, atherosclerosis and autoimmune diseases.1–3 Leukocytes in the inflamed tissue secrete pro-inflammatory mediators, reactive oxygen species and proteases that cause tissue damage, inflammation and disease pathogenesis.4–6 Pharmaceutical companies are designing drugs to limit leukocyte mediated inflammatory response. However, current anti-inflammatory drugs show limited efficacy and exhibit severe side effects.7 Therefore, more specific and potent anti-inflammatory drugs are needed urgently.
Migration of leukocytes from circulating blood to the site of infection or injury is a key event in the inflammatory response that occurs through multiple step processes, which involve sequential capture on, rolling along and firm adhesion to the microvascular endothelium, followed by transmigration through the vessel wall and further migration in extravascular tissue. Rolling and extravasations of leukocytes are largely mediated by the surface expression of cell adhesion molecules (CAM) that includes E-selectin, ICAM-1 and VCAM-1 on endothelial cells.8 Pharmacological inhibition of CAM on endothelial cells is a promising strategy for therapeutic intervention of inflammatory disorders.9
Oxindoles, particularly COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundisatin (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound1H-indole-2,3-dione) are endogenous compounds ubiquitously present in blood, central nervous system, body fluids and other human tissues that show a wide range of biological activities including antibacterial, antifungal, anticonvulsant, antiviral, and antiproliferative activity.10 A variety of N-protected COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundisatin derivatives have been reported in the literature exhibiting a broad range of biological activities.11–18N-alkyl and N-acyl isatin derivatives with bromo and chloro substituents and oxime derivatives have exhibited a broad-spectrum of biological activities including anti-inflammatory activity.19,20
Recognizing the beneficial pharmacological activities of 2,3-oxindole derivatives and their Schiff and Mannich bases, we have synthesized novel N-protected derivatives of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundisatin having different halogen substituents at the C-5 position in the COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundisatin ring and evaluated their anti-inflammatory activities using our cell based assay that measures inhibition of TNF-α induced ICAM-1 expression on human endothelial cells. The present study reports anti-inflammatory activities and structure–activity relationship of the novel COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundisatin compounds.
Results and discussion
Different N-protected derivatives 3a–p were prepared in 70–80% yields by treating compounds 2a–d with different acylating/alkylating agents in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundtert-butanol using COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundpotassium tert-butoxide as a base (Scheme 1). Compounds 2a–d were obtained exclusively as E-isomers by treating the commercially available different substituted isatins 1a–d with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethoxycarbonylmethylene-triphenyl-phosphorane in glacial COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundacetic acid for 4 h at 80 °C in 65–72% yields (Scheme 1).21–23 The stereochemistry of the compounds 2a–d was established from their 2D-NOESY and 1D-NOE NMR studies. As shown in Fig. 1, compounds 2a–d could be formed as either E- or Z-isomers. Careful study of the interactions of 2D-NOESY and 1D-NOE NMR spectral experiments on compounds 2a–d (Fig. 1) revealed that there is no interaction between the alkene proton and the aromatic proton at the C-4 position of the benzene ring, thereby affirming the exclusive formation of the E-isomers of the compounds 2a–d. This is also reported in the literature23—a detailed study of the 1D-NOE NMR spectra of compounds having similar structures was carried out and these were found to have E-geometry around the double bond.23 If the geometry around the double bond in these four compounds had been Z, there might have been interactions between the alkene proton and the aromatic ring proton at the C-4 position (Fig. 1), and the NOE effect would have been observed between these two protons in the 2D-NOESY and 1D-NOE NMR spectral experiments on compounds 2a–d. Compound COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound2c is novel and its melting point and spectral data have not been reported earlier.
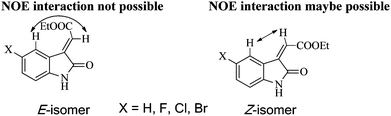 |
| | Fig. 1 NOE interactions in E- and Z-isomers of compounds 2a–d. | |
All the synthesized compounds 3a–p are novel and were characterized by spectroscopic techniques like NMR, IR, mass spectrometry, etc. The known compound COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound3a was characterized by comparing its spectral data and melting point with that reported in the literature.24 The synthesized compounds 2a–2d and 3a–3p were then screened for their anti-inflammatory activities by measuring TNF-α induced ICAM-1 expression inhibition and the structure–activity relationship was studied. Compounds 3a–p potently inhibited ICAM-1 expression. Compound 3p showed highest inhibition (∼93%) of ICAM-1 expression at the maximal tolerable dose of 100 μM (Table 1) with an IC50 value of 10 μM. It is important to point out that the IC50 values of 3a–p, particularly of 3p, are found to be much lower than the commonly used anti-inflammatory agents, e.g., COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundaspirin (IC50 5 mM), COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundN-acetyl cysteine (IC50 10 mM), COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compounddiclofenac (IC50 0.75 mM) etc. indicating that these compounds could be useful as COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundlead molecules.25–27
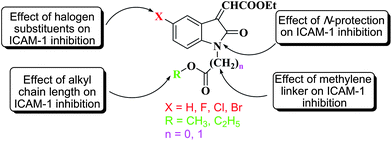
Structure–activity relationship
To examine the role of different modifications (Fig. 2), such as the effect of: (i) N-protection in compounds 2a–d, (ii) halogen substituents at the C-5 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcarbon of the COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundisatin ring, (iii) the length of the alkyl chain in the alcohol part of the N-protected COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundisatin derivatives and (iv) the methylene linker between the ester COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcarbonyl moiety and the nitrogen atom of the COMPOUND LINKS
Read more about this on ChemSpider
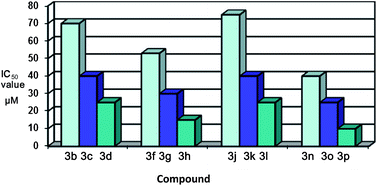
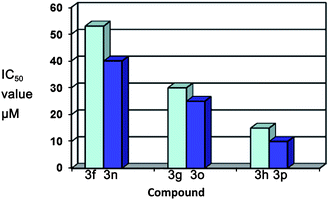
Download mol file of compoundisatin ring on the ICAM-1 expression inhibition, we have prepared different N-protected derivatives, i.e.3a–p of the substituted isatins 1a–d. The results showed a good structure–activity relationship that would be useful in understanding the functional components responsible for the inhibitory activity of these compounds.
(i) Effect of N-protection.
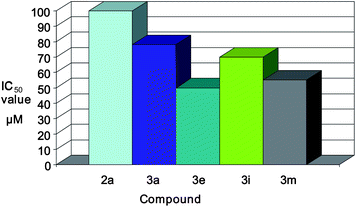
On comparing compound 2a with compounds COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound3a, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound3e, 3i and 3m in which the nitrogen atom of the COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundisatin ring has been protected (Table 1), we have found in each case a decrease in IC50 value (Fig. 3), suggesting that ICAM-1 expression inhibitory activity increases with the protection of the nitrogen atom of the COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundisatin ring. Similar observations were revealed on comparing the compound 2b with the compounds COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound3b, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound3f, 3j and 3n, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound2c with the compounds COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound3c, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound3g, 3k and 3o; and the compound 2d with the compounds COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound3d, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound3h, 3l and 3p.
Time kinetics of ICAM-1 inhibition by compound 3p
The time kinetics of ICAM-1 inhibition by the most active compound of the series, 3p on endothelial cells was also investigated. For this, the cells were incubated with the maximum tolerable dose (100 μM) of 3p for time points before, simultaneously and after TNF-α induction followed by the Cell-ELISA for measurement of ICAM-1 expression. A time-dependent ICAM-1 inhibition pattern was observed where the maximum inhibition of ICAM-1 expression occurred when 3p was added prior to induction with TNF-α (Fig. 6A).
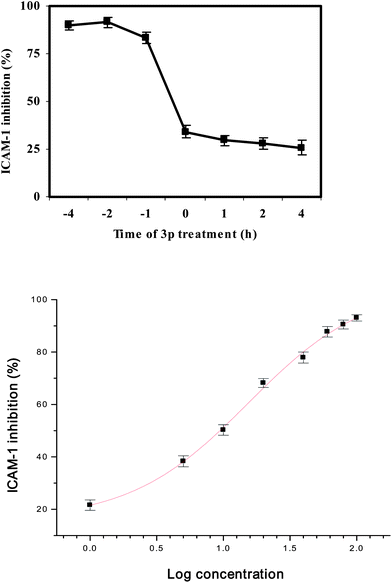 |
| | Fig. 6
A. Time kinetics of ICAM-1 inhibition by 3p. The endothelial cells were treated with 3p at various time points followed by induction with TNF-α for 16 h. The ICAM-1 expression levels were measured by Cell-ELISA. B. Dose-dependent inhibition of ICAM-1 expression on human endothelial cells by 3p. The cells were treated with 3p at various concentrations followed by induction with TNF-α for 16 h. The ICAM-1 expression levels were measured by Cell-ELISA. The results are expressed as mean ± SEM. | |
Dose-dependent inhibition of ICAM-1 expression by compound 3p
The dose-dependent effect of the most active compound of the series, 3p on ICAM-1 expression was also investigated. For this, the cells were pre-treated with various concentrations of 3p for 2 h followed by TNF-α induction for 16 h. The ICAM-1 protein levels were measured by Cell-ELISA. The percentage ICAM-1 inhibition at each concentration of 3p were calculated. These percentages were plotted against the log concentrations of compound 3p to get a dose-response curve. The results showed that compound 3p inhibited the TNF-α-induced ICAM-1 expression on human endothelial cells in a dose-dependent manner with an IC50 value of 10 μM (Fig. 6B).
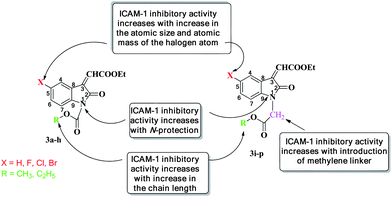
In summary, the ICAM-1 expression inhibitory activity of the N-protected derivatives of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundisatin (i) increases with protection of the nitrogen atom (N-1) of the COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundisatin ring, (ii) increases with increase in the atomic size of the halogen atom at the C-5 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcarbon atom of the COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundisatin ring, (iii) increases with increase in chain length of the alcohol moiety, and (iv) increases with the introduction of a methylene linker between the ester COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcarbonyl moiety and the nitrogen atom of the COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundisatin ring (Fig. 7).
Conclusions
We have synthesized fifteen novel bioactive N-protected derivatives of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundisatin. We report for the first time, the anti-inflammatory activity of these novel compounds. Compound 3p was found to be the most potent in inhibiting the ICAM-1 expression in a concentration- and time-dependent manner. The structure–activity relationship for these compounds has been discussed extensively in the present study.
Experimental
Analytical TLCs were performed on Merck COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundsilica gel 60 F254 plates. All flash chromatographic separations were performed on 100–200 mesh COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundsilica gel. IR spectra were recorded on a Perkin-Elmer 2000 FT-IR spectrometer. The 1H NMR and 13C NMR spectra (in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3) were recorded on a Bruker AC-300 Avance spectrometer operating at 300 MHz and at 75.5 MHz, respectively using TMS as internal standard. The NOE 1H NMR and NOESY 1H NMR spectra were recorded on 400, 500 and 700 MHz instrument. The chemical shift values are on δ scale and the coupling constants (J) are in Hz. The HRMS determinations were made in FAB positive mode on a JEOL JMS-AX505W high-resolution mass spectrometer using COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundbis-hydroxyethyldisulfide (HEDS) doped with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundsodium acetate as matrix. Microwave reactions were performed in a microwave oven of 850 W 1.2 Cft (33 L, Infodisplay, Sharp Carosel). Melting points were recorded in a COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundsulfuric acid bath and are uncorrected.
Materials
Materials were procured from commercial vendors and were used without further purification unless otherwise noted. Petroleum ether and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate were distilled over P2O5 and K2CO3, respectively prior to use.
General method for the preparation of compounds 2a–d
A mixture of compound 1a–d (0.05 mol) and commercially available COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethoxycarbonylmethylene-triphenyl-phosphorane (0.05 mol) in glacial COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundacetic acid (60 mL) was heated for 4 h at 80 °C.18 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundAcetic acid was removed under vacuum and the residue was washed onto a filter funnel with a small quantity of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundmethanol. Recrystallization from COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethanol gave compounds 2a–d as orange solids in 65–72% yields.21–23
Obtained as an orange solid (17.2 gm, 71% yield), mp 160–162 °C (from petroleum ether–COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate), Rf: 0.45(4:1 petroleum ether–COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate); νmax(KBr)/cm−1: 3166, 1710, 1648, 1613, 1453, 1209, 1027, 819; 1H NMR (300 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 1.29(3H, t, J = 6.9 Hz, = CHCOOCH2CH3), 4.25(2H, q, J = 6.9 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 6.59(1H, s,
CHCOOCH2CH3), 6.59(1H, s, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO–), 6.85(1H, d, J = 8.4 Hz, C-7H), 7.39(1H, d, J = 6.6 Hz, C-6H), 8.35(1H, s, C-4H), 8.53(1H, s, NH); 13C NMR (75.5 MHz, COMPOUND LINKS
CHCO–), 6.85(1H, d, J = 8.4 Hz, C-7H), 7.39(1H, d, J = 6.6 Hz, C-6H), 8.35(1H, s, C-4H), 8.53(1H, s, NH); 13C NMR (75.5 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 13.9(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 61.2(
CHCOOCH2CH3), 61.2(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 111.7(C-7), 120.9(C-5), 122.2(
CHCOOCH2CH3), 111.7(C-7), 120.9(C-5), 122.2(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 125.7(C-4), 127.5(C-3), 132.3(C-6), 137.4(C-8), 143.7(C-9), 164.9(
CH–), 125.7(C-4), 127.5(C-3), 132.3(C-6), 137.4(C-8), 143.7(C-9), 164.9(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOC2H5) and 167.4(C-2). HRMS m/z Calcd for C12H10ClNO3Na [M + Na]+: 274.0241. Found: 274.0238.
Compound 2a–d (0.001 mol) was dissolved in COMPOUND LINKS
CHCOOC2H5) and 167.4(C-2). HRMS m/z Calcd for C12H10ClNO3Na [M + Na]+: 274.0241. Found: 274.0238.
Compound 2a–d (0.001 mol) was dissolved in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundtert-butanol (15 mL) and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundpotassium tert-butoxide (0.001 mol) was added to it in an ice bath. The reaction mixture was stirred for 15 min followed by dropwise addition of the appropriate acylating/alkylating agent (0.001 mol) for 15 min.
The reaction mixture was then heated at 60 °C for 5–6 h and the progress of the reaction was monitored by TLC; the reaction mixture was then chromatographed over COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundsilica gel using COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate–petroleum ether (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70) as eluent to afford the compounds 3a–p as yellow to orange solids in 70–80% yields.
70) as eluent to afford the compounds 3a–p as yellow to orange solids in 70–80% yields.
Obtained as a bright yellow solid (210 mg, 75%), mp 109–111 °C (lit. mp24 110–112 °C).
Obtained as a dark yellow solid (270 mg, 72% yield), mp 96–98 °C (from petroleum ether–COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate), Rf: 0.54(4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether–COMPOUND LINKS
1 petroleum ether–COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate); νmax(KBr)/cm−1: 3418, 2926, 1765, 1733, 1643, 1604, 1471, 1343, 1199, 1154, 1025; 1H NMR (300 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 1.39(3H, t, J = 7.2 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 4.04(3H, s, –OCH3), 4.32(2H, q, J = 7.2 Hz,
CHCOOCH2CH3), 4.04(3H, s, –OCH3), 4.32(2H, q, J = 7.2 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 6.98(1H, s,
CHCOOCH2CH3), 6.98(1H, s, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO–), 7.14–7.21(1H, m, C-6H), 7.97–8.02(1H, m, C-4H), 8.48–8.52(1H, m, C-7H); 13C NMR (75.5 MHz, COMPOUND LINKS
CHCO–), 7.14–7.21(1H, m, C-6H), 7.97–8.02(1H, m, C-4H), 8.48–8.52(1H, m, C-7H); 13C NMR (75.5 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 14.1(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 54.1(–OCH3), 61.6(
CHCOOCH2CH3), 54.1(–OCH3), 61.6(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 115.4(d, J = 26.4 Hz, C-4), 116.2(d, J = 8.3 Hz, C-6), 119.4(d, J = 23.4 Hz, C-7), 121.3(d, J = 10.5 Hz, C-3), 125.1(
CHCOOCH2CH3), 115.4(d, J = 26.4 Hz, C-4), 116.2(d, J = 8.3 Hz, C-6), 119.4(d, J = 23.4 Hz, C-7), 121.3(d, J = 10.5 Hz, C-3), 125.1(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 135.5(d, J = 3.2 Hz, C-8), 137.4(C-9), 151.0(NCOOCH3), 159.7(d, J = 243.8 Hz, C-5), 164.9 (C-2) and 165.1(
CH–), 135.5(d, J = 3.2 Hz, C-8), 137.4(C-9), 151.0(NCOOCH3), 159.7(d, J = 243.8 Hz, C-5), 164.9 (C-2) and 165.1(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOC2H5). HRMS m/z Calcd for C14H13FNO5 [M + H]+: 294.0772. Found: 294.0767.
Obtained as a light yellow solid (480 mg, 78% yield), mp 124–126 °C (from petroleum ether–COMPOUND LINKS
CHCOOC2H5). HRMS m/z Calcd for C14H13FNO5 [M + H]+: 294.0772. Found: 294.0767.
Obtained as a light yellow solid (480 mg, 78% yield), mp 124–126 °C (from petroleum ether–COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate), Rf: 0.51(4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether–COMPOUND LINKS
1 petroleum ether–COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate); νmax(KBr)/cm−1: 3449, 2925, 1765, 1734, 1713, 1640, 1600, 1460, 1352, 1197, 1026, 774; 1H NMR (300 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 1.40(3H, t, J = 7.2 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 4.04(3H, s, –OCH3), 4.34(2H, q, J = 7.2 Hz,
CHCOOCH2CH3), 4.04(3H, s, –OCH3), 4.34(2H, q, J = 7.2 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 6.97(1H, s,
CHCOOCH2CH3), 6.97(1H, s, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO–), 7.41–7.45(1H, m, C-6H), 7.95(1H, d, J = 9.0 Hz, C-4H), 8.74(1H, d, J = 2.1 Hz, C-7H); 13C NMR (75.5 MHz, COMPOUND LINKS
CHCO–), 7.41–7.45(1H, m, C-6H), 7.95(1H, d, J = 9.0 Hz, C-4H), 8.74(1H, d, J = 2.1 Hz, C-7H); 13C NMR (75.5 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 14.1(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 54.2(–OCH3), 61.7(
CHCOOCH2CH3), 54.2(–OCH3), 61.7(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 116.2(C-7), 121.4(C-5), 125.2(
CHCOOCH2CH3), 116.2(C-7), 121.4(C-5), 125.2(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 128.2(C-4), 130.6(C-3), 132.5(C-6), 135.0(C-8), 139.7(C-9), 150.8(NCOOCH3) and 164.9(C-2 and = CHCOOC2H5). HRMS m/z Calcd for C14H13ClNO5 [M + H]+: 310.0477. Found: 310.0472.
Obtained as a light yellow solid (280 mg, 78% yield), mp 136–138 °C (from petroleum ether–COMPOUND LINKS
CH–), 128.2(C-4), 130.6(C-3), 132.5(C-6), 135.0(C-8), 139.7(C-9), 150.8(NCOOCH3) and 164.9(C-2 and = CHCOOC2H5). HRMS m/z Calcd for C14H13ClNO5 [M + H]+: 310.0477. Found: 310.0472.
Obtained as a light yellow solid (280 mg, 78% yield), mp 136–138 °C (from petroleum ether–COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate), Rf: 0.52(4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether–COMPOUND LINKS
1 petroleum ether–COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate); νmax(KBr)/cm−1: 3449, 2924, 1766, 1734, 1712, 1638, 1595, 1458, 1352, 1195, 1107, 1026, 774; 1H NMR (300 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 1.40(3H, t, J = 7.2 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 4.04(3H, s, –OCH3), 4.33–4.40(2H, m,
CHCOOCH2CH3), 4.04(3H, s, –OCH3), 4.33–4.40(2H, m, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 6.97(1H, s,
CHCOOCH2CH3), 6.97(1H, s, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO–), 7.58(1H, d, J = 8.7 Hz, C-6H), 7.91(1H, d, J = 8.7 Hz, C-7H), 8.89(1H, brs, C-4H); 13C NMR (75.5 MHz, COMPOUND LINKS
CHCO–), 7.58(1H, d, J = 8.7 Hz, C-6H), 7.91(1H, d, J = 8.7 Hz, C-7H), 8.89(1H, brs, C-4H); 13C NMR (75.5 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 14.1(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 54.2(–OCH3), 61.7(
CHCOOCH2CH3), 54.2(–OCH3), 61.7(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 116.5(C-7), 118.1(C-5), 121.8(C-4), 125.2(
CHCOOCH2CH3), 116.5(C-7), 118.1(C-5), 121.8(C-4), 125.2(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 131.1(C-6), 134.8(C-3), 135.4(C-8), 140.2(C-9), 150.8(NCOOCH3), 164.7(C-2) and 164.9(
CH–), 131.1(C-6), 134.8(C-3), 135.4(C-8), 140.2(C-9), 150.8(NCOOCH3), 164.7(C-2) and 164.9(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOC2H5). HRMS m/z Calcd for C14H13BrNO5 [M + H]+: 353.9972. Found: 353.9969.
Obtained as a light yellow solid (110 mg, 73% yield), mp 74–76 °C (from petroleum ether–COMPOUND LINKS
CHCOOC2H5). HRMS m/z Calcd for C14H13BrNO5 [M + H]+: 353.9972. Found: 353.9969.
Obtained as a light yellow solid (110 mg, 73% yield), mp 74–76 °C (from petroleum ether–COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate), Rf: 0.58(4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether–COMPOUND LINKS
1 petroleum ether–COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate); νmax(KBr)/cm−1: 3425, 2926, 1758, 1734, 1710, 1638, 1597, 1464, 1371, 1198, 1090, 1027, 788; 1H NMR (300 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 1.31 & 1.39 (6H, 2t, J = 6.9 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –COOCH2CH3), 4.27 & 4.42(4H, 2q, J = 7.2` Hz,
CHCOOCH2CH3 & –COOCH2CH3), 4.27 & 4.42(4H, 2q, J = 7.2` Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –COOCH2CH3), 6.86(1H, s,
CHCOOCH2CH3 & –COOCH2CH3), 6.86(1H, s, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO–), 7.12–7.19(1H, m, C-5H), 7.38(1H, t, J = 7.8 Hz, C-6H), 7.90(1H, d, J = 8.1 Hz, C-4H), 8.62(1H, d, J = 7.8 Hz, C-7H); 13C NMR (75.5 MHz, COMPOUND LINKS
CHCO–), 7.12–7.19(1H, m, C-5H), 7.38(1H, t, J = 7.8 Hz, C-6H), 7.90(1H, d, J = 8.1 Hz, C-4H), 8.62(1H, d, J = 7.8 Hz, C-7H); 13C NMR (75.5 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 13.1 & 13.2(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –COOCH2CH3), 60.4 & 62.6(
CHCOOCH2CH3 & –COOCH2CH3), 60.4 & 62.6(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –COOCH2CH3), 114.0(C-7), 119.1(C-6), 122.5(C-4), 123.8(
CHCOOCH2CH3 & –COOCH2CH3), 114.0(C-7), 119.1(C-6), 122.5(C-4), 123.8(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 127.3(C-5), 131.8(C-3), 135.0(C-8), 140.4(C-9), 149.4(NCOOC2H5), 164.2(C-2) and 164.5(
CH–), 127.3(C-5), 131.8(C-3), 135.0(C-8), 140.4(C-9), 149.4(NCOOC2H5), 164.2(C-2) and 164.5(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOC2H5). HRMS m/z Calcd for C15H15NO5Na [M + Na]+: 312.0842. Found: 312.0832.
Obtained as a light yellow solid (182 mg, 79% yield), mp 66–68 °C (from petroleum ether–COMPOUND LINKS
CHCOOC2H5). HRMS m/z Calcd for C15H15NO5Na [M + Na]+: 312.0842. Found: 312.0832.
Obtained as a light yellow solid (182 mg, 79% yield), mp 66–68 °C (from petroleum ether–COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate), Rf: 0.53(4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether–COMPOUND LINKS
1 petroleum ether–COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate); νmax(KBr)/cm−1: 3447, 2924, 1763, 1735, 1712, 1642, 1598, 1476, 1371, 1334, 1208, 1159, 1027, 828; 1H NMR (300 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 1.39 & 1.46 (6H, 2t, J = 7.2 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –COOCH2CH3), 4.35 & 4.49 (4H, 2q, J = 7.2 Hz,
CHCOOCH2CH3 & –COOCH2CH3), 4.35 & 4.49 (4H, 2q, J = 7.2 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –COOCH2CH3), 6.96 (1H, s,
CHCOOCH2CH3 & –COOCH2CH3), 6.96 (1H, s, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO–), 7.13–7.20 (1H, m, C-6H), 7.94–7.99 (1H, m, C-4H), 8.48–8.51 (1H, m, C-7H); 13C NMR (75.5 MHz, COMPOUND LINKS
CHCO–), 7.13–7.20 (1H, m, C-6H), 7.94–7.99 (1H, m, C-4H), 8.48–8.51 (1H, m, C-7H); 13C NMR (75.5 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 14.1 & 14.2 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –COOCH2CH3), 61.6 & 63.7 (
CHCOOCH2CH3 & –COOCH2CH3), 61.6 & 63.7 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –COOCH2CH3), 115.4 (d, J = 26.4 Hz, C-4), 116.1 (d, J = 7.55 Hz, C-6), 119.3 (d, J = 24.1 Hz, C-7), 121.3 (d, J = 9.8 Hz, C-3), 124.8 (
CHCOOCH2CH3 & –COOCH2CH3), 115.4 (d, J = 26.4 Hz, C-4), 116.1 (d, J = 7.55 Hz, C-6), 119.3 (d, J = 24.1 Hz, C-7), 121.3 (d, J = 9.8 Hz, C-3), 124.8 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 135.6 (d, J = 3.0 Hz, C-8), 137.5 (d, J = 2.2 Hz, C-9), 150.4 (NCOOC2H5), 159.6 (d, J = 243.1 Hz, C-5), 164.9 (C-2) and 165.1(
CH–), 135.6 (d, J = 3.0 Hz, C-8), 137.5 (d, J = 2.2 Hz, C-9), 150.4 (NCOOC2H5), 159.6 (d, J = 243.1 Hz, C-5), 164.9 (C-2) and 165.1(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOC2H5). HRMS m/z Calcd for C15H15FNO5 [M + H]+: 308.0929. Found: 308.0925.
Obtained as a light yellow solid (300 mg, 77% yield), mp 110–112 °C (from petroleum ether–COMPOUND LINKS
CHCOOC2H5). HRMS m/z Calcd for C15H15FNO5 [M + H]+: 308.0929. Found: 308.0925.
Obtained as a light yellow solid (300 mg, 77% yield), mp 110–112 °C (from petroleum ether–COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate), Rf: 0.55(4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether–COMPOUND LINKS
1 petroleum ether–COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate); νmax(KBr)/cm−1: 3426, 2925, 1765, 1731, 1703, 1642, 1594, 1458, 1371, 1323, 1206, 1171, 1107, 1029, 776; 1H NMR (300 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 1.39 & 1.46 (6H, 2t, J = 7.2 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –COOCH2CH3), 4.35 & 4.44 (4H, 2q, J = 7.2 Hz,
CHCOOCH2CH3 & –COOCH2CH3), 4.35 & 4.44 (4H, 2q, J = 7.2 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –COOCH2CH3), 6.97 (1H, s,
CHCOOCH2CH3 & –COOCH2CH3), 6.97 (1H, s, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO–), 7.41–7.45 (1H, m, C-6H), 7.93–7.99 (1H, m, C-4H), 8.74 (1H, d, J = 2.1 Hz, C-7H); 13C NMR (75.5 MHz, COMPOUND LINKS
CHCO–), 7.41–7.45 (1H, m, C-6H), 7.93–7.99 (1H, m, C-4H), 8.74 (1H, d, J = 2.1 Hz, C-7H); 13C NMR (75.5 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 14.1 & 14.2 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –COOCH2CH3), 61.7 & 63.8 (
CHCOOCH2CH3 & –COOCH2CH3), 61.7 & 63.8 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –COOCH2CH3), 116.2(C-7), 121.4(C-5), 125.0(
CHCOOCH2CH3 & –COOCH2CH3), 116.2(C-7), 121.4(C-5), 125.0(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 128.2(C-4), 130.5(C-3), 132.5(C-6), 135.1(C-8), 139.8(C-9), 150.3(NCOOC2H5) and 164.9(C-2 and
CH–), 128.2(C-4), 130.5(C-3), 132.5(C-6), 135.1(C-8), 139.8(C-9), 150.3(NCOOC2H5) and 164.9(C-2 and ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOC2H5). HRMS m/z Calcd for C15H15ClNO5 [M + H]+: 324.0533. Found: 324.0522.
Obtained as a dark yellow solid (250 mg, 67% yield), mp 124–126 °C (from petroleum ether–COMPOUND LINKS
CHCOOC2H5). HRMS m/z Calcd for C15H15ClNO5 [M + H]+: 324.0533. Found: 324.0522.
Obtained as a dark yellow solid (250 mg, 67% yield), mp 124–126 °C (from petroleum ether–COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate), Rf: 0.51(4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether–COMPOUND LINKS
1 petroleum ether–COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate); νmax(KBr)/cm−1: 3436, 2925, 1767, 1733, 1705, 1633, 1593, 1459, 1369, 1324, 1204, 1105, 1024, 821; 1H NMR (300 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 1.4 & 1.46(6H, 2t, J = 7.2 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –COOCH2CH3), 4.36 & 4.49 (4H, 2q, J = 7.2 Hz,
CHCOOCH2CH3 & –COOCH2CH3), 4.36 & 4.49 (4H, 2q, J = 7.2 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –COOCH2CH3), 6.96(1H, s,
CHCOOCH2CH3 & –COOCH2CH3), 6.96(1H, s, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO–), 7.58(1H, dd, J = 1.8, 2.1 Hz, C-6H), 7.88(1H, d, J = 8.7 Hz, C-4H), 8.88(1H, d, J = 2.1 Hz, C-7H); 13C NMR (75.5 MHz, COMPOUND LINKS
CHCO–), 7.58(1H, dd, J = 1.8, 2.1 Hz, C-6H), 7.88(1H, d, J = 8.7 Hz, C-4H), 8.88(1H, d, J = 2.1 Hz, C-7H); 13C NMR (75.5 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 14.1 & 14.2 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –COOCH2CH3), 61.7 & 63.8 (
CHCOOCH2CH3 & –COOCH2CH3), 61.7 & 63.8 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –COOCH2CH3), 116.5 (C-7), 118.0 (C-5), 121.7 (C-4), 125.0 (
CHCOOCH2CH3 & –COOCH2CH3), 116.5 (C-7), 118.0 (C-5), 121.7 (C-4), 125.0 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 131.0 (C-6), 134.9 (C-3), 135.4 (C-8), 140.3 (C-9), 150.3 (NCOOC2H5), 164.8 (C-2) and 164.9 (
CH–), 131.0 (C-6), 134.9 (C-3), 135.4 (C-8), 140.3 (C-9), 150.3 (NCOOC2H5), 164.8 (C-2) and 164.9 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOC2H5). HRMS m/z Calcd for C15H15BrNO5 [M + H]+: 368.0128. Found: 368.0119.
CHCOOC2H5). HRMS m/z Calcd for C15H15BrNO5 [M + H]+: 368.0128. Found: 368.0119.
(E)-Methyl 2-[3-(2-ethoxy-2-oxoethylidene)-2-oxoindoline-1-yl] acetate (3i)
Obtained as a light yellow solid (160 mg, 71% yield), mp 123–125 °C (from petroleum ether–COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate), Rf: 0.55(4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether–COMPOUND LINKS
1 petroleum ether–COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate); νmax(KBr)/cm−1: 3423, 2959, 1740, 1713, 1652, 1611, 1473, 1345, 1227, 1199, 1016, 755; 1H NMR (300 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 1.37 (3H, t, J = 7.2 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 3.75 (3H, s, –CH2COOCH3), 4.33 (2H, q, J = 7.2 Hz,
CHCOOCH2CH3), 3.75 (3H, s, –CH2COOCH3), 4.33 (2H, q, J = 7.2 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 4.50 (2H, s, –CH2COOCH3), 6.69 (1H, d, J = 7.8 Hz, C-7H), 6.94 (1H, s,
CHCOOCH2CH3), 4.50 (2H, s, –CH2COOCH3), 6.69 (1H, d, J = 7.8 Hz, C-7H), 6.94 (1H, s, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO–), 7.09 (1H, t, J = 7.8 Hz, C-5H), 7.35 (1H, t, J = 7.8 Hz, C-6H), 8.60 (1H, d, J = 7.8 Hz, C-4H); 13C NMR (75.5 MHz, COMPOUND LINKS
CHCO–), 7.09 (1H, t, J = 7.8 Hz, C-5H), 7.35 (1H, t, J = 7.8 Hz, C-6H), 8.60 (1H, d, J = 7.8 Hz, C-4H); 13C NMR (75.5 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 14.1 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 41.2 (–CH2COOCH3), 52.6 (–CH2COOCH3), 61.2 (
CHCOOCH2CH3), 41.2 (–CH2COOCH3), 52.6 (–CH2COOCH3), 61.2 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 108.1 (C-7), 119.8 (C-6), 123.0 (C-4), 123.2 (
CHCOOCH2CH3), 108.1 (C-7), 119.8 (C-6), 123.0 (C-4), 123.2 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 128.9 (C-5), 132.4 (C-3), 137.1 (C-8), 144.5 (C-9), 165.4 (–CH2COOCH3), 167.53(C-2) and 167.8(
CH–), 128.9 (C-5), 132.4 (C-3), 137.1 (C-8), 144.5 (C-9), 165.4 (–CH2COOCH3), 167.53(C-2) and 167.8(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOC2H5). HRMS m/z Calcd for C15H15NO5Na [M + Na]+: 312.0842. Found: 312.0838.
CHCOOC2H5). HRMS m/z Calcd for C15H15NO5Na [M + Na]+: 312.0842. Found: 312.0838.
(E)-Methyl 2-[3-(2-ethoxy-2-oxoethylidene)-5-flouro-2-oxoindoline-1-yl] acetate (3j)
Obtained as a light yellow solid (410 mg, 78% yield), mp 155–157 °C (from petroleum ether–COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate), Rf: 0.51(4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether–COMPOUND LINKS
1 petroleum ether–COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate); νmax(KBr)/cm−1: 3423, 2925, 1766, 1749, 1709, 1652, 1617, 1491, 1351, 1204, 821; 1H NMR (300 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 1.38 (3H, t, J = 7.2 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 3.76 (3H, s, –CH2COOCH3), 4.34 (2H, q, J = 6.8 Hz,
CHCOOCH2CH3), 3.76 (3H, s, –CH2COOCH3), 4.34 (2H, q, J = 6.8 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 4.49 (2H, s, –CH2COOCH3), 6.60–6.64 (1H, m, C-7H), 6.97 (1H, s,
CHCOOCH2CH3), 4.49 (2H, s, –CH2COOCH3), 6.60–6.64 (1H, m, C-7H), 6.97 (1H, s, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO–), 7.04–7.10 (1H, m, C-6H), 8.40–8.44 (1H, m, C-4H); 13C NMR (75.5 MHz, COMPOUND LINKS
CHCO–), 7.04–7.10 (1H, m, C-6H), 8.40–8.44 (1H, m, C-4H); 13C NMR (75.5 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 14.1 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 41.3 (–CH2COOCH3), 52.7 (–CH2COOCH3), 61.4 (
CHCOOCH2CH3), 41.3 (–CH2COOCH3), 52.7 (–CH2COOCH3), 61.4 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 108.5 (d, J = 7.5 Hz, C-4), 116.6 (d, J = 27.1 Hz, C-6), 118.7 (d, J = 24.1 Hz, C-7), 120.8 (d, J = 9.8 Hz, C-3), 124.4 (
CHCOOCH2CH3), 108.5 (d, J = 7.5 Hz, C-4), 116.6 (d, J = 27.1 Hz, C-6), 118.7 (d, J = 24.1 Hz, C-7), 120.8 (d, J = 9.8 Hz, C-3), 124.4 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 136.8 (d, J = 3.0 Hz, C-8), 140.6 (C-9), 159.1 (d, J = 240.8 Hz, C-5), 165.2 (–CH2COOCH3), 167.2 (C-2) and 167.6 (
CH–), 136.8 (d, J = 3.0 Hz, C-8), 140.6 (C-9), 159.1 (d, J = 240.8 Hz, C-5), 165.2 (–CH2COOCH3), 167.2 (C-2) and 167.6 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOC2H5). HRMS m/z Calcd for C15H14FNO5Na [M + Na]+: 330.0748. Found: 330.0741.
CHCOOC2H5). HRMS m/z Calcd for C15H14FNO5Na [M + Na]+: 330.0748. Found: 330.0741.
(E)-Methyl 2-[5-chloro-3-(2-ethoxy-2-oxoethylidene)-2-oxoindoline-1-yl] acetate (3k)
Obtained as a light yellow solid (340 mg, 66% yield), mp 134–136 °C (from petroleum ether–COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate), Rf: 0.52(4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether–COMPOUND LINKS
1 petroleum ether–COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate); νmax(KBr)/cm−1: 3427, 2987, 1755, 1713, 1651, 1607, 1477, 1347, 1200, 1025, 826; 1H NMR (300 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 1.38 (3H, t, J = 7.2 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 3.76 (3H, s, –CH2COOCH3), 4.35 (2H, q, J = 7.2 Hz,
CHCOOCH2CH3), 3.76 (3H, s, –CH2COOCH3), 4.35 (2H, q, J = 7.2 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 4.49 (2H, s, –CH2COOCH3), 6.63 (1H, d, J = 8.4 Hz, C-7H), 6.97 (1H, s,
CHCOOCH2CH3), 4.49 (2H, s, –CH2COOCH3), 6.63 (1H, d, J = 8.4 Hz, C-7H), 6.97 (1H, s, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO–), 7.31–7.35 (1H, m, C-6H), 8.63 (1H, d, J = 2.1 Hz, C-4H); 13C NMR (75.5 MHz, COMPOUND LINKS
CHCO–), 7.31–7.35 (1H, m, C-6H), 8.63 (1H, d, J = 2.1 Hz, C-4H); 13C NMR (75.5 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 14.1 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 41.2 (–CH2COOCH3), 52.7 (–CH2COOCH3), 61.5 (
CHCOOCH2CH3), 41.2 (–CH2COOCH3), 52.7 (–CH2COOCH3), 61.5 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 109.0 (C-7), 121.0 (C-5), 124.5 (
CHCOOCH2CH3), 109.0 (C-7), 121.0 (C-5), 124.5 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 128.6 (C-3), 129.0 (C-4), 132.0 (C-6), 136.2 (C-8), 142.9 (C-9), 165.1 (–CH2COOCH3), 167.0 (C-2) and 167.5 (
CH–), 128.6 (C-3), 129.0 (C-4), 132.0 (C-6), 136.2 (C-8), 142.9 (C-9), 165.1 (–CH2COOCH3), 167.0 (C-2) and 167.5 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOC2H5). HRMS m/z Calcd for C15H14ClNO5Na [M + Na]+: 346.0453. Found: 346.0451.
CHCOOC2H5). HRMS m/z Calcd for C15H14ClNO5Na [M + Na]+: 346.0453. Found: 346.0451.
(E)-Methyl 2-[5-bromo-3-(2-ethoxy-2-oxoethylidene)-2-oxoindoline-1-yl] acetate (3l)
Obtained as a dark yellow solid (410 mg, 66% yield), mp 158–160 °C (from petroleum ether–COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate), Rf: 0.50(4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether–COMPOUND LINKS
1 petroleum ether–COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate); νmax(KBr)/cm−1: 3426, 2925, 1754, 1712, 1650, 1603, 1438, 1345, 1199, 1025, 823; 1H NMR (300 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 1.39 (3H, t, J = 7.2 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 3.76 (3H, s, –CH2COOCH3), 4.35 (2H, q, J = 6.9 Hz,
CHCOOCH2CH3), 3.76 (3H, s, –CH2COOCH3), 4.35 (2H, q, J = 6.9 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 4.49 (2H, s, –CH2COOCH3), 6.58 (1H, d, J = 8.4 Hz, C-7H), 6.97 (1H, s,
CHCOOCH2CH3), 4.49 (2H, s, –CH2COOCH3), 6.58 (1H, d, J = 8.4 Hz, C-7H), 6.97 (1H, s, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO–), 7.47–7.50 (1H, m, C-6H), 8.78 (1H, s, C-4H); 13C NMR (75.5 MHz, COMPOUND LINKS
CHCO–), 7.47–7.50 (1H, m, C-6H), 8.78 (1H, s, C-4H); 13C NMR (75.5 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 14.1 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 41.2 (–CH2COOCH3), 52.7 (–CH2COOCH3), 61.5 (
CHCOOCH2CH3), 41.2 (–CH2COOCH3), 52.7 (–CH2COOCH3), 61.5 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 109.5 (C-7), 115.9 (C-5), 121.4 (C-4), 124.6 (
CHCOOCH2CH3), 109.5 (C-7), 115.9 (C-5), 121.4 (C-4), 124.6 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 131.8 (C-6), 134.9 (C-3), 136.1 (C-8), 143.4 (C-9), 165.1 (–CH2COOCH3), 166.9 (C-2) and 167.5 (
CH–), 131.8 (C-6), 134.9 (C-3), 136.1 (C-8), 143.4 (C-9), 165.1 (–CH2COOCH3), 166.9 (C-2) and 167.5 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOC2H5). HRMS m/z Calcd for C15H15BrNO5 [M + H]+: 368.0128. Found: 368.0118.
CHCOOC2H5). HRMS m/z Calcd for C15H15BrNO5 [M + H]+: 368.0128. Found: 368.0118.
(E)-Ethyl 2-[3-(2-ethoxy-2-oxoethylidene)-2-oxoindoline-1-yl] acetate (3m)
Obtained as a light yellow solid (210 mg, 75% yield), mp 118–120 °C (from petroleum ether–COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate), Rf: 0.61(4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether–COMPOUND LINKS
1 petroleum ether–COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate); νmax(KBr)/cm−1: 3423, 2929, 1735, 1711, 1649, 1606, 1474, 1344, 1222, 1025, 755; 1H NMR (300 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 1.26 & 1.37 (6H, 2t, J = 7.2 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –CH2COOCH2CH3), 4.21 & 4.33 (4H, 2q, J = 7.2 Hz,
CHCOOCH2CH3 & –CH2COOCH2CH3), 4.21 & 4.33 (4H, 2q, J = 7.2 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –CH2COOCH2CH3), 4.48 (2H, s, –CH2COOCH2CH3), 6.69 (1H, d, J = 7.8 Hz, C-7H), 6.94 (1H, s,
CHCOOCH2CH3 & –CH2COOCH2CH3), 4.48 (2H, s, –CH2COOCH2CH3), 6.69 (1H, d, J = 7.8 Hz, C-7H), 6.94 (1H, s, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO–), 7.08 (1H, t, J = 7.5 Hz, C-5H), 7.35 (1H, t, J = 7.5 Hz, C-6), 8.59 (1H, d, J = 7.8 Hz, C-4H); 13C NMR (75.5 MHz, COMPOUND LINKS
CHCO–), 7.08 (1H, t, J = 7.5 Hz, C-5H), 7.35 (1H, t, J = 7.5 Hz, C-6), 8.59 (1H, d, J = 7.8 Hz, C-4H); 13C NMR (75.5 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 14.0 & 14.1 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –CH2COOCH2CH3), 41.4 (–CH2COOCH2CH3), 61.2 & 61.8 (
CHCOOCH2CH3 & –CH2COOCH2CH3), 41.4 (–CH2COOCH2CH3), 61.2 & 61.8 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –CH2COOCH2CH3), 108.1 (C-7), 119.8 (C-6), 123.0 (C-4), 123.1 (
CHCOOCH2CH3 & –CH2COOCH2CH3), 108.1 (C-7), 119.8 (C-6), 123.0 (C-4), 123.1 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 128.9 (C-5), 132.3 (C-3), 137.2 (C-8), 144.6 (C-9), 165.5 (–CH2COOC2H5), 167.2 (C-2) and 167.5 (
CH–), 128.9 (C-5), 132.3 (C-3), 137.2 (C-8), 144.6 (C-9), 165.5 (–CH2COOC2H5), 167.2 (C-2) and 167.5 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOC2H5). HRMS m/z Calcd for C16H17NO5Na [M + Na]+: 326.0999. Found: 326.0985.
CHCOOC2H5). HRMS m/z Calcd for C16H17NO5Na [M + Na]+: 326.0999. Found: 326.0985.
(E)-Ethyl 2-[3-(2-ethoxy-2-oxoethylidene)-5-flouro-2-oxoindoline-1-yl] acetate (3n)
Obtained as a light yellow solid (550 mg, 80% yield), mp 130–131 °C (from petroleum ether–COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate), Rf: 0.58 (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether–COMPOUND LINKS
1 petroleum ether–COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate); νmax(KBr)/cm−1: 3423, 2925, 1741, 1709, 1654, 1617, 1492, 1372, 1351, 1217, 1025, 777; 1H NMR (300 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 1.26 & 1.38 (6H, 2t, J = 7.2 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –CH2COOCH2CH3), 4.22 & 4.34 (4H, 2q, J = 7.2 Hz,
CHCOOCH2CH3 & –CH2COOCH2CH3), 4.22 & 4.34 (4H, 2q, J = 7.2 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –CH2COOCH2CH3), 4.47 (2H, s, –CH2COOCH2CH3), 6.60–6.64 (1H, m, C-7H), 6.97 (1H, s,
CHCOOCH2CH3 & –CH2COOCH2CH3), 4.47 (2H, s, –CH2COOCH2CH3), 6.60–6.64 (1H, m, C-7H), 6.97 (1H, s, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO–), 7.04–7.10 (1H, m, C-6H), 8.40–8.43 (1H, m, C-4H); 13C NMR (75.5 MHz, COMPOUND LINKS
CHCO–), 7.04–7.10 (1H, m, C-6H), 8.40–8.43 (1H, m, C-4H); 13C NMR (75.5 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 14.0 & 14.1 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –CH2COOCH2CH3), 41.4 (–CH2COOCH2CH3), 61.4 & 61.9 (
CHCOOCH2CH3 & –CH2COOCH2CH3), 41.4 (–CH2COOCH2CH3), 61.4 & 61.9 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –CH2COOCH2CH3), 108.6 (d, J = 8.3 Hz, C-4), 116.6 (d, J = 36.9 Hz, C-6), 118.7 (d, J = 24.9 Hz, C-7), 120.7 (d, J = 7.1 Hz, C-3), 124.3 (
CHCOOCH2CH3 & –CH2COOCH2CH3), 108.6 (d, J = 8.3 Hz, C-4), 116.6 (d, J = 36.9 Hz, C-6), 118.7 (d, J = 24.9 Hz, C-7), 120.7 (d, J = 7.1 Hz, C-3), 124.3 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 136.8 (d, J = 2.2 Hz, C-8), 140.7 (C-9), 159.0 (d, J = 240.0 Hz, C-5), 165.2 (–CH2COOC2H5), 167.1 (C-2) and 167.3 (
CH–), 136.8 (d, J = 2.2 Hz, C-8), 140.7 (C-9), 159.0 (d, J = 240.0 Hz, C-5), 165.2 (–CH2COOC2H5), 167.1 (C-2) and 167.3 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOC2H5). HRMS m/z Calcd for C16H16FNO5Na [M + Na]+: 344.0905. Found: 344.0897.
CHCOOC2H5). HRMS m/z Calcd for C16H16FNO5Na [M + Na]+: 344.0905. Found: 344.0897.
(E)-Ethyl 2-[5-chloro-3-(2-ethoxy-2-oxoethylidene)-2-oxoindoline-1-yl] acetate (3o)
Obtained as a light yellow solid (450 mg, 67% yield), mp 163–165 °C (from petroleum ether–COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate), Rf: 0.58 (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether–COMPOUND LINKS
1 petroleum ether–COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate); νmax(KBr)/cm−1: 3424, 2928, 1741, 1709, 1652, 1610, 1443, 1369, 1202, 1020, 817; 1H NMR (300 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 1.27 & 1.39 (6H, 2t, J = 6.9, 7.2 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –CH2COOCH2CH3), 4.23 & 4.36 (4H, 2q, J = 6.9 Hz,
CHCOOCH2CH3 & –CH2COOCH2CH3), 4.23 & 4.36 (4H, 2q, J = 6.9 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –CH2COOCH2CH3), 4.48 (2H, s, –CH2COOCH2CH3), 6.64 (1H, d, J = 8.4 Hz, C-7H), 6.98 (1H, s,
CHCOOCH2CH3 & –CH2COOCH2CH3), 4.48 (2H, s, –CH2COOCH2CH3), 6.64 (1H, d, J = 8.4 Hz, C-7H), 6.98 (1H, s, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO–), 7.32–7.36 (1H, m, C-6H), 8.65 (1H, d, J = 1.8 Hz, C-4H); 13C NMR (75.5 MHz, COMPOUND LINKS
CHCO–), 7.32–7.36 (1H, m, C-6H), 8.65 (1H, d, J = 1.8 Hz, C-4H); 13C NMR (75.5 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 14.0 & 14.1 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –CH2COOCH2CH3), 41.4 (–CH2COOCH2CH3), 61.5 & 61.9 (
CHCOOCH2CH3 & –CH2COOCH2CH3), 41.4 (–CH2COOCH2CH3), 61.5 & 61.9 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –CH2COOCH2CH3), 109.1 (C-7), 121.0 (C-5), 124.5 (
CHCOOCH2CH3 & –CH2COOCH2CH3), 109.1 (C-7), 121.0 (C-5), 124.5 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 128.6 (C-3), 129.0 (C-4), 132.0 (C-6), 136.3 (C-8), 143.0 (C-9), 165.1 (–CH2COOC2H5), 167.0 (C-2) and 167.1 (
CH–), 128.6 (C-3), 129.0 (C-4), 132.0 (C-6), 136.3 (C-8), 143.0 (C-9), 165.1 (–CH2COOC2H5), 167.0 (C-2) and 167.1 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOC2H5). HRMS m/z Calcd for C16H17ClNO5 [M + H]+: 338.0790. Found: 338.0782.
CHCOOC2H5). HRMS m/z Calcd for C16H17ClNO5 [M + H]+: 338.0790. Found: 338.0782.
(E)-Ethyl 2-[5-bromo-3-(2-ethoxy-2-oxoethylidene)-2-oxoindoline-1-yl] acetate (3p)
Obtained as a dark yellow solid (480 mg, 74% yield), mp 152–154 °C (from petroleum ether–COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate), Rf: 0.59 (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether–COMPOUND LINKS
1 petroleum ether–COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl acetate); νmax(KBr)/cm−1: 3423, 2926, 1741, 1709, 1607, 1437, 1371, 1202, 1123, 1025, 816; 1H NMR (300 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 1.26 & 1.38 (6H, 2t, J = 7.2 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –CH2COOCH2CH3), 4.19 & 4.35 (4H, 2q, J = 6.9, 7.5 Hz,
CHCOOCH2CH3 & –CH2COOCH2CH3), 4.19 & 4.35 (4H, 2q, J = 6.9, 7.5 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –CH2COOCH2CH3), 4.47 (2H, s, –CH2COOCH2CH3), 6.58 (1H, d, J = 8.1 Hz, C-7H), 6.96 (1H, s,
CHCOOCH2CH3 & –CH2COOCH2CH3), 4.47 (2H, s, –CH2COOCH2CH3), 6.58 (1H, d, J = 8.1 Hz, C-7H), 6.96 (1H, s, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO–), 7.46–7.49 (1H, m, C-6H), 8.77 (1H, d, J = 1.5 Hz, C-4H); 13C NMR (75.5 MHz, COMPOUND LINKS
CHCO–), 7.46–7.49 (1H, m, C-6H), 8.77 (1H, d, J = 1.5 Hz, C-4H); 13C NMR (75.5 MHz, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCDCl3): δ 14.0 & 14.1 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –CH2COOCH2CH3), 41.4 (–CH2COOCH2CH3), 61.5 & 61.9 (
CHCOOCH2CH3 & –CH2COOCH2CH3), 41.4 (–CH2COOCH2CH3), 61.5 & 61.9 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –CH2COOCH2CH3), 109.6 (C-7), 115.8 (C-5), 121.4 (C-4), 124.5 (
CHCOOCH2CH3 & –CH2COOCH2CH3), 109.6 (C-7), 115.8 (C-5), 121.4 (C-4), 124.5 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 131.7 (C-6), 134.8 (C-3), 136.1 (C-8), 143.5 (C-9), 165.1 (–CH2COOC2H5) and 166.9 (C-2 and
CH–), 131.7 (C-6), 134.8 (C-3), 136.1 (C-8), 143.5 (C-9), 165.1 (–CH2COOC2H5) and 166.9 (C-2 and ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOC2H5). HRMS m/z Calcd for C16H17BrNO5 [M + H]+: 382.0285. Found: 382.0286.
Primary endothelial cells were isolated from human umbilical cord using mild trypsinization.28 The cells were grown in M199 medium supplemented with 15% heat inactivated fetal calf serum, 2 mM COMPOUND LINKS
CHCOOC2H5). HRMS m/z Calcd for C16H17BrNO5 [M + H]+: 382.0285. Found: 382.0286.
Primary endothelial cells were isolated from human umbilical cord using mild trypsinization.28 The cells were grown in M199 medium supplemented with 15% heat inactivated fetal calf serum, 2 mM COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundL-glutamine, 100 units ml−1 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundpenicillin, 100 μg ml−1 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundstreptomycin, 0.25 μg ml−1 amphotericin B, endothelial cell growth factor (50 μg ml−1). At confluence, the cells were subcultured using 0.05% trypsin-0.01 M EDTA solution and were used between passages three to four.
Cell viability assay
The cytotoxicity of these compounds was analyzed by colorimetric MTT (methylthiazolydiphenyl-tetrazolium bromide) assay as described.28 Briefly, endothelial cells were treated with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO alone (0.25% as vehicle) or with different concentrations of compounds for 24 h. The medium was removed and 100 μl MTT (2.5 mg ml−1 in serum free medium) was added to each well. The MTT was removed after 4 h, cells were washed out with PBS and 100 μl COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO was added to each well to dissolve COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundwater insoluble MTT-formazan crystals. Absorbance was recorded at 570 nm in an ELISA reader (Bio-Rad, Model 680, USA). All experiments were performed at least 3 times in triplicate wells.
Cell based-ELISA for measurement of ICAM-1 activity
Cell-ELISA was used for measuring the expression of ICAM-1 on surface of endothelial cells.28 Endothelial cells were incubated with or without the test compounds at desired concentrations for the required period, followed by treatment with TNF-α (10 ng ml−1) for 16 h for ICAM-1 expression. The cells were fixed with 1.0% COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundglutaraldehyde. Non-specific binding of antibody was blocked by using skimmed milk (3.0% in PBS). Cells were incubated overnight at 4 °C with anti-ICAM-1 mAb, diluted in blocking buffer, the cells were further washed with PBS and incubated with peroxidase-conjugated goat anti-mouse secondary Abs. After washings, cells were exposed to the peroxidase substrate (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundo-phenylenediamine dihydrochloride 40 mg/100 ml in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcitrate phosphate buffer, pH 4.5). Reaction was stopped by the addition of 2 N COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundsulfuric acid and absorbance at 490 nm was measured using a microplate reader (Spectramax 190, Molecular Devices, USA). The potencies of the compounds were compared on the basis of their IC50 values calculated from the dose-response curves.28–30
Time kinetics of ICAM-1 inhibition by 3p
The confluent monolayer of human endothelial cells were treated with or without 100 μM of 3p at time points ranging from 4 h, 2 h and 1 h before TNF-α stimulation (pre-treatment), simultaneously along with TNF-α stimulation (co-treatment) and 4 h, 2 h and 1 h after the TNF-α stimulation (post-treatment). The cells were incubated in the presence of TNF-α for 16 h before ICAM-1 expression was measured by Cell-ELISA. The data are representative of three independent experiments.
Acknowledgements
Authors acknowledge the help of St. Stephen's Hospital, Delhi for providing umbilical cords. This work was partly funded by the Council of Scientific and Industrial Research, India grant NWP0033 (to B.G.), the NIH grants HL081205, HL095420 (SCCOR), NIEHS-P50ES01590 & GM079239 (to S.B.) and the University of Delhi grant under the Strengthening R&D Doctoral Research Programme and the Department of Scientific & Industrial Research (DSIR), Ministry of Science & Technology, India (to V.S.P. and A.K.P.).
Notes and references
- T. A. Springer, Cell, 1994, 76, 301 CAS.
- T. Collins, M. A. Read, A. S. Neish, M. Z. Whitley, D. Thanos and T. Maniatis, FASEB J., 1995, 9, 899 CAS.
- L. Osborn, Cell, 1990, 62, 3 CrossRef CAS.
- C. E. Butcher, Cell, 1991, 67, 1033 CrossRef CAS.
- A. Mantovani, F. Bussolino and M. Introna, Immunol. Today, 1997, 18, 231 CrossRef CAS.
- A. Gorski, Immunol. Today, 1994, 15, 251 Search PubMed.
- C. Brojstan, J. Anrather, V. Csizmadia, G. Natrajan and H. Winkler, J. Immunol., 1997, 58, 3836 Search PubMed.
- B. Madan, S. Batra and B. Ghosh, Mol. Pharmacol., 2000, 58, 534 Search PubMed.
-
M. R. Weiser, S. A. L. Gibbs and H. B. Hechtman, In Adhesion Molecules in Health and Disease; L. C. Paul andT. B. Issekutz, ed.; Marcel Dekker: NewYork, 1997, p 55 Search PubMed.
- J. F. M. Silva, S. J. Garden and A. C. Pinto, J. Braz. Chem. Soc., 2001, 12, 273 CAS.
- G. J. Kapadia, Y. N. Shukla and S. P. Basak, Tetrahedron, 1980, 36, 2441 CrossRef CAS.
- J. Bergman, J. Lindstrom and U. Tilstam, Tetrahedron, 1985, 41, 2879 CrossRef CAS.
- S. E. Webber, J. Tikhe, S. T. Worland, S. A. Fuhrman, T. F. Hendrickson, D. A. Matthews, R. A. Love, A. K. Patick, J. W. Meador, R. A. Ferre, E. L. Brown, D. M. DeLisle, C. E. Ford and S. L. Binford, J. Med. Chem., 1996, 39, 5072 Search PubMed.
- G. Filomeni, G. Cerchiaro, F. Da Costa, M. Ana, A. De Martino, J. Z. Pedersen, G. Rotilio and M. R. Ciriolo, J. Biol. Chem., 2007, 282, 12010 CAS.
- V. A. Muthukumar, S. George and V. Vaidhyalingam, Biol. Pharm. Bull., 2008, 31, 1461 Search PubMed.
- G. Filomeni, S. Piccirillo, I. Graziani, S. Cardaci, F. Da Costa, M. Ana, G. Rotilio and M. R. Ciriolo, Carcinogenesis, 2009, 30, 1115 Search PubMed.
- G. Kiran, G. Rajyalakshmi, J. V. Rao and M. Sarangapani, Pharmacologyonline, 2009, 1, 303 Search PubMed.
-
F. G. Salituro, G. W. Bemis, S. Wilke, J. Green, J. Cao, H. Gao and E. M. Harrington, PCT Int. Appl. WO Pat. 0064872.
- M. Verma, S. N. Pandeya, K. N. Singh and J. P. Stables, Acta Pharm., 2004, 54, 49 Search PubMed.
- S. K. Sridhar, S. N. Pandeya, J. P. Stables and A. Ramesh, Eur. J. Pharm. Sci., 2002, 16, 129 Search PubMed.
- H. A. Bradman, J. Heterocycl. Chem., 1973, 10, 383 CrossRef.
- P. L. Julian, H. C. Printy, R. Ketcham and R. Doone, J. Am. Chem. Soc., 1953, 75, 5305 Search PubMed.
- R. Shimazawa, M. Kuriyama and R. Shirai, Bioorg. Med. Chem. Lett., 2008, 18, 3350 Search PubMed.
- B. M. Trost, N. Cramer and S. M. Silverman, J. Am. Chem. Soc., 2007, 129, 12396 CrossRef CAS.
- C. Weber, W. Erl, A. Pietsch and P. C Weber, Circulation, 1995, 91, 1914 CAS.
- D. M. Radomska-Leśniewska, A. M. Sadowska, F. J. Van Overveld, U. Demkow, J. Zieliński and W. A. De Backer, Physiol. Pharmacol., 2006, 57, 325 Search PubMed.
- A. Sakai, Life Sci., 1996, 58, 2377 Search PubMed.
- S. Kumar, P. Arya, C. Mukherjee, B. K. Singh, N. Singh, V. S. Parmar, A. K. Prasad and B. Ghosh, Biochemistry, 2005, 44, 15944 Search PubMed.
- S. Kumar, B. K. Singh, A. K. Pandey, A. Kumar, S. K. Sharma, H. G. Raj, A. K. Prasad, E. Van der Eycken, V. S. Parmar and B. Ghosh, Bioorg. Med. Chem., 2007, 15, 2952 Search PubMed.
- L. Pasquinucci, O. Prezzavento, A. Marrazzo, E. Amata, S. Ronsisvalle, Z. Georgoussi, D. D. Fourla, G. M. Scoto, C. Parenti, G. Aricò and G. Ronsisvalle, Bioorg. Med. Chem., 2010, 18, 4975 Search PubMed.
Footnote |
† Electronic supplementary information (ESI) available: 1H-NMR & 13C-NMR spectra of compounds COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound3b, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound3c, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound3d, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound3e, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound3f, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound3g, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound3h, 3i, 3j, 3l, 3m, 3n, 3o and 3p and NOE-1H NMR and NOESY-1H NMR of compounds 2a, 2b, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound2c and 2d. See DOI: 10.1039/c0md00262c |
|
| This journal is © The Royal Society of Chemistry 2011 |
Click here to see how this site uses Cookies. View our privacy policy here. 


![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 6.59(1H, s,
CHCOOCH2CH3), 6.59(1H, s, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO–), 6.85(1H, d, J = 8.4 Hz, C-7H), 7.39(1H, d, J = 6.6 Hz, C-6H), 8.35(1H, s, C-4H), 8.53(1H, s, NH); 13C NMR (75.5 MHz, CDCl3): δ 13.9(
CHCO–), 6.85(1H, d, J = 8.4 Hz, C-7H), 7.39(1H, d, J = 6.6 Hz, C-6H), 8.35(1H, s, C-4H), 8.53(1H, s, NH); 13C NMR (75.5 MHz, CDCl3): δ 13.9(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 61.2(
CHCOOCH2CH3), 61.2(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 111.7(C-7), 120.9(C-5), 122.2(
CHCOOCH2CH3), 111.7(C-7), 120.9(C-5), 122.2(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 125.7(C-4), 127.5(C-3), 132.3(C-6), 137.4(C-8), 143.7(C-9), 164.9(
CH–), 125.7(C-4), 127.5(C-3), 132.3(C-6), 137.4(C-8), 143.7(C-9), 164.9(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOC2H5) and 167.4(C-2). HRMS m/z Calcd for C12H10ClNO3Na [M + Na]+: 274.0241. Found: 274.0238.
CHCOOC2H5) and 167.4(C-2). HRMS m/z Calcd for C12H10ClNO3Na [M + Na]+: 274.0241. Found: 274.0238.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70) as eluent to afford the compounds 3a–p as yellow to orange solids in 70–80% yields.
70) as eluent to afford the compounds 3a–p as yellow to orange solids in 70–80% yields.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether–ethyl acetate); νmax(KBr)/cm−1: 3418, 2926, 1765, 1733, 1643, 1604, 1471, 1343, 1199, 1154, 1025; 1H NMR (300 MHz, CDCl3): δ 1.39(3H, t, J = 7.2 Hz,
1 petroleum ether–ethyl acetate); νmax(KBr)/cm−1: 3418, 2926, 1765, 1733, 1643, 1604, 1471, 1343, 1199, 1154, 1025; 1H NMR (300 MHz, CDCl3): δ 1.39(3H, t, J = 7.2 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 4.04(3H, s, –OCH3), 4.32(2H, q, J = 7.2 Hz,
CHCOOCH2CH3), 4.04(3H, s, –OCH3), 4.32(2H, q, J = 7.2 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 6.98(1H, s,
CHCOOCH2CH3), 6.98(1H, s, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO–), 7.14–7.21(1H, m, C-6H), 7.97–8.02(1H, m, C-4H), 8.48–8.52(1H, m, C-7H); 13C NMR (75.5 MHz, CDCl3): δ 14.1(
CHCO–), 7.14–7.21(1H, m, C-6H), 7.97–8.02(1H, m, C-4H), 8.48–8.52(1H, m, C-7H); 13C NMR (75.5 MHz, CDCl3): δ 14.1(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 54.1(–OCH3), 61.6(
CHCOOCH2CH3), 54.1(–OCH3), 61.6(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 115.4(d, J = 26.4 Hz, C-4), 116.2(d, J = 8.3 Hz, C-6), 119.4(d, J = 23.4 Hz, C-7), 121.3(d, J = 10.5 Hz, C-3), 125.1(
CHCOOCH2CH3), 115.4(d, J = 26.4 Hz, C-4), 116.2(d, J = 8.3 Hz, C-6), 119.4(d, J = 23.4 Hz, C-7), 121.3(d, J = 10.5 Hz, C-3), 125.1(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 135.5(d, J = 3.2 Hz, C-8), 137.4(C-9), 151.0(NCOOCH3), 159.7(d, J = 243.8 Hz, C-5), 164.9 (C-2) and 165.1(
CH–), 135.5(d, J = 3.2 Hz, C-8), 137.4(C-9), 151.0(NCOOCH3), 159.7(d, J = 243.8 Hz, C-5), 164.9 (C-2) and 165.1(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOC2H5). HRMS m/z Calcd for C14H13FNO5 [M + H]+: 294.0772. Found: 294.0767.
CHCOOC2H5). HRMS m/z Calcd for C14H13FNO5 [M + H]+: 294.0772. Found: 294.0767.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether–ethyl acetate); νmax(KBr)/cm−1: 3449, 2925, 1765, 1734, 1713, 1640, 1600, 1460, 1352, 1197, 1026, 774; 1H NMR (300 MHz, CDCl3): δ 1.40(3H, t, J = 7.2 Hz,
1 petroleum ether–ethyl acetate); νmax(KBr)/cm−1: 3449, 2925, 1765, 1734, 1713, 1640, 1600, 1460, 1352, 1197, 1026, 774; 1H NMR (300 MHz, CDCl3): δ 1.40(3H, t, J = 7.2 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 4.04(3H, s, –OCH3), 4.34(2H, q, J = 7.2 Hz,
CHCOOCH2CH3), 4.04(3H, s, –OCH3), 4.34(2H, q, J = 7.2 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 6.97(1H, s,
CHCOOCH2CH3), 6.97(1H, s, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO–), 7.41–7.45(1H, m, C-6H), 7.95(1H, d, J = 9.0 Hz, C-4H), 8.74(1H, d, J = 2.1 Hz, C-7H); 13C NMR (75.5 MHz, CDCl3): δ 14.1(
CHCO–), 7.41–7.45(1H, m, C-6H), 7.95(1H, d, J = 9.0 Hz, C-4H), 8.74(1H, d, J = 2.1 Hz, C-7H); 13C NMR (75.5 MHz, CDCl3): δ 14.1(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 54.2(–OCH3), 61.7(
CHCOOCH2CH3), 54.2(–OCH3), 61.7(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 116.2(C-7), 121.4(C-5), 125.2(
CHCOOCH2CH3), 116.2(C-7), 121.4(C-5), 125.2(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 128.2(C-4), 130.6(C-3), 132.5(C-6), 135.0(C-8), 139.7(C-9), 150.8(NCOOCH3) and 164.9(C-2 and = CHCOOC2H5). HRMS m/z Calcd for C14H13ClNO5 [M + H]+: 310.0477. Found: 310.0472.
CH–), 128.2(C-4), 130.6(C-3), 132.5(C-6), 135.0(C-8), 139.7(C-9), 150.8(NCOOCH3) and 164.9(C-2 and = CHCOOC2H5). HRMS m/z Calcd for C14H13ClNO5 [M + H]+: 310.0477. Found: 310.0472.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether–ethyl acetate); νmax(KBr)/cm−1: 3449, 2924, 1766, 1734, 1712, 1638, 1595, 1458, 1352, 1195, 1107, 1026, 774; 1H NMR (300 MHz, CDCl3): δ 1.40(3H, t, J = 7.2 Hz,
1 petroleum ether–ethyl acetate); νmax(KBr)/cm−1: 3449, 2924, 1766, 1734, 1712, 1638, 1595, 1458, 1352, 1195, 1107, 1026, 774; 1H NMR (300 MHz, CDCl3): δ 1.40(3H, t, J = 7.2 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 4.04(3H, s, –OCH3), 4.33–4.40(2H, m,
CHCOOCH2CH3), 4.04(3H, s, –OCH3), 4.33–4.40(2H, m, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 6.97(1H, s,
CHCOOCH2CH3), 6.97(1H, s, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO–), 7.58(1H, d, J = 8.7 Hz, C-6H), 7.91(1H, d, J = 8.7 Hz, C-7H), 8.89(1H, brs, C-4H); 13C NMR (75.5 MHz, CDCl3): δ 14.1(
CHCO–), 7.58(1H, d, J = 8.7 Hz, C-6H), 7.91(1H, d, J = 8.7 Hz, C-7H), 8.89(1H, brs, C-4H); 13C NMR (75.5 MHz, CDCl3): δ 14.1(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 54.2(–OCH3), 61.7(
CHCOOCH2CH3), 54.2(–OCH3), 61.7(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 116.5(C-7), 118.1(C-5), 121.8(C-4), 125.2(
CHCOOCH2CH3), 116.5(C-7), 118.1(C-5), 121.8(C-4), 125.2(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 131.1(C-6), 134.8(C-3), 135.4(C-8), 140.2(C-9), 150.8(NCOOCH3), 164.7(C-2) and 164.9(
CH–), 131.1(C-6), 134.8(C-3), 135.4(C-8), 140.2(C-9), 150.8(NCOOCH3), 164.7(C-2) and 164.9(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOC2H5). HRMS m/z Calcd for C14H13BrNO5 [M + H]+: 353.9972. Found: 353.9969.
CHCOOC2H5). HRMS m/z Calcd for C14H13BrNO5 [M + H]+: 353.9972. Found: 353.9969.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether–ethyl acetate); νmax(KBr)/cm−1: 3425, 2926, 1758, 1734, 1710, 1638, 1597, 1464, 1371, 1198, 1090, 1027, 788; 1H NMR (300 MHz, CDCl3): δ 1.31 & 1.39 (6H, 2t, J = 6.9 Hz,
1 petroleum ether–ethyl acetate); νmax(KBr)/cm−1: 3425, 2926, 1758, 1734, 1710, 1638, 1597, 1464, 1371, 1198, 1090, 1027, 788; 1H NMR (300 MHz, CDCl3): δ 1.31 & 1.39 (6H, 2t, J = 6.9 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –COOCH2CH3), 4.27 & 4.42(4H, 2q, J = 7.2` Hz,
CHCOOCH2CH3 & –COOCH2CH3), 4.27 & 4.42(4H, 2q, J = 7.2` Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –COOCH2CH3), 6.86(1H, s,
CHCOOCH2CH3 & –COOCH2CH3), 6.86(1H, s, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO–), 7.12–7.19(1H, m, C-5H), 7.38(1H, t, J = 7.8 Hz, C-6H), 7.90(1H, d, J = 8.1 Hz, C-4H), 8.62(1H, d, J = 7.8 Hz, C-7H); 13C NMR (75.5 MHz, CDCl3): δ 13.1 & 13.2(
CHCO–), 7.12–7.19(1H, m, C-5H), 7.38(1H, t, J = 7.8 Hz, C-6H), 7.90(1H, d, J = 8.1 Hz, C-4H), 8.62(1H, d, J = 7.8 Hz, C-7H); 13C NMR (75.5 MHz, CDCl3): δ 13.1 & 13.2(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –COOCH2CH3), 60.4 & 62.6(
CHCOOCH2CH3 & –COOCH2CH3), 60.4 & 62.6(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –COOCH2CH3), 114.0(C-7), 119.1(C-6), 122.5(C-4), 123.8(
CHCOOCH2CH3 & –COOCH2CH3), 114.0(C-7), 119.1(C-6), 122.5(C-4), 123.8(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 127.3(C-5), 131.8(C-3), 135.0(C-8), 140.4(C-9), 149.4(NCOOC2H5), 164.2(C-2) and 164.5(
CH–), 127.3(C-5), 131.8(C-3), 135.0(C-8), 140.4(C-9), 149.4(NCOOC2H5), 164.2(C-2) and 164.5(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOC2H5). HRMS m/z Calcd for C15H15NO5Na [M + Na]+: 312.0842. Found: 312.0832.
CHCOOC2H5). HRMS m/z Calcd for C15H15NO5Na [M + Na]+: 312.0842. Found: 312.0832.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether–ethyl acetate); νmax(KBr)/cm−1: 3447, 2924, 1763, 1735, 1712, 1642, 1598, 1476, 1371, 1334, 1208, 1159, 1027, 828; 1H NMR (300 MHz, CDCl3): δ 1.39 & 1.46 (6H, 2t, J = 7.2 Hz,
1 petroleum ether–ethyl acetate); νmax(KBr)/cm−1: 3447, 2924, 1763, 1735, 1712, 1642, 1598, 1476, 1371, 1334, 1208, 1159, 1027, 828; 1H NMR (300 MHz, CDCl3): δ 1.39 & 1.46 (6H, 2t, J = 7.2 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –COOCH2CH3), 4.35 & 4.49 (4H, 2q, J = 7.2 Hz,
CHCOOCH2CH3 & –COOCH2CH3), 4.35 & 4.49 (4H, 2q, J = 7.2 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –COOCH2CH3), 6.96 (1H, s,
CHCOOCH2CH3 & –COOCH2CH3), 6.96 (1H, s, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO–), 7.13–7.20 (1H, m, C-6H), 7.94–7.99 (1H, m, C-4H), 8.48–8.51 (1H, m, C-7H); 13C NMR (75.5 MHz, CDCl3): δ 14.1 & 14.2 (
CHCO–), 7.13–7.20 (1H, m, C-6H), 7.94–7.99 (1H, m, C-4H), 8.48–8.51 (1H, m, C-7H); 13C NMR (75.5 MHz, CDCl3): δ 14.1 & 14.2 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –COOCH2CH3), 61.6 & 63.7 (
CHCOOCH2CH3 & –COOCH2CH3), 61.6 & 63.7 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –COOCH2CH3), 115.4 (d, J = 26.4 Hz, C-4), 116.1 (d, J = 7.55 Hz, C-6), 119.3 (d, J = 24.1 Hz, C-7), 121.3 (d, J = 9.8 Hz, C-3), 124.8 (
CHCOOCH2CH3 & –COOCH2CH3), 115.4 (d, J = 26.4 Hz, C-4), 116.1 (d, J = 7.55 Hz, C-6), 119.3 (d, J = 24.1 Hz, C-7), 121.3 (d, J = 9.8 Hz, C-3), 124.8 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 135.6 (d, J = 3.0 Hz, C-8), 137.5 (d, J = 2.2 Hz, C-9), 150.4 (NCOOC2H5), 159.6 (d, J = 243.1 Hz, C-5), 164.9 (C-2) and 165.1(
CH–), 135.6 (d, J = 3.0 Hz, C-8), 137.5 (d, J = 2.2 Hz, C-9), 150.4 (NCOOC2H5), 159.6 (d, J = 243.1 Hz, C-5), 164.9 (C-2) and 165.1(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOC2H5). HRMS m/z Calcd for C15H15FNO5 [M + H]+: 308.0929. Found: 308.0925.
CHCOOC2H5). HRMS m/z Calcd for C15H15FNO5 [M + H]+: 308.0929. Found: 308.0925.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether–ethyl acetate); νmax(KBr)/cm−1: 3426, 2925, 1765, 1731, 1703, 1642, 1594, 1458, 1371, 1323, 1206, 1171, 1107, 1029, 776; 1H NMR (300 MHz, CDCl3): δ 1.39 & 1.46 (6H, 2t, J = 7.2 Hz,
1 petroleum ether–ethyl acetate); νmax(KBr)/cm−1: 3426, 2925, 1765, 1731, 1703, 1642, 1594, 1458, 1371, 1323, 1206, 1171, 1107, 1029, 776; 1H NMR (300 MHz, CDCl3): δ 1.39 & 1.46 (6H, 2t, J = 7.2 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –COOCH2CH3), 4.35 & 4.44 (4H, 2q, J = 7.2 Hz,
CHCOOCH2CH3 & –COOCH2CH3), 4.35 & 4.44 (4H, 2q, J = 7.2 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –COOCH2CH3), 6.97 (1H, s,
CHCOOCH2CH3 & –COOCH2CH3), 6.97 (1H, s, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO–), 7.41–7.45 (1H, m, C-6H), 7.93–7.99 (1H, m, C-4H), 8.74 (1H, d, J = 2.1 Hz, C-7H); 13C NMR (75.5 MHz, CDCl3): δ 14.1 & 14.2 (
CHCO–), 7.41–7.45 (1H, m, C-6H), 7.93–7.99 (1H, m, C-4H), 8.74 (1H, d, J = 2.1 Hz, C-7H); 13C NMR (75.5 MHz, CDCl3): δ 14.1 & 14.2 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –COOCH2CH3), 61.7 & 63.8 (
CHCOOCH2CH3 & –COOCH2CH3), 61.7 & 63.8 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –COOCH2CH3), 116.2(C-7), 121.4(C-5), 125.0(
CHCOOCH2CH3 & –COOCH2CH3), 116.2(C-7), 121.4(C-5), 125.0(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 128.2(C-4), 130.5(C-3), 132.5(C-6), 135.1(C-8), 139.8(C-9), 150.3(NCOOC2H5) and 164.9(C-2 and
CH–), 128.2(C-4), 130.5(C-3), 132.5(C-6), 135.1(C-8), 139.8(C-9), 150.3(NCOOC2H5) and 164.9(C-2 and ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOC2H5). HRMS m/z Calcd for C15H15ClNO5 [M + H]+: 324.0533. Found: 324.0522.
CHCOOC2H5). HRMS m/z Calcd for C15H15ClNO5 [M + H]+: 324.0533. Found: 324.0522.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether–ethyl acetate); νmax(KBr)/cm−1: 3436, 2925, 1767, 1733, 1705, 1633, 1593, 1459, 1369, 1324, 1204, 1105, 1024, 821; 1H NMR (300 MHz, CDCl3): δ 1.4 & 1.46(6H, 2t, J = 7.2 Hz,
1 petroleum ether–ethyl acetate); νmax(KBr)/cm−1: 3436, 2925, 1767, 1733, 1705, 1633, 1593, 1459, 1369, 1324, 1204, 1105, 1024, 821; 1H NMR (300 MHz, CDCl3): δ 1.4 & 1.46(6H, 2t, J = 7.2 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –COOCH2CH3), 4.36 & 4.49 (4H, 2q, J = 7.2 Hz,
CHCOOCH2CH3 & –COOCH2CH3), 4.36 & 4.49 (4H, 2q, J = 7.2 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –COOCH2CH3), 6.96(1H, s,
CHCOOCH2CH3 & –COOCH2CH3), 6.96(1H, s, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO–), 7.58(1H, dd, J = 1.8, 2.1 Hz, C-6H), 7.88(1H, d, J = 8.7 Hz, C-4H), 8.88(1H, d, J = 2.1 Hz, C-7H); 13C NMR (75.5 MHz, CDCl3): δ 14.1 & 14.2 (
CHCO–), 7.58(1H, dd, J = 1.8, 2.1 Hz, C-6H), 7.88(1H, d, J = 8.7 Hz, C-4H), 8.88(1H, d, J = 2.1 Hz, C-7H); 13C NMR (75.5 MHz, CDCl3): δ 14.1 & 14.2 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –COOCH2CH3), 61.7 & 63.8 (
CHCOOCH2CH3 & –COOCH2CH3), 61.7 & 63.8 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –COOCH2CH3), 116.5 (C-7), 118.0 (C-5), 121.7 (C-4), 125.0 (
CHCOOCH2CH3 & –COOCH2CH3), 116.5 (C-7), 118.0 (C-5), 121.7 (C-4), 125.0 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 131.0 (C-6), 134.9 (C-3), 135.4 (C-8), 140.3 (C-9), 150.3 (NCOOC2H5), 164.8 (C-2) and 164.9 (
CH–), 131.0 (C-6), 134.9 (C-3), 135.4 (C-8), 140.3 (C-9), 150.3 (NCOOC2H5), 164.8 (C-2) and 164.9 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOC2H5). HRMS m/z Calcd for C15H15BrNO5 [M + H]+: 368.0128. Found: 368.0119.
CHCOOC2H5). HRMS m/z Calcd for C15H15BrNO5 [M + H]+: 368.0128. Found: 368.0119.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether–ethyl acetate); νmax(KBr)/cm−1: 3423, 2959, 1740, 1713, 1652, 1611, 1473, 1345, 1227, 1199, 1016, 755; 1H NMR (300 MHz, CDCl3): δ 1.37 (3H, t, J = 7.2 Hz,
1 petroleum ether–ethyl acetate); νmax(KBr)/cm−1: 3423, 2959, 1740, 1713, 1652, 1611, 1473, 1345, 1227, 1199, 1016, 755; 1H NMR (300 MHz, CDCl3): δ 1.37 (3H, t, J = 7.2 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 3.75 (3H, s, –CH2COOCH3), 4.33 (2H, q, J = 7.2 Hz,
CHCOOCH2CH3), 3.75 (3H, s, –CH2COOCH3), 4.33 (2H, q, J = 7.2 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 4.50 (2H, s, –CH2COOCH3), 6.69 (1H, d, J = 7.8 Hz, C-7H), 6.94 (1H, s,
CHCOOCH2CH3), 4.50 (2H, s, –CH2COOCH3), 6.69 (1H, d, J = 7.8 Hz, C-7H), 6.94 (1H, s, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO–), 7.09 (1H, t, J = 7.8 Hz, C-5H), 7.35 (1H, t, J = 7.8 Hz, C-6H), 8.60 (1H, d, J = 7.8 Hz, C-4H); 13C NMR (75.5 MHz, CDCl3): δ 14.1 (
CHCO–), 7.09 (1H, t, J = 7.8 Hz, C-5H), 7.35 (1H, t, J = 7.8 Hz, C-6H), 8.60 (1H, d, J = 7.8 Hz, C-4H); 13C NMR (75.5 MHz, CDCl3): δ 14.1 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 41.2 (–CH2COOCH3), 52.6 (–CH2COOCH3), 61.2 (
CHCOOCH2CH3), 41.2 (–CH2COOCH3), 52.6 (–CH2COOCH3), 61.2 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 108.1 (C-7), 119.8 (C-6), 123.0 (C-4), 123.2 (
CHCOOCH2CH3), 108.1 (C-7), 119.8 (C-6), 123.0 (C-4), 123.2 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 128.9 (C-5), 132.4 (C-3), 137.1 (C-8), 144.5 (C-9), 165.4 (–CH2COOCH3), 167.53(C-2) and 167.8(
CH–), 128.9 (C-5), 132.4 (C-3), 137.1 (C-8), 144.5 (C-9), 165.4 (–CH2COOCH3), 167.53(C-2) and 167.8(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOC2H5). HRMS m/z Calcd for C15H15NO5Na [M + Na]+: 312.0842. Found: 312.0838.
CHCOOC2H5). HRMS m/z Calcd for C15H15NO5Na [M + Na]+: 312.0842. Found: 312.0838.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether–ethyl acetate); νmax(KBr)/cm−1: 3423, 2925, 1766, 1749, 1709, 1652, 1617, 1491, 1351, 1204, 821; 1H NMR (300 MHz, CDCl3): δ 1.38 (3H, t, J = 7.2 Hz,
1 petroleum ether–ethyl acetate); νmax(KBr)/cm−1: 3423, 2925, 1766, 1749, 1709, 1652, 1617, 1491, 1351, 1204, 821; 1H NMR (300 MHz, CDCl3): δ 1.38 (3H, t, J = 7.2 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 3.76 (3H, s, –CH2COOCH3), 4.34 (2H, q, J = 6.8 Hz,
CHCOOCH2CH3), 3.76 (3H, s, –CH2COOCH3), 4.34 (2H, q, J = 6.8 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 4.49 (2H, s, –CH2COOCH3), 6.60–6.64 (1H, m, C-7H), 6.97 (1H, s,
CHCOOCH2CH3), 4.49 (2H, s, –CH2COOCH3), 6.60–6.64 (1H, m, C-7H), 6.97 (1H, s, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO–), 7.04–7.10 (1H, m, C-6H), 8.40–8.44 (1H, m, C-4H); 13C NMR (75.5 MHz, CDCl3): δ 14.1 (
CHCO–), 7.04–7.10 (1H, m, C-6H), 8.40–8.44 (1H, m, C-4H); 13C NMR (75.5 MHz, CDCl3): δ 14.1 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 41.3 (–CH2COOCH3), 52.7 (–CH2COOCH3), 61.4 (
CHCOOCH2CH3), 41.3 (–CH2COOCH3), 52.7 (–CH2COOCH3), 61.4 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 108.5 (d, J = 7.5 Hz, C-4), 116.6 (d, J = 27.1 Hz, C-6), 118.7 (d, J = 24.1 Hz, C-7), 120.8 (d, J = 9.8 Hz, C-3), 124.4 (
CHCOOCH2CH3), 108.5 (d, J = 7.5 Hz, C-4), 116.6 (d, J = 27.1 Hz, C-6), 118.7 (d, J = 24.1 Hz, C-7), 120.8 (d, J = 9.8 Hz, C-3), 124.4 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 136.8 (d, J = 3.0 Hz, C-8), 140.6 (C-9), 159.1 (d, J = 240.8 Hz, C-5), 165.2 (–CH2COOCH3), 167.2 (C-2) and 167.6 (
CH–), 136.8 (d, J = 3.0 Hz, C-8), 140.6 (C-9), 159.1 (d, J = 240.8 Hz, C-5), 165.2 (–CH2COOCH3), 167.2 (C-2) and 167.6 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOC2H5). HRMS m/z Calcd for C15H14FNO5Na [M + Na]+: 330.0748. Found: 330.0741.
CHCOOC2H5). HRMS m/z Calcd for C15H14FNO5Na [M + Na]+: 330.0748. Found: 330.0741.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether–ethyl acetate); νmax(KBr)/cm−1: 3427, 2987, 1755, 1713, 1651, 1607, 1477, 1347, 1200, 1025, 826; 1H NMR (300 MHz, CDCl3): δ 1.38 (3H, t, J = 7.2 Hz,
1 petroleum ether–ethyl acetate); νmax(KBr)/cm−1: 3427, 2987, 1755, 1713, 1651, 1607, 1477, 1347, 1200, 1025, 826; 1H NMR (300 MHz, CDCl3): δ 1.38 (3H, t, J = 7.2 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 3.76 (3H, s, –CH2COOCH3), 4.35 (2H, q, J = 7.2 Hz,
CHCOOCH2CH3), 3.76 (3H, s, –CH2COOCH3), 4.35 (2H, q, J = 7.2 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 4.49 (2H, s, –CH2COOCH3), 6.63 (1H, d, J = 8.4 Hz, C-7H), 6.97 (1H, s,
CHCOOCH2CH3), 4.49 (2H, s, –CH2COOCH3), 6.63 (1H, d, J = 8.4 Hz, C-7H), 6.97 (1H, s, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO–), 7.31–7.35 (1H, m, C-6H), 8.63 (1H, d, J = 2.1 Hz, C-4H); 13C NMR (75.5 MHz, CDCl3): δ 14.1 (
CHCO–), 7.31–7.35 (1H, m, C-6H), 8.63 (1H, d, J = 2.1 Hz, C-4H); 13C NMR (75.5 MHz, CDCl3): δ 14.1 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 41.2 (–CH2COOCH3), 52.7 (–CH2COOCH3), 61.5 (
CHCOOCH2CH3), 41.2 (–CH2COOCH3), 52.7 (–CH2COOCH3), 61.5 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 109.0 (C-7), 121.0 (C-5), 124.5 (
CHCOOCH2CH3), 109.0 (C-7), 121.0 (C-5), 124.5 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 128.6 (C-3), 129.0 (C-4), 132.0 (C-6), 136.2 (C-8), 142.9 (C-9), 165.1 (–CH2COOCH3), 167.0 (C-2) and 167.5 (
CH–), 128.6 (C-3), 129.0 (C-4), 132.0 (C-6), 136.2 (C-8), 142.9 (C-9), 165.1 (–CH2COOCH3), 167.0 (C-2) and 167.5 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOC2H5). HRMS m/z Calcd for C15H14ClNO5Na [M + Na]+: 346.0453. Found: 346.0451.
CHCOOC2H5). HRMS m/z Calcd for C15H14ClNO5Na [M + Na]+: 346.0453. Found: 346.0451.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether–ethyl acetate); νmax(KBr)/cm−1: 3426, 2925, 1754, 1712, 1650, 1603, 1438, 1345, 1199, 1025, 823; 1H NMR (300 MHz, CDCl3): δ 1.39 (3H, t, J = 7.2 Hz,
1 petroleum ether–ethyl acetate); νmax(KBr)/cm−1: 3426, 2925, 1754, 1712, 1650, 1603, 1438, 1345, 1199, 1025, 823; 1H NMR (300 MHz, CDCl3): δ 1.39 (3H, t, J = 7.2 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 3.76 (3H, s, –CH2COOCH3), 4.35 (2H, q, J = 6.9 Hz,
CHCOOCH2CH3), 3.76 (3H, s, –CH2COOCH3), 4.35 (2H, q, J = 6.9 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 4.49 (2H, s, –CH2COOCH3), 6.58 (1H, d, J = 8.4 Hz, C-7H), 6.97 (1H, s,
CHCOOCH2CH3), 4.49 (2H, s, –CH2COOCH3), 6.58 (1H, d, J = 8.4 Hz, C-7H), 6.97 (1H, s, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO–), 7.47–7.50 (1H, m, C-6H), 8.78 (1H, s, C-4H); 13C NMR (75.5 MHz, CDCl3): δ 14.1 (
CHCO–), 7.47–7.50 (1H, m, C-6H), 8.78 (1H, s, C-4H); 13C NMR (75.5 MHz, CDCl3): δ 14.1 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 41.2 (–CH2COOCH3), 52.7 (–CH2COOCH3), 61.5 (
CHCOOCH2CH3), 41.2 (–CH2COOCH3), 52.7 (–CH2COOCH3), 61.5 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3), 109.5 (C-7), 115.9 (C-5), 121.4 (C-4), 124.6 (
CHCOOCH2CH3), 109.5 (C-7), 115.9 (C-5), 121.4 (C-4), 124.6 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 131.8 (C-6), 134.9 (C-3), 136.1 (C-8), 143.4 (C-9), 165.1 (–CH2COOCH3), 166.9 (C-2) and 167.5 (
CH–), 131.8 (C-6), 134.9 (C-3), 136.1 (C-8), 143.4 (C-9), 165.1 (–CH2COOCH3), 166.9 (C-2) and 167.5 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOC2H5). HRMS m/z Calcd for C15H15BrNO5 [M + H]+: 368.0128. Found: 368.0118.
CHCOOC2H5). HRMS m/z Calcd for C15H15BrNO5 [M + H]+: 368.0128. Found: 368.0118.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether–ethyl acetate); νmax(KBr)/cm−1: 3423, 2929, 1735, 1711, 1649, 1606, 1474, 1344, 1222, 1025, 755; 1H NMR (300 MHz, CDCl3): δ 1.26 & 1.37 (6H, 2t, J = 7.2 Hz,
1 petroleum ether–ethyl acetate); νmax(KBr)/cm−1: 3423, 2929, 1735, 1711, 1649, 1606, 1474, 1344, 1222, 1025, 755; 1H NMR (300 MHz, CDCl3): δ 1.26 & 1.37 (6H, 2t, J = 7.2 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –CH2COOCH2CH3), 4.21 & 4.33 (4H, 2q, J = 7.2 Hz,
CHCOOCH2CH3 & –CH2COOCH2CH3), 4.21 & 4.33 (4H, 2q, J = 7.2 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –CH2COOCH2CH3), 4.48 (2H, s, –CH2COOCH2CH3), 6.69 (1H, d, J = 7.8 Hz, C-7H), 6.94 (1H, s,
CHCOOCH2CH3 & –CH2COOCH2CH3), 4.48 (2H, s, –CH2COOCH2CH3), 6.69 (1H, d, J = 7.8 Hz, C-7H), 6.94 (1H, s, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO–), 7.08 (1H, t, J = 7.5 Hz, C-5H), 7.35 (1H, t, J = 7.5 Hz, C-6), 8.59 (1H, d, J = 7.8 Hz, C-4H); 13C NMR (75.5 MHz, CDCl3): δ 14.0 & 14.1 (
CHCO–), 7.08 (1H, t, J = 7.5 Hz, C-5H), 7.35 (1H, t, J = 7.5 Hz, C-6), 8.59 (1H, d, J = 7.8 Hz, C-4H); 13C NMR (75.5 MHz, CDCl3): δ 14.0 & 14.1 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –CH2COOCH2CH3), 41.4 (–CH2COOCH2CH3), 61.2 & 61.8 (
CHCOOCH2CH3 & –CH2COOCH2CH3), 41.4 (–CH2COOCH2CH3), 61.2 & 61.8 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –CH2COOCH2CH3), 108.1 (C-7), 119.8 (C-6), 123.0 (C-4), 123.1 (
CHCOOCH2CH3 & –CH2COOCH2CH3), 108.1 (C-7), 119.8 (C-6), 123.0 (C-4), 123.1 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 128.9 (C-5), 132.3 (C-3), 137.2 (C-8), 144.6 (C-9), 165.5 (–CH2COOC2H5), 167.2 (C-2) and 167.5 (
CH–), 128.9 (C-5), 132.3 (C-3), 137.2 (C-8), 144.6 (C-9), 165.5 (–CH2COOC2H5), 167.2 (C-2) and 167.5 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOC2H5). HRMS m/z Calcd for C16H17NO5Na [M + Na]+: 326.0999. Found: 326.0985.
CHCOOC2H5). HRMS m/z Calcd for C16H17NO5Na [M + Na]+: 326.0999. Found: 326.0985.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether–ethyl acetate); νmax(KBr)/cm−1: 3423, 2925, 1741, 1709, 1654, 1617, 1492, 1372, 1351, 1217, 1025, 777; 1H NMR (300 MHz, CDCl3): δ 1.26 & 1.38 (6H, 2t, J = 7.2 Hz,
1 petroleum ether–ethyl acetate); νmax(KBr)/cm−1: 3423, 2925, 1741, 1709, 1654, 1617, 1492, 1372, 1351, 1217, 1025, 777; 1H NMR (300 MHz, CDCl3): δ 1.26 & 1.38 (6H, 2t, J = 7.2 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –CH2COOCH2CH3), 4.22 & 4.34 (4H, 2q, J = 7.2 Hz,
CHCOOCH2CH3 & –CH2COOCH2CH3), 4.22 & 4.34 (4H, 2q, J = 7.2 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –CH2COOCH2CH3), 4.47 (2H, s, –CH2COOCH2CH3), 6.60–6.64 (1H, m, C-7H), 6.97 (1H, s,
CHCOOCH2CH3 & –CH2COOCH2CH3), 4.47 (2H, s, –CH2COOCH2CH3), 6.60–6.64 (1H, m, C-7H), 6.97 (1H, s, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO–), 7.04–7.10 (1H, m, C-6H), 8.40–8.43 (1H, m, C-4H); 13C NMR (75.5 MHz, CDCl3): δ 14.0 & 14.1 (
CHCO–), 7.04–7.10 (1H, m, C-6H), 8.40–8.43 (1H, m, C-4H); 13C NMR (75.5 MHz, CDCl3): δ 14.0 & 14.1 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –CH2COOCH2CH3), 41.4 (–CH2COOCH2CH3), 61.4 & 61.9 (
CHCOOCH2CH3 & –CH2COOCH2CH3), 41.4 (–CH2COOCH2CH3), 61.4 & 61.9 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –CH2COOCH2CH3), 108.6 (d, J = 8.3 Hz, C-4), 116.6 (d, J = 36.9 Hz, C-6), 118.7 (d, J = 24.9 Hz, C-7), 120.7 (d, J = 7.1 Hz, C-3), 124.3 (
CHCOOCH2CH3 & –CH2COOCH2CH3), 108.6 (d, J = 8.3 Hz, C-4), 116.6 (d, J = 36.9 Hz, C-6), 118.7 (d, J = 24.9 Hz, C-7), 120.7 (d, J = 7.1 Hz, C-3), 124.3 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 136.8 (d, J = 2.2 Hz, C-8), 140.7 (C-9), 159.0 (d, J = 240.0 Hz, C-5), 165.2 (–CH2COOC2H5), 167.1 (C-2) and 167.3 (
CH–), 136.8 (d, J = 2.2 Hz, C-8), 140.7 (C-9), 159.0 (d, J = 240.0 Hz, C-5), 165.2 (–CH2COOC2H5), 167.1 (C-2) and 167.3 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOC2H5). HRMS m/z Calcd for C16H16FNO5Na [M + Na]+: 344.0905. Found: 344.0897.
CHCOOC2H5). HRMS m/z Calcd for C16H16FNO5Na [M + Na]+: 344.0905. Found: 344.0897.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether–ethyl acetate); νmax(KBr)/cm−1: 3424, 2928, 1741, 1709, 1652, 1610, 1443, 1369, 1202, 1020, 817; 1H NMR (300 MHz, CDCl3): δ 1.27 & 1.39 (6H, 2t, J = 6.9, 7.2 Hz,
1 petroleum ether–ethyl acetate); νmax(KBr)/cm−1: 3424, 2928, 1741, 1709, 1652, 1610, 1443, 1369, 1202, 1020, 817; 1H NMR (300 MHz, CDCl3): δ 1.27 & 1.39 (6H, 2t, J = 6.9, 7.2 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –CH2COOCH2CH3), 4.23 & 4.36 (4H, 2q, J = 6.9 Hz,
CHCOOCH2CH3 & –CH2COOCH2CH3), 4.23 & 4.36 (4H, 2q, J = 6.9 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –CH2COOCH2CH3), 4.48 (2H, s, –CH2COOCH2CH3), 6.64 (1H, d, J = 8.4 Hz, C-7H), 6.98 (1H, s,
CHCOOCH2CH3 & –CH2COOCH2CH3), 4.48 (2H, s, –CH2COOCH2CH3), 6.64 (1H, d, J = 8.4 Hz, C-7H), 6.98 (1H, s, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO–), 7.32–7.36 (1H, m, C-6H), 8.65 (1H, d, J = 1.8 Hz, C-4H); 13C NMR (75.5 MHz, CDCl3): δ 14.0 & 14.1 (
CHCO–), 7.32–7.36 (1H, m, C-6H), 8.65 (1H, d, J = 1.8 Hz, C-4H); 13C NMR (75.5 MHz, CDCl3): δ 14.0 & 14.1 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –CH2COOCH2CH3), 41.4 (–CH2COOCH2CH3), 61.5 & 61.9 (
CHCOOCH2CH3 & –CH2COOCH2CH3), 41.4 (–CH2COOCH2CH3), 61.5 & 61.9 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –CH2COOCH2CH3), 109.1 (C-7), 121.0 (C-5), 124.5 (
CHCOOCH2CH3 & –CH2COOCH2CH3), 109.1 (C-7), 121.0 (C-5), 124.5 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 128.6 (C-3), 129.0 (C-4), 132.0 (C-6), 136.3 (C-8), 143.0 (C-9), 165.1 (–CH2COOC2H5), 167.0 (C-2) and 167.1 (
CH–), 128.6 (C-3), 129.0 (C-4), 132.0 (C-6), 136.3 (C-8), 143.0 (C-9), 165.1 (–CH2COOC2H5), 167.0 (C-2) and 167.1 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOC2H5). HRMS m/z Calcd for C16H17ClNO5 [M + H]+: 338.0790. Found: 338.0782.
CHCOOC2H5). HRMS m/z Calcd for C16H17ClNO5 [M + H]+: 338.0790. Found: 338.0782.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum ether–ethyl acetate); νmax(KBr)/cm−1: 3423, 2926, 1741, 1709, 1607, 1437, 1371, 1202, 1123, 1025, 816; 1H NMR (300 MHz, CDCl3): δ 1.26 & 1.38 (6H, 2t, J = 7.2 Hz,
1 petroleum ether–ethyl acetate); νmax(KBr)/cm−1: 3423, 2926, 1741, 1709, 1607, 1437, 1371, 1202, 1123, 1025, 816; 1H NMR (300 MHz, CDCl3): δ 1.26 & 1.38 (6H, 2t, J = 7.2 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –CH2COOCH2CH3), 4.19 & 4.35 (4H, 2q, J = 6.9, 7.5 Hz,
CHCOOCH2CH3 & –CH2COOCH2CH3), 4.19 & 4.35 (4H, 2q, J = 6.9, 7.5 Hz, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –CH2COOCH2CH3), 4.47 (2H, s, –CH2COOCH2CH3), 6.58 (1H, d, J = 8.1 Hz, C-7H), 6.96 (1H, s,
CHCOOCH2CH3 & –CH2COOCH2CH3), 4.47 (2H, s, –CH2COOCH2CH3), 6.58 (1H, d, J = 8.1 Hz, C-7H), 6.96 (1H, s, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO–), 7.46–7.49 (1H, m, C-6H), 8.77 (1H, d, J = 1.5 Hz, C-4H); 13C NMR (75.5 MHz, CDCl3): δ 14.0 & 14.1 (
CHCO–), 7.46–7.49 (1H, m, C-6H), 8.77 (1H, d, J = 1.5 Hz, C-4H); 13C NMR (75.5 MHz, CDCl3): δ 14.0 & 14.1 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –CH2COOCH2CH3), 41.4 (–CH2COOCH2CH3), 61.5 & 61.9 (
CHCOOCH2CH3 & –CH2COOCH2CH3), 41.4 (–CH2COOCH2CH3), 61.5 & 61.9 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOCH2CH3 & –CH2COOCH2CH3), 109.6 (C-7), 115.8 (C-5), 121.4 (C-4), 124.5 (
CHCOOCH2CH3 & –CH2COOCH2CH3), 109.6 (C-7), 115.8 (C-5), 121.4 (C-4), 124.5 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 131.7 (C-6), 134.8 (C-3), 136.1 (C-8), 143.5 (C-9), 165.1 (–CH2COOC2H5) and 166.9 (C-2 and
CH–), 131.7 (C-6), 134.8 (C-3), 136.1 (C-8), 143.5 (C-9), 165.1 (–CH2COOC2H5) and 166.9 (C-2 and ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOC2H5). HRMS m/z Calcd for C16H17BrNO5 [M + H]+: 382.0285. Found: 382.0286.
CHCOOC2H5). HRMS m/z Calcd for C16H17BrNO5 [M + H]+: 382.0285. Found: 382.0286.
