Tacrine-mefenamic acid hybrids for inhibition of acetylcholinesterase†
Joshua J.
Bornstein
c,
Todd J.
Eckroat
ac,
Jacob L.
Houghton
ac,
Christopher K.
Jones
bc,
Keith D.
Green
c and
Sylvie
Garneau-Tsodikova
*abc
aDepartment of Medicinal Chemistry in the College of Pharmacy, University of Michigan, Ann Arbor, MI 48109-2216, USA. E-mail: sylviegt@umich.edu; Fax: +734-615-5521; Tel: +734-615-2736
bChemical Biology Doctoral Program, University of Michigan, Ann Arbor, MI 48109-2216, USA
cLife Sciences Institute, University of Michigan, Ann Arbor, MI 48109-2216, USA
First published on 9th March 2011
Abstract
Alzheimer's disease (AD) is a complex syndrome characterized by the degeneration of the brain and central nervous system that may be caused by an assortment of genetic and environmental factors. Consequently, a conjunctive approach targeting multiple affecters of AD could lead to improved drug candidates for the treatment of AD. A convergent chemical synthetic approach yielded a series of tacrine-mefenamic acid hybrids that were evaluated for their ability to inhibit acetylcholinesterase (AChE). A majority of the compounds tested showed low nanomolar IC50 values, an improvement over the parent compound, tacrine, suggesting that they could be effective in increasing cholinergic function. Additionally, an assay to evaluate the compounds upon exposure to reactive oxygen species was performed, the results of which may suggest a role for the mefenamic acid moiety in the inhibition of AChE. Molecular modeling studies were performed to rationalize the experimental results.
Introduction
Alzheimer's disease (AD) is a progressive neurodegenerative disorder characterized by the atrophy of cholinergic neurons in areas of the brain vital for cognitive function, leading to symptoms that range from memory loss to the loss of motor abilities and eventually death. Pathologies associated with AD include the degeneration of brain cells due to the development of plaques and tangles associated with the aggregation of the protein fragment amyloid-β (Aβ) and the irregular phosphorylation of the Tau protein, respectively. Additionally, concurrent deficits in excitatory amino acid (EAA) transmission via acetylcholine (ACh) are observed due to substantial deficits in choline acetyltransferase (ChAT),1–3 the enzyme responsible for the formation of ACh in the brain.One of the primary pharmacological strategies employed in the treatment of AD has been inhibition of cholinesterases (ChEs). These therapies aim to decrease the rate of decomposition of ACh at synapses in the brain thereby raising the potential for increased levels of EAA transmission and improved cognitive function. This strategy has proved moderately successful, yielding potent reversible acetylcholinesterase inhibitors (AChEi) such as tacrine (1) (Fig. 1), donepezil, and galantamine, as well as substrate mimics such as neostigmine and pyridostigmine, all of which have shown benefit in the management of AD symptoms to varying degrees. However, despite the moderate clinical successes that have been observed, it has been suggested that these treatments, highlighted by clinical studies of donepezil, are not cost-effective and that an alternative approach may provide better outcomes.4,5 Nonetheless, interest in developing improved ChE inhibitors for treatment of AD has increased recently, likely due to the body of empirical evidence showing the benefits of ChE inhibitors in AD patients as well as the lack of successful alternative approaches.
 | ||
| Fig. 1 Structures of tacrine (1) and mefenamic acid (2). | ||
Other factors responsible for the onset and progression of AD have been identified, including inflammatory responses and the resultant increased oxidative stress in the form of free-radicals. The increase in oxidative stress related to the natural decline in an aging body's defense mechanisms often precedes the onset of the previously mentioned indications associated with AD. There is substantial epidemiological evidence linking the development of AD to inflammatory processes in the brain6 as well as evidence that treatment with non-steroidal anti-inflammatory drugs (NSAIDs) may improve cognition and delay the progression of AD.7–10 Given this information, it is evident that treatment of only a single determinant of the pathology of AD is not an effective approach. Furthermore, the evidence implies that treatment of multiple determinants of the disease, especially those related to oxidative stress, may provide synergistic effects.
One solution to this type of problem that has recently emerged is a conjunctive approach in which two biologically active molecules with similar or dissimilar mechanisms of action are combined into a single molecule to improve potency and/or exhibit multiple modes of action, resulting in a synergistic effect. Tacrine-based dimers and hybrids with improved pharmacological properties have been the target of a number of discovery efforts in the past decade, leading in several cases to compounds with desirable synergy and improved potency.11–15
Based on the potential of reported tacrine-based molecules, we decided to take a similar approach in identifying compounds with the potential to serve as multi-functioning therapeutics. The ability of NSAIDS such as mefenamic acid (2) to inactivate enzymes, including AChE, in the presence of peroxidases and their potential for AD treatment has been well-characterized in the literature (Fig. 1).9,16–18 Studies have shown that mefenamic acid is capable of decreasing the occurence of free-radicals and attenuating Aβ peptide-induced neurotoxicity while improving cognitive impairments.10 Additionally, it has been suggested that AChE may accelerate the formation of stable amyloid fibrils and stable Aβ complexes.19 This role is attributed to the peripheral anionic site (PAS) as propidium iodide, a PAS binding molecule, has proved effective in reducing Aβ aggregation while there are no similar reports implicating CAS inhibitors.20 The numerous desirable properties of mefenamic acid led us to believe that it would be an ideal scaffold to incorporate into a series of tacrine-based hybrid molecules aimed at both the CAS and PAS, taking advantage of tacrine's affinity for the CAS and using it to guide the mefenamic acid portion of the molecules to the PAS. This type of strategy has been widely discussed in the literature and was recently reviewed.21
Herein, we report the synthesis and evaluation of a series of tacrine- and 6-chlorotacrine-mefenamic acid hybrid molecules aimed at combining the AChE inhibitor properties of tacrine with the antioxidant and AChE modulating properties of mefenamic acid into a single, dual-action molecule for the treatment of AD. This conjunctive medicinal chemistry approach led to the identification of nanomolar and sub-nanomolar inhibitors of AChE.
Results and discussion
Synthesis
Scheme 1 illustrates the convergent synthetic approach to the synthesis of tacrine-based bifunctional inhibitors of AChE comprised of mefenamic acid (2) connected to tacrine (1) or 6-chlorotacrine via a hydrophobic linker moiety which in some cases (Series B) contains an amide functionality. This approach allows for the fine tuning of the linker region in order to assess the optimal spacing between the two active molecules. The starting compounds 9-chlorotacrine (3) and 6,9-dichlorotacrine (4) were synthesized as previously reported (Scheme 1A).22 The tacrine-containing portion of the bifunctional molecules (Series A and B) was prepared by an established method.23 Nucleophilic aromatic substitution of various diamines at the 9-Cl position of tacrine derivatives 3 and 4 gave 5a–h and 6a–h in yields from 18–83% (Scheme 1A). The mefenamic acid-containing portion of the bifunctional molecules of Series B was prepared by coupling methyl esters 7a–d to 2 to yield 8a–d in low to moderate yield. The subsequent hydrolysis of esters 8a–d yielded compounds 9a–d quantitatively (Scheme 1B). Compound 7a was commercially available whereas 7b–d were prepared using stoichiometric hydrochloric acid in methanol followed by evaporation of the solvents to yield the HCl salts in quantitative yield. The coupling of 5a–h and 6a–h with mefenamic acid or with 9a–d gave compounds 10a–f and 11a–f (Series A) as well as compounds 12a–t and 13a–t (Series B), respectively (Scheme 1C). After purification by chromatographic techniques, the final bifunctional inhibitors were obtained in varying yields, 10–94%.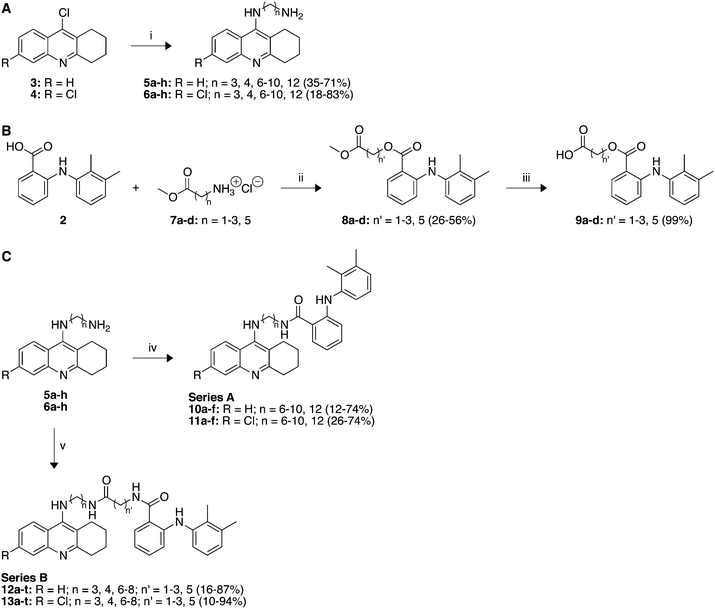 | ||
| Scheme 1 Synthetic schemes for the preparation of A tacrine-containing portion of bifunctional molecules, B mefenamic acid-containing portion of Series B of bifunctional compounds, and C Series A and B of tacrine and chlorotacrine-mefenamic acid bifunctional molecules. Reagents and conditions: (i) H2N(CH2)nNH2 (3 eq), 1-pentanol, Δ. (ii) DCC (3 eq), HOBt (3 eq), DIPEA (7 eq), DMF. (iii) 1 M NaOH (H2O), THF. (iv) 2, EDAC·HCl (1.1 eq), HOBt (1.1 eq), Et3N (1.8 eq), DMF. (v) 9a–d, EDAC·HCl (1.1 eq), HOBt (1.1 eq), Et3N (1.8 eq), DMF. | ||
Acetylcholinesterase (AChE) inhibition
In order to evaluate the potential of the bifunctional compounds as therapeutic agents for the symptoms of AD, their IC50 values were determined via two biochemical assays (Table 1 and Fig. 2). The first assay, which employed the method of Ellman,24 was used to determine the inhibitory potential of each molecule towards AChE from Torpedo californica (TcAChE). All compounds of interest were incubated in the presence of the enzyme for 10 min before initiation of the enzymatic reaction with acetylcholine, allowing for binding of the potential inhibitors. Additionally, a ROS inhibition assay was performed according to the method of Muraoka and Miura to assess the potential of ROS-induced inactivation of AChE.16 The compounds of interest were incubated in the presence of horseradish peroxidase and hydrogen peroxide prior to incubation with AChE in order to generate radical species. It is thought that mefenamic acid radical species generated by peroxidases that are triggered during inflammatory responses are capable of effectively inactivating AChE.16 We hypothesized that by inhibiting the enzyme with tacrine while simultaneously using the tacrine moiety to direct the mefenamic acid radical to the vicinity of the AChE active site, we may see a synergistic effect evidenced by a decrease in the IC50 value relative to that obtained without ROS. In fact, this trend was observed for most of the tested compounds – all ROS IC50 values were within one order of magnitude or showed improvement relative to their performance using the Ellman method. Interestingly, this trend is opposite of that seen with the parent compound, tacrine, suggesting the mefenamic acid moiety is contributing to the increased potency of the molecules.| Compda | Series | R | n | n′ | IC50 (nM)b | ROS IC50 (nM) |
|---|---|---|---|---|---|---|
| a See Scheme 1 for chemical structures. b AChE from eel was used. | ||||||
| 1 | 52.4 ± 7.3 | 183 ± 21 | ||||
| 2 | > 1.25 mM | 6120 ± 680 | ||||
1 and 2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mix) 1 mix) |
68.7 ± 3.8 | 83.9 ± 0.4 | ||||
| 3 | > 25 μM | |||||
| 4 | > 50 μM | |||||
| Neostigmine | 4.6 ± 1.0 | |||||
| Pyridostigmine | 82.0 ± 1.5 | |||||
| 10a | A | H | 6 | 2.24 ± 0.11 | 0.129 ± 0.030 | |
| 10b | A | H | 7 | 1.39 ± 0.21 | 25.2 ± 4.5 | |
| 10c | A | H | 8 | 75.5 ± 19.2 | 0.045 ± 0.015 | |
| 10d | A | H | 9 | 1.54 ± 0.17 | 15.1 ± 0.2 | |
| 10e | A | H | 10 | 385 ± 48 | 0.908 ± 0.267 | |
| 10f | A | H | 12 | 50.9 ± 1.4 | 6.94 ± 1.42 | |
| 11a | A | Cl | 6 | 7230 ± 187 | 1.02 ± 0.36 | |
| 11b | A | Cl | 7 | 1380 ± 340 | 29.8 ± 4.0 | |
| 11c | A | Cl | 8 | 0.495 ± 0.064 | 1.49 ± 0.30 | |
| 11d | A | Cl | 9 | 6.94 ± 0.66 | 6.72 ± 0.78 | |
| 11e | A | Cl | 10 | 0.776 ± 0.108 | 1.85 ± 0.11 | |
| 11f | A | Cl | 12 | 2360 ± 830 | 16.2 ± 1.8 | |
| 12a | B | H | 3 | 1 | 87.3 ± 33.6 | 47.5 ± 11.5 |
| 12b | B | H | 3 | 2 | 3800 ± 280 | 7.92 ± 1.96 |
| 12c | B | H | 3 | 3 | 3730 ± 253 | 23.0 ± 9.0 |
| 12d | B | H | 3 | 5 | 811 ± 70 | 20.2 ± 6.5 |
| 12e | B | H | 4 | 1 | 262 ± 68 | 53.6 ± 3.8 |
| 12f | B | H | 4 | 2 | 426 ± 86 | 2.43 ± 0.36 |
| 12g | B | H | 4 | 3 | 89.7 ± 11.3 | 14.6 ± 2.4 |
| 12h | B | H | 4 | 5 | 1860 ± 290 | 34.0 ± 9.0 |
| 12i | B | H | 6 | 1 | 18.3 ± 4.0 | 54.8 ± 4.7 |
| 12j | B | H | 6 | 2 | 985 ± 95 | 40.2 ± 8.4 |
| 12k | B | H | 6 | 3 | 195 ± 27 | 29.9 ± 11.1 |
| 12l | B | H | 6 | 5 | 1440 ± 197 | 34.0 ± 2.4 |
| 12m | B | H | 7 | 1 | 5.55 ± 1.21 | 15.3 ± 6.6 |
| 12n | B | H | 7 | 2 | 13.9 ± 1.4 | 1.02 ± 0.36 |
| 12o | B | H | 7 | 3 | 8.09 ± 0.91 | 44.6 ± 1.2 |
| 12p | B | H | 7 | 5 | 3.60 ± 0.16 | 3.66 ± 0.24 |
| 12q | B | H | 8 | 1 | 3.30 ± 0.75 | 17.2 ± 4.3 |
| 12r | B | H | 8 | 2 | 8.58 ± 1.42 | 4.07 ± 0.56 |
| 12s | B | H | 8 | 3 | 17.4 ± 6.2 | 14.6 ± 5.2 |
| 12t | B | H | 8 | 5 | 18.7 ± 5.0 | 6.26 ± 1.79 |
| 13a | B | Cl | 3 | 1 | 28.1 ± 5.2 | 3.10 ± 0.94 |
| 13b | B | Cl | 3 | 2 | 20.3 ± 1.4 | 21.3 ± 3.3 |
| 13c | B | Cl | 3 | 3 | 7.13 ± 0.41 | 1.03 ± 0.22 |
| 13d | B | Cl | 3 | 5 | 1.65 ± 0.33 | 7.85 ± 2.45 |
| 13e | B | Cl | 4 | 1 | 156 ± 34 | 89.4 ± 6.2 |
| 13f | B | Cl | 4 | 2 | 2470 ± 98 | 33.0 ± 9.3 |
| 13g | B | Cl | 4 | 3 | 13.6 ± 1.8 | 12.4 ± 1.8 |
| 13h | B | Cl | 4 | 5 | 37.4 ± 9.4 | 0.299 ± 0.067 |
| 13i | B | Cl | 6 | 1 | 7.65 ± 0.24 | 3.46 ± 0.49 |
| 13j | B | Cl | 6 | 2 | 1.14 ± 0.31 | 2.96 ± 0.41 |
| 13k | B | Cl | 6 | 3 | 41.7 ± 11.5 | 15.6 ± 1.5 |
| 13l | B | Cl | 6 | 5 | 2.94 ± 0.45 | 7.39 ± 1.51 |
| 13m | B | Cl | 7 | 1 | 0.418 ± 0.025 | 0.009 ± 0.003 |
| 13n | B | Cl | 7 | 2 | 6.67 ± 1.82 | 10.0 ± 1.6 |
| 13o | B | Cl | 7 | 3 | 7.91 ± 0.69 | 6.55 ± 1.66 |
| 13p | B | Cl | 7 | 5 | 11.1 ± 1.1 | 13.4 ± 3.9 |
| 13q | B | Cl | 8 | 1 | 39.8 ± 3.3 | 18.1 ± 3.7 |
| 13r | B | Cl | 8 | 2 | 18.3 ± 5.5 | 4.97 ± 0.26 |
| 13s | B | Cl | 8 | 3 | 17.0 ± 3.0 | 6.59 ± 0.83 |
| 13t | B | Cl | 8 | 5 | 80.1 ± 16.6 | 8.67 ± 0.73 |
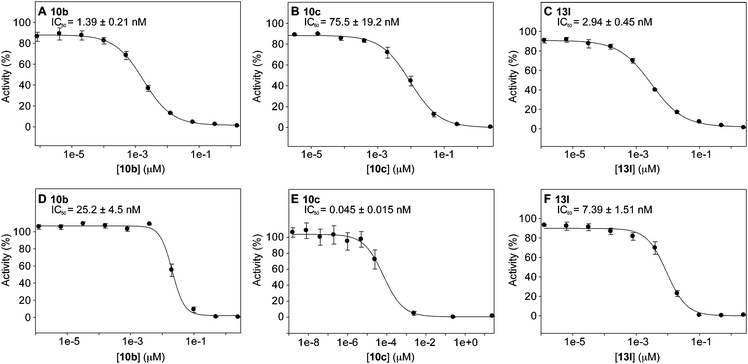 | ||
| Fig. 2 Representative examples of IC50 curves (A–C) and ROS IC50 curves (D–E) for selected compounds from Series A (compounds 10b and 10c) and B (compound 13l). | ||
An additional set of experiments were completed in which the assays were performed with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 equimolar ratio of mefenamic acid and tacrine in order to confirm that linking these two molecules was indeed beneficial. The results reported in Table 1 show that the best inhibitors did in fact outperform a 1
1 equimolar ratio of mefenamic acid and tacrine in order to confirm that linking these two molecules was indeed beneficial. The results reported in Table 1 show that the best inhibitors did in fact outperform a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture, with compound 13m being over 150-fold and 9300-fold more potent in the TcAChE and ROS assays, respectively. These results confirm that linking the two molecules yields more potent inhibitors than concurrent exposure to the two parent compounds.
1 mixture, with compound 13m being over 150-fold and 9300-fold more potent in the TcAChE and ROS assays, respectively. These results confirm that linking the two molecules yields more potent inhibitors than concurrent exposure to the two parent compounds.
Series A showed an overall better performance in the assays, consistently yielding potent compounds, particularly in the ROS assay. All compounds tested were in the low nanomolar range with several molecules that were active at picomolar concentrations, showing drastic improvement over tacrine (AChE IC50 = 52.4 ± 7.3 nM, ROS AChE IC50 = 183 ± 21 nM). The data for Series A indicate that compounds with a 6-chlorotacrine moiety are more potent than their non-chlorinated tacrine counterparts, a trend observed in similar studies with tacrine hybrids.12,15,25 A linker region comprised of 8 to 10 aliphatic carbons between the amine and amide nitrogen atoms of the respective parent molecules was found to be ideal for this series as several of these compounds showed sub-nanomolar IC50 values, the best being compounds 11c and 11e (Table 1).
Close examination of the data for Series B revealed several trends. The 6-chlorotacrine derivatives consistently outperformed their non-halogenated counterparts, similarly to Series A, a trend observed in a number of other studies.12,15,25 Previous studies have also shown that the 6-Cl moiety may cause an increase in selectivity for AChE over butyrylcholinesterase (BChE), thus decreasing the occurrence of unwanted side effects.11,12,15 An optimal length for the amide containing methylene linker between 1 and 2 was determined to be about ten atoms, which was determined by direct comparison after adding the total number of methylene units to the amide atoms (n + n′ + 2 = number of atoms in linker). An optimal length for the methylene linker between tacrine and the amide nitrogen was determined to be n = 7 by a direct comparison of all molecules with a value of n = 3, 4, 5, 6, 7, and 8. Without exception, all compounds with n = 7 showed IC50 values below 50 nM. Following these two trends led to the most potent compound 13m (AChE IC50 = 0.418 ± 0.025 nM, ROS AChE IC50 = 0.009 ± 0.003 nM). This compound exhibits >100-fold increase in potency in the AChE assay and >20,000-fold increase in the AChE ROS assay compared to the parent compound, tacrine (1).
In order to investigate the mode of inhibition of the tacrine-mefenamic acid hybrids, representative compounds from each series A and B were selected and evaluated at constant inhibitor concentration and varying substrate concentrations. Surprisingly, Lineweaver–Burk analysis of the most potent compounds suggested that those inhibitors are non-competitive (Fig. 3). This is an interesting result considering what is known about tacrine's competitive mode of inhibition with regards to the natural acetylcholine substrate. A more likely scenario than the observed non-competitive inhibition, given what is known of tacrine, is that mixed inhibition is observed to some degree but the non-competitive aspect dominates under the given reaction conditions. The inhibition assay indicated that tacrine acted as a competitive inhibitor. It may not be ruled out as a possibility that tacrine is interacting with the CAS, disrupting the enzyme function, and in fact, analysis of a selection of weaker inhibitors (data not shown) indicated mixed inhibition patterns in a Lineweaver–Burk analysis. Studies of similar compounds, in which a class of hydrocarbon-linked tacrine dimers were co-crystallized with TcAChE, suggested that the tacrine moieties may also interact via π–π stacking interactions with the heterocyclic residues of the PAS.26 Given this crystallographic evidence, another plausible scenario in which the reported compounds interact with the PAS but do not form additional contacts with the CAS could explain this non-competitive pattern of inhibition. Interestingly, a recent study of tacrine-ferulic acid hybrids showed a similar non-competitive inhibition profile while many other hybrids' mode of inhibition was not reported, but rather assigned based on docking studies.27 However, the exact mode of interaction may not be strictly defined based on the current study, and will be the target of future research efforts with this interesting set of compounds.
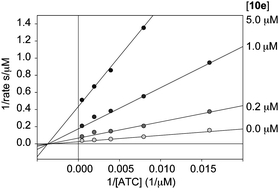 | ||
| Fig. 3 Representative plot showing the non-competitive inhibition with respect to acetylthiocholine (ATC) with compound 10e using four concentrations of inhibitor 10e and four concentrations of ATC. | ||
Molecular modeling
In order to elucidate the trends observed in the inhibition data, molecular modeling experiments were performed with a selection of the most potent molecules. A structure of TcAChE complexed with tacrine (PDB: 1ACJ)28 was used for the modeling studies as the enzyme from the same organism was used in the in vitro biochemical assays presented above, and no human AChE (hAChE) complexed with tacrine is available. This strategy allowed for direct comparison of the modeling results between the hybrid molecules and the parent molecule, tacrine (1), and also gave the best opportunity to find correlation with our assay results. Although the following results remain speculative in the absence of crystallographic data, they do provide a basis for rationalizing the observed trends.Our goal was to visualize the interactions of the three sections of our inhibitors with their suspected regions of interaction in the TcAChE active site: the tacrine moiety, the linker region, and the mefenamic acid moiety with the catalytic active site (CAS), the mid-gorge region, and the PAS, respectively. Modeling was performed using Autodock. The best scoring conformations in terms of energetics were selected after 100 docking iterations focused around the active site (see experimental section for further details). Models of several tacrine-mefenamic acid hybrids were constructed, all of which suggested strong interactions between the energy minimized hybrids and the enzyme complex. A selection of these results are depicted in Fig. 4.
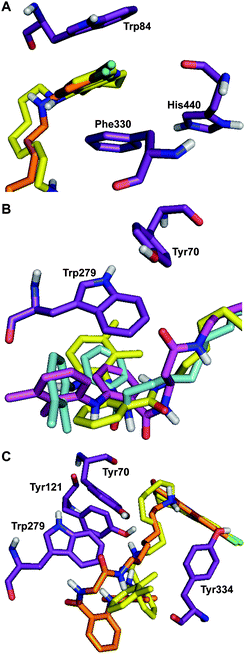 | ||
| Fig. 4 A selection of hybrid molecules docked in TcAChE showing A the interaction of the 6-chlorotacrine moiety of compounds 11c (yellow) and 13m (orange) with Trp84, Phe330, and His440 in the CAS, B the proximity of the mefenamic acid moiety in compounds 10c (yellow), 11e (blue), and 13j (magenta) to Tyr70 and Trp279 of the PAS, and C the proximity of the mefenamic acid moiety of compounds 11c (yellow) and 13m (orange) to Trp279 and Tyr residues (70, 121, and 334) near the PAS. Oxygen, nitrogen, and hydrogen atoms are shown in red, blue, and white, respectively. | ||
The energy-minimized complexes of all molecules modeled predicted that the tacrine portion would be situated in the CAS. In accordance with similar docking studies of tacrine containing molecules, the quinoline ring system is stacked between Trp84 and Phe330 while the nitrogen atom of the ring is positioned such that it indicates the formation of a hydrogen bond with the backbone carbonyl of His440 (Fig. 4A). This is consistent with previous docking studies that used tacrine-based molecules with TcAChE25,29 and suggests that these tacrine-mefenamic acid hybrids could be capable of acting as substrate competitive inhibitors like tacrine (Fig. 4A–C). We suspect that the non-competitive or mixed-mode of inhibition may arise from additional beneficial interactions that are not predicted by these modeling experiments.
The methylene linker portion of both series spans the active-site gorge, allowing the tacrine and mefenamic acid to interact with the CAS and PAS, repsectively. There are approximately 16 Å between the two binding sites from the quinoline ring nitrogen atom30 and a similar distance is observed in the TcAChE structure used in this study. As mentioned, a linker length of approximately 10 atoms showed the strongest inhibition in the biochemical assays. This trend is not perfectly defined across the two series of inhibitors with regards to inhibition data and the same is true of the modeling studies, which fail to further elucidate specific beneficial interactions. Yet, it is quite clear that a linker length of approximately 8–10 atoms, with or without an amide bond, would allow for the mefenamic acid portion to interact with the PAS if the tacrine moiety bound in the CAS.
A great deal of variability in the orientation of the mefenamic acid moiety was observed in the molecules docked. Variations in the interactions of the linker section may explain the inconsistencies observed with the mefenamic acid moiety. One trend that is immediately apparent is the ability of the tacrine moiety, if bound in the CAS, to direct the mefenamic acid to the vicinity of the PAS (Fig. 4B). Mefenamic acid is thought to deactivate AChE through a free-radical mechanism, not through specific interaction or modification of the CAS.16 Our modeling studies showed that the mefenamic acid moiety would be in close proximity to Trp279 as well as tyrosines (Tyr70, 121, and 334) near the PAS (Fig. 4C), which may have two implications.
Since IC50 values were dependent on the enzyme concentration, it was hypothesized that the low IC50 values observed in the ROS assay may be due to the inactivation of some amount of the enzyme by radicals through interaction with tyrosine or tryptophan residues proximal to the PAS, which provides another possible explanation for the non-competitive or mixed-mode of inhibition (Fig. 4B,C). It is known that AChE has an adhesion function, located at the PAS, that governs AChE's interactions with Aβ and is believed to induce Aβ fibril formation.19,31,32 Perturbation of the PAS by a number of small molecules and antibodies has been shown to inhibit Aβ fibril formation, presumably by blocking the interaction at the PAS.19,33 Consequently, we believe that the tacrine-mefenamic acid hybrids reported herein may be capable of disrupting Aβ fibril formation and further experiments will be carried out to determine whether or not this is the case.
Conclusions
Tacrine-mefenamic acid hybrid molecules were synthesized via an easily accessible, convergent synthetic route and evaluated as inhibitors of AChE in two biochemical assays. The compounds appeared to act as non-competitive or mixed-mode inhibitors with respect to acetylthiocholine in the instances tested, and most were capable of inducing a half-maximal enzymatic response at low nanomolar concentrations with instances of picomolar IC50 values observed. One plausible explanation is that mixed inhibition is observed with a predominantly non-competitive mode of inhibition being observed for the most potent inhibitors tested. Several sub-nanomolar inhibitors were identified and selected for molecular modeling experiments. Taken together, the results suggest that the tacrine portion of the inhibitors may be capable of binding in the AChE CAS, spanning the active-site gorge via a methylene-based linker, and positioning the mefenamic acid moiety to interact with the PAS. While there is some discrepancy between the modeling studies and analysis of the mode of inhibition, these compounds provide the framework for the development of novel AChE inhibitors that may be capable of alleviating the symptoms of AD associated with a decrease in cholinergic function and may also be capable of diminishing Aβ aggregation to the extent that AChE is involved. Along with a more throrough investigation aimed at determining the mechanism by which AChE function is reduced by these inhibitors, further optimization studies aimed at improving potency and determining the possible secondary effects of the hybrid molecules are currently underway.Acknowledgements
This work was supported by the Life Sciences Institute and the College of Pharmacy at the University of Michigan (SGT). The ACS Division of Medicinal Chemistry and Eli Lilly are acknowledged for a predoctoral fellowship to JLH. JLH is also supported by the Cellular Biotechnology Training Program (CBTP) and by a Rackham Merit Fellowship at the University of Michigan. JJB was supported by a 2010 Margaret and Herman Sokol Endowment Award, provided through the UM chemistry department. We thank Hao Xu for the initial preparation of compound 4. We thank Alaina Detoma for help with AutoDock.Notes and references
- K. D. Green, V. R. Porter, Y. Zhang and S. Garneau-Tsodikova, Biochemistry, 2010, 49, 6219–6227 CrossRef CAS
.
- K. D. Green, M. Fridman and S. Garneau-Tsodikova, ChemBioChem, 2009, 10, 2191–2194 CrossRef CAS
.
- A. R. Kim, R. J. Rylett and B. H. Shilton, Biochemistry, 2006, 45, 14621–14631 CrossRef CAS
.
- R. C. Petersen, R. G. Thomas, M. Grundman, D. Bennett, R. Doody, S. Ferris, D. Galasko, S. Jin, J. Kaye, A. Levey, E. Pfeiffer, M. Sano, C. H. van Dyck and L. J. Thal, N. Engl. J. Med., 2005, 352, 2379–2388 CrossRef CAS
.
- C. Courtney, D. Farrell, R. Gray, R. Hills, L. Lynch, E. Sellwood, S. Edwards, W. Hardyman, J. Raftery, P. Crome, C. Lendon, H. Shaw and P. Bentham, Lancet, 2004, 363, 2105–2115 CrossRef CAS
.
- P. L. McGeer, M. Schulzer and E. G. McGeer, Neurology, 1996, 47, 425–432 CAS
.
- P. L. McGeer and E. G. McGeer, Neurobiol. Aging, 2007, 28, 639–647 CrossRef CAS
.
- P. L. McGeer, J. Rogers and E. G. McGeer, J. Alzheimers Dis., 2006, 9, 271–276 CAS
.
- C. A. Szekely, T. Town and P. P. Zandi, Subcellular Biochemistry, 2007, 42, 229–248 Search PubMed
.
- Y. Joo, H. S. Kim, R. S. Woo, C. H. Park, K. Y. Shin, J. P. Lee, K. A. Chang, S. Kim and Y. H. Suh, Mol Pharmacol, 2006, 69, 76–84 CAS
.
- P. Camps, X. Formosa, D. Munoz-Torrero, J. Petrignet, A. Badia and M. V. Clos, J. Med. Chem., 2005, 48, 1701–1704 CrossRef CAS
.
- D. Alonso, I. Dorronsoro, L. Rubio, P. Munoz, E. Garcia-Palomero, M. Del Monte, A. Bidon-Chanal, M. Orozco, F. J. Luque, A. Castro, M. Medina and A. Martinez, Bioorg. Med. Chem., 2005, 13, 6588–6597 CrossRef CAS
.
- S. Butini, E. Guarino, G. Campiani, M. Brindisi, S. S. Coccone, I. Fiorini, E. Novellino, T. Belinskaya, A. Saxena and S. Gemma, Bioorg. Med. Chem. Lett., 2008, 18, 5213–5216 CrossRef CAS
.
- D. Shao, C. Zou, C. Luo, X. Tang and Y. Li, Bioorg. Med. Chem. Lett., 2004, 14, 4639–4642 CrossRef CAS
.
- M. I. Rodriguez-Franco, M. I. Fernandez-Bachiller, C. Perez, B. Hernandez-Ledesma and B. Bartolome, J. Med. Chem., 2006, 49, 459–462 CrossRef CAS
.
- S. Muraoka and T. Miura, Life Sci., 2009, 84, 272–277 CrossRef CAS
.
- S. Muraoka and T. Miura, Life Sci., 2003, 72, 1897–1907 CrossRef CAS
.
- T. Thomas, T. G. Nadackal and K. Thomas, NeuroReport, 2001, 12, 3263–3267 CrossRef CAS
.
- N. C. Inestrosa, A. Alvarez, C. A. Perez, R. D. Moreno, M. Vicente, C. Linker, O. I. Casanueva, C. Soto and J. Garrido, Neuron, 1996, 16, 881–891 CrossRef CAS
.
- M. Bartolini, C. Bertucci, V. Cavrini and V. Andrisano, Biochem. Pharmacol., 2003, 65, 407–416 CrossRef CAS
.
- D. M. Du and P. R. Carlier, Curr. Pharm. Des., 2004, 10, 3141–3156 CrossRef CAS
.
- M. K. Hu, L. J. Wu, G. Hsiao and M. H. Yen, J. Med. Chem., 2002, 45, 2277–2282 CrossRef
.
- P. R. Carlier, E. S. Chow, Y. Han, J. Liu, J. El Yazal and Y. P. Pang, J. Med. Chem., 1999, 42, 4225–4231 CrossRef CAS
.
- G. L. Ellman, K. D. Courtney, V. Andres, Jr. and R. M. Feather-Stone, Biochem. Pharmacol., 1961, 7, 88–95 CrossRef CAS
.
- M. I. Fernandez-Bachiller, C. Perez, N. E. Campillo, J. A. Paez, G. C. Gonzalez-Munoz, P. Usan, E. Garcia-Palomero, M. G. Lopez, M. Villarroya, A. G. Garcia, A. Martinez and M. I. Rodriguez-Franco, ChemMedChem, 2009, 4, 828–841 CrossRef CAS
.
- E. H. Rydberg, B. Brumshtein, H. M. Greenblatt, D. M. Wong, D. Shaya, L. D. Williams, P. R. Carlier, Y. P. Pang, I. Silman and J. L. Sussman, J. Med. Chem., 2006, 49, 5491–5500 CrossRef CAS
.
- L. Fang, B. Kraus, J. Lehmann, J. Heilmann, Y. Zhang and M. Decker, Bioorg. Med. Chem. Lett., 2008, 18, 2905–2909 CrossRef CAS
.
- M. Harel, I. Schalk, L. Ehret-Sabatier, F. Bouet, M. Goeldner, C. Hirth, P. H. Axelsen, I. Silman and J. L. Sussman, Proc. Natl. Acad. Sci. U. S. A., 1993, 90, 9031–9035 CAS
.
- C. H. da Silva, V. L. Campo, I. Carvalho and C. A. Taft, J. Mol. Graphics Modell., 2006, 25, 169–175 CrossRef CAS
.
- Y. P. Pang, P. Quiram, T. Jelacic, F. Hong and S. Brimijoin, J. Biol. Chem., 1996, 271, 23646–23649 CrossRef CAS
.
- G. Johnson and S. W. Moore, Biochem. Biophys. Res. Commun., 1999, 258, 758–762 CrossRef CAS
.
- A. Alvarez, C. Opazo, R. Alarcon, J. Garrido and N. C. Inestrosa, J. Mol. Biol., 1997, 272, 348–361 CrossRef CAS
.
- A. E. Reyes, D. R. Perez, A. Alvarez, J. Garrido, M. K. Gentry, B. P. Doctor and N. C. Inestrosa, Biochem. Biophys. Res. Commun., 1997, 232, 652–655 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic, biochemical, and computational procedures as well as characterization of all novel compounds. See DOI: 10.1039/c0md00256a |
| This journal is © The Royal Society of Chemistry 2011 |
