Discovery and pharmacological characterization of a selective delta opiate receptor antagonist (CP-646,777)†
Spiros
Liras
*a,
Stanton F.
McHardy‡
a,
Martin P.
Allen
a,
Barb E.
Segelstein
a,
Steven D.
Heck
a,
Dianne K.
Bryce
b,
Anne W.
Schmidt
b,
Rebecca
O'Connor
b,
Michelle
Vanase-Frawley
b,
Ernesto
Callegari
c and
Stafford
McLean
b
aNeuroscience Medicinal Chemistry, Pfizer Worldwide Research and Development, Eastern Point Road, Groton, CT 06340, USA. E-mail: spiros.liras@pfizer.com
bNeuroscience Biology, Pfizer Worldwide Research and Development, Eastern Point Road, Groton, CT 06340, USA
cPharmacokinetics, Dynamics and Metabolism, Pfizer Worldwide Research and Development, Eastern Point Road, Groton, CT 06340, USA
First published on 9th March 2011
Abstract
CP-646,777 (compound 2a) is identified as a potent and selective delta opioid receptor antagonist. The synthesis, pharmacological evaluation, disposition characteristics and pharmacokinetic properties of this compound are reported herein. An approach for reducing clearance as measured by human liver microsomes is demonstrated. The significance of p-glycoprotein efflux on CNS disposition and on the pharmacological action of CP-646,777 is also discussed.
Introduction
Opioid receptor antagonists are known to decrease ethanol intake in preclinical and clinical studies. It has been postulated that antagonism of opioid receptors interferes with the reward system associated with alcohol consumption.1 The clinical evidence is based on the efficacy in ethanol intake reduction shown by naltrexone and other nonselective agents such as nalmefene and naloxone.2 The scientific deliberation regarding the relative significance and contribution of opioid receptor subtypes to the reduction in ethanol intake is current and rigorous.3 Since compounds such as naltrexone and naloxone are nonselective antagonists for the mu, delta and kappa receptors, it is not clear which receptor subtype(s) drives the reduction in ethanol intake in the clinic. Recently, there have been numerous reports which suggest that delta antagonists reduce ethanol consumption.4 Given the intense interest of the scientific community on the issue of pharmacological relevance of the opioid receptors we set out to deliver novel, selective and centrally active delta opioid receptor antagonists. We intended to pharmacologically characterize the antagonists in mechanism and disease relevant models.In an earlier communication5 we disclosed the design, synthesis and in vitro biological evaluation of biaryl piperidines. We reported that biaryl piperidines are potent and selective delta opioid receptor ligands. As we reported earlier, compounds from this class of biaryl piperidines were limited as potential probes due to high clearance as measured in vitro by human liver microsomes. In this report we disclose the design and synthesis of a delta receptor antagonist with improved pharmacokinetic properties for clearance, sufficient central exposure, and the pharmacological evaluation of this antagonistin vitro and in vivo.
Results and discussion
Compound 1 (Fig. 1) represents a lead biaryl piperidine. The compound exhibited good affinity for the delta receptor with a Ki of 11 nM and selectivity over both the mu receptor (Ki >890 nM) and the kappa receptor (Ki 150 nM). Compound 1 was characterized as a delta receptor antagonist with an IC50 of 72 nM and was found to exhibit high in vitro clearance in human liver microsomes (80 mL min−1 kg−1). Thus, our first research objective was to optimize the pharmacokinetic properties of this lead. To achieve this we employed a medicinal chemistry strategy which aimed to increase the polarity of the lead, as determined by cLogP, without increasing the overall molecular weight. We envisaged that pyrimidines of general structure 2 would deliver the project objectives of potent, selective, and metabolically stable delta receptor antagonists. It was anticipated that the non phenolic aromatic ring in the lead compound 1 functions as a spacer moiety, based on the design strategy which employed elements of the message-address principle.6 Thus, we rationalized that polarization of the spacer would be not detrimental to the affinity and selectivity attributes of the lead.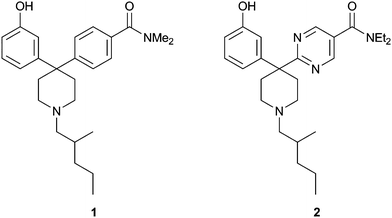 | ||
| Fig. 1 Biaryl piperidine 1 and target pyrimidine 2. | ||
To access the desired pyrimidines (e.g. compound 2) we investigated the synthetic plan described in Schemes 1 and 2. Our initial plan involved the construction of the piperidine ring first. As we had envisioned, dialkylation of 3-methoxyphenyl acetonitrile with bis-(2-chloroethyl) methylamine in aqueous sodium hydroxide7 yielded piperidine nitrile 3 in high yield. Conversion, however, of the nitrile 3 to amidine 4 under numerous conditions was met with failure and this approach was abandoned. Consequently, we pursued the preparation of a precursor amidine through the more flexible nitrile 5. Thus, we proceeded with dialkylation of the phenyl acetonitrile with allyl bromide to deliver compound 5. Subsequent treatment with trimethylaluminium and ammonium chloride in refluxing toluene8 yielded the desired amidine 6.
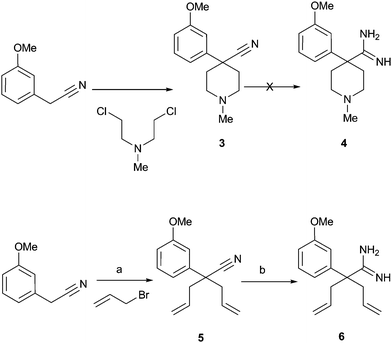 | ||
| Scheme 1 Reagents and conditions: a) 50% NaOH(aq), (C16H33)P(n-Bu)3Br, 89% overall; b) Al(Me)3, NH4Cl, toluene, reflux, then silica gel, CHCl3, 49%. | ||
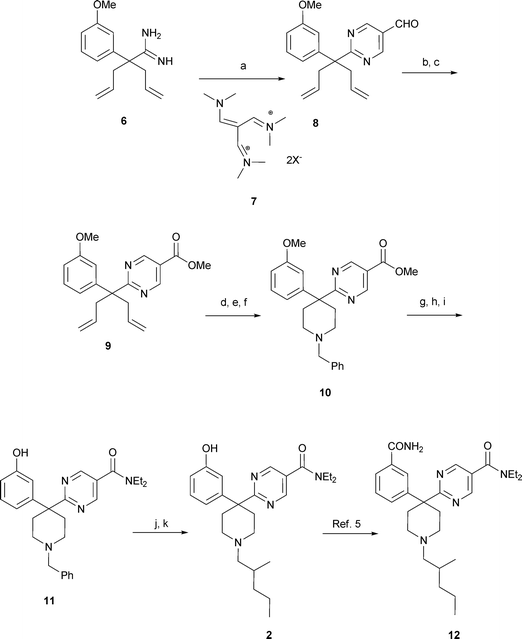 | ||
| Scheme 2 Reagents and conditions: a) NaOEt; EtOH, 0 to 80 °C, then 1 M HCl, 81%; b) NaClO2, KH2PO4, t-BuOH, 2-methyl-2-butene, −5 °C to rt, 94%; c) CDI, CH3OH, rt, 84%; d) OsO4, NMO, acetone-H2O; e) NaIO4, NMO, EtOH–H2O, rt f) BnNH2, NaBH(OAc)3, CH3COOH, CH2Cl2, rt, 50% for steps d, e, f; g) LiOH, MeOH–H2O, rt; h) CDI, Et2NH, CH2Cl2, rt, 95% for steps g and h; i) EtSH, NaH, DMF, 120 °C, 75%; j) alpha-chloroethyl chloroformate, K2CO3, (CH2)2Cl2 then CH3OH, reflux, 90%; k) 2-methyl valeraldehyde, NaBH(OAc)3, (CH2)2Cl2, rt, 77%. | ||
With the desired amidine 6 in hand, efforts commenced to complete the pyrimidine synthesis (Scheme 2). Reaction of the amidine 6 with vinamidinium salt 7 yielded the pyrimidine 8 in high yield.9 During the course of this investigation we discovered that the bis perchlorate vinamidinium salts possessed remarkably high thermal energy and high shock sensitivity, precluding the use of this reagent for multi-gram preparation. We subsequently demonstrated that the bis tetrafluoroborate salt was equally effective in performing the pyrimidine formation reaction and possessed substantially lower thermal energy; hence we employed that reagent to deliver the requisite pyrimidine in high yield on large scale.10Oxidation of the aldehyde and conversion to the ester proceeded smoothly to yield diene 9 which was subsequently treated with osmium tetroxide, sodium periodate, and benzyl amine with sodium triacetoxy borohydride to yield piperidine 10. Conversion of 10 to amide 11 took place after hydrolysis to the acid, subsequent coupling with diethylamine and deprotection of the methyl ether with sodium hydride and ethanethiol in DMF.11Debenzylation with α-chloro ethyl chloroformate, subsequent methanolysis and reductive alkylation with α-methyl valeraldehyde under standard conditions yielded the final product 2. As we reported earlier we sought phenol replacements since we anticipated that the moiety could be responsible for secondary metabolismviaconjugation. Thus, we investigated the potential of substituting the phenol with carboxamides. Based on a previously reported series of transformations we produced compound 12.5
Compound 2 possessed the desired in vitro pharmacology attributes.12,13 It exhibited high in vitro affinity for the delta receptor (6 nM) and excellent selectivity over the mu and kappa receptors (>100x). Compound 2 is a delta opiate receptor antagonist with an IC50 of 21 nM as determined in a GTP-γ S assay. The above data confirmed the hypothesis that chemical manipulation of the spacer aromatic ring would be not detrimental to affinity and selectivity. The strategy to optimize in vitro human microsomal clearance by reducing lipophilicity via heteroatom insertions in the spacer phenyl ring was also validated. The lead compound was significantly more stable than its predecessor biphenyl piperidine. Intrinsic clearance as measured in vitro by human liver microsomes was improved to 40 mL min−1 kg−1. In contrast, compound 12 retained high affinity for the delta receptor, reduced selectivity over kappa and excellent selectivity over the mu receptor. Significantly, 12 demonstrated greatly improved metabolic stability relative to 1 and 2 with an in vitro clearance of 13 mL min−1 kg−1, consistent with a reduced cLogP value, which further supports the importance of reducing lipophilicity.
The second objective of our investigation was to establish sufficient CNS exposure for pharmacological action with the leading compounds. Compounds 2 and 12 were predicted to be efflux substrates, based on in vitro data generated via an assay utilizing the MDCK-MDR1 cell line (an MDCK line stably transfected with the MDR1 gene that expresses a functionally active human P-gp).14 Compound 2 demonstrated a high BA/AB efflux ratio of 13. Compound 12 similarly demonstrated a high BA/AB efflux ratio of 13. Generally, a compound with a ratio of >2 is classified as a P-gp substrate.15 We decided to further investigate the significance of the efflux potential with in vivo disposition and pharmacology studies. Key in vitro and physical properties data for compounds 2 and 12 are presented in Table 1.
| Compound | 2 | 12 |
|---|---|---|
| Delta Ki (nM) | 6.0 | 2.0 |
| Mu Ki (nM) | >890 | >890 |
| Kappa Ki (nM) | >890 | 65 |
| Functional IC50 (nM) GTP-γ S | 21 | 35 |
| clogP | 3.6 | 2.8 |
| TPSA | 70 | 93 |
| HLM (mL min−1 kg−1) | 40 | 13 |
| MDR BA/AB | 13 | 13 |
Based on the balance of the overall properties we selected to focus on the preferred enantiomer of compound 2 for further evaluation in vivo. The enantioselective synthesis of the pair of enantiomers was achieved with the utilization of optically pure 2-methyl valeraldehyde.16 The desired compounds were produced according to the conditions for reductive alkylation as reported in Scheme 2 in good yields (74–80%) and enantiomeric excess (>95% ee). In vitro pharmacological studies revealed that the enantiomerically pure 2a (Fig. 2) (R enantiomer, CP-646,777) had excellent affinity for the delta receptor (Ki 2 nM), excellent selectivity over the mu (Ki 830 nM) and kappa receptors (Ki 142 nM) and an IC50 of 35 nM as a delta receptor antagonist.17
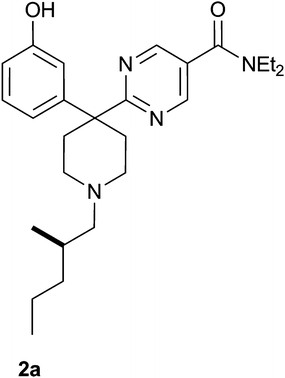 | ||
| Fig. 2 Compound 2a. | ||
To investigate the magnitude of P-gp efflux in vivo with compound 2a we conducted brain disposition measurements with P-gp deficient (KO) and wild type (WT) mice. In this experiment KO and WT mice were dosed (sc) with 2a and plasma and brain concentrations were determined between 0 and 4 h. The brain to plasma exposure (AUC) ratios in WT and KO were calculated and it was determined that compound 2a exhibited a six fold asymmetry between the KO and WT mice with higher brain to plasma exposure observed in KO mice. Hence, we concluded that 2a is a strong P-gp substrate in vivo in mice. We intended to further evaluate the impact of P-gp efflux on in vivo rat pharmacology models. Toward that goal, plasma and CSF concentrations were determined at a single time point, 30 min post dose, which was consistent with the pharmacology model treatment course. Compound 2a achieved high concentration in rat CSF (300 nM at 32 mg kg−1. 103 nM at 10 mg kg−1), despite being an efflux substrate. Based on microdialysis experiments we determined that extra cellular fluid (ECF) levels18 were in equilibrium with CSF levels, hence CSF concentration was identified as a surrogate for free brain concentration. Based on the CSF concentration relative to a 2 nM delta receptor Ki ([CSF]/Ki ratio of 150 at 32 mg kg−1) it was determined that the compound represented an adequate pharmacological tool and so it was profiled in additional pharmacology studies. Compound 2a was evaluated in vivo in a hypothermia reversal model which is linked to delta receptor pharmacology.19 Delta opioid receptors are present in the preoptic anterior hypothalamus, an area thought to play a key role in thermoregulation, and likely mediate the hypothermia elicited by the selective delta agonist SNC-80.20 However, a minor contribution from peripherally located delta receptors cannot be ruled out. We proceeded to investigate the potential of reversing SNC-80 induced hypothermia with selective delta opioid antagonist 2a. In a typical experiment male ICR mice (17–19 g) upon arrival were group housed (10/box) with ad lib access to food and water. Subjects were acclimated to the facility for at least 1 week prior to testing. On the day of the experiment, mice were injected with vehicle (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90, sc), or test compound (usually 10 mg kg−1, sc). Fifteen minutes later, mice were injected with vehicle (0.3% tartaric acid) or SNC-80 (10 mg kg−1, ip). 30 min post 2nd injection, rectal temperatures were taken in all mice and recorded to the nearest tenth of a degree. Body temperatures were determined with a VWR digital thermometer and flexible thermistor, which was inserted 2–3 cm rectally. The ambient temperature during testing ranged from 19–21 degrees centigrade. Data was analyzed by one-way ANOVA with PLSD post-hoc comparisons following significant main effects. Reversal (%) from the SNC-80 effect was typically graphically represented.
90, sc), or test compound (usually 10 mg kg−1, sc). Fifteen minutes later, mice were injected with vehicle (0.3% tartaric acid) or SNC-80 (10 mg kg−1, ip). 30 min post 2nd injection, rectal temperatures were taken in all mice and recorded to the nearest tenth of a degree. Body temperatures were determined with a VWR digital thermometer and flexible thermistor, which was inserted 2–3 cm rectally. The ambient temperature during testing ranged from 19–21 degrees centigrade. Data was analyzed by one-way ANOVA with PLSD post-hoc comparisons following significant main effects. Reversal (%) from the SNC-80 effect was typically graphically represented.
Compound 2a demonstrated good activity in the hypothermia assay with an ED50 of 3 mg kg−1 (15 fold the receptor Ki, in CSF based on linear exposures).21 Key in vitro and in vivo characteristics of compound 2a are shown in Table 2.
| Delta Ki (nM) | 2 |
| Mu Ki (nM) | 830 |
| Kappa Ki (nM) | 142 |
| Functional IC50 (nM) | 35 |
| Clint (HLM) mL min−1 kg−1 | 48 |
| MDR BA/AB | 13 |
| P-GP KO/WT | 6.5 |
| Plasma concentration (nM) (32 mg kg−1 dose) | 7500 |
| % Fraction unbound (rat) | 30 |
| CSF concentration (nM) (32 and 10 mg kg−1 dose) | 300, 103 |
| Hypothermia ED50 (mg kg−1) | 3 |
The in depth profiling of compound 2a allowed us to quantitatively assess the impact of P-gp efflux. It is clearly possible with P-gp efflux substrate compounds to reach concentrations in the central compartment with pharmacological relevance. There is, however, a concomitant requirement for higher concentration of compounds in the periphery. In the particular case of compound 2a, efficacy in the hypothermia model was realized with CSF concentrations approximately 15-fold over the receptor Ki and at plasma free concentrations close to 100 fold over the receptor Ki. Unnecessarily high concentrations in the periphery are likely to narrow safety related therapeutic margins. Consequently, eliminating active efflux has become a key objective of our research. Toward this end we are investigating several hypotheses including reduction of molecular weight, reduction of overall hydrogen bond donor count, and modulation of the pKa of strongly basic amines.
Conclusions
In summary, we have demonstrated that insertion of heteroatoms in the spacer of the 4,4-biaryl piperidines delivered delta opioid antagonists with high affinity, selectivity and improved pharmacokinetic properties. Compound 2a from this investigation served as a valuable pharmacological tool was utilized in implementing a complete screening sequence for evaluating delta antagonists preclinically. While there are some limitations associated with the tool compound, such as efflux, the compound achieved appreciable central exposure measured by CSF concentrations and demonstrated efficacy in a pharmacologically relevant model. The in depth characterization of 2a enabled us to develop a thorough understanding of the risk posed by P-gp efflux. Efforts to eliminate P-gp efflux by modulation of key properties such as hydrogen bond donor number, molecular weight and basic amine pKa and additional profiling of compounds in assays measuring ethanol intake will be reported in due course.Notes and references
- (a) D. Benjamin, E. R. Grant and L. A. Pohorecky, Brain Res., 1993, 621(1), 137 CrossRef CAS; (b) L. M. Oswald and G. S. Wand, Physiol. Behav., 2004, 81(2), 339 CrossRef CAS.
- (a) S. S. O'Malley, Alcohol Alcohol. Suppl., 1996, 1, 77 Search PubMed; (b) G. O'Leary, J. Borrow and R. D. Weiss, Curr. Psychiatry Rep., 2001, 3(6), 484 Search PubMed.
- (a) J. R. Barson, A. J. Carr, J. E. Soun, N. C. Sobhani, S. F. Leibowitz and B. G. Hoebel, Physiol. Behav., 2009, 98, 453 CrossRef CAS; (b) P. W. Marinelli, D. Funk, S. Harding, Z. Li, W. Juzytsch and A. D. Le, Eur. J. Neurosci., 2009, 30, 671 CrossRef.
- C. K. Nielsen, J. A. Simms, H. B. Pierson, R. Li, S. K. Saini, S. Ananthan and S. E. Bartlett, Biol. Psychiatry, 2008, 64, 974 CrossRef CAS.
- For representative references please see S. Liras, S. F. McHardy, M. P. Allen, B. E. Segelstein, S. D. Heck, D. K. Bryce, A. W. Schmidt, M. Vanase-Frawley, E. Callegari and S. McLean, Bioorg. Med. Chem. Lett., 2010, 20, 503 Search PubMed The biaryl piperidine class is an evolution of privileged opiares such as phenyl piperidines and merepidine., (a) C. H. Mitch, J. D. Leander, L. D. Mendelsohn, W. N. Shaw, D. T. Wong, B. E. Cantrell, B. G. Johnson, J. K. Reel, J. D. Snoddy, A. E. Takemori and D. M. Zimmerman, J. Med. Chem., 1993, 36, 1836; (b) S. A. Lomenzo, J. B. Rhoden, S. Izenwasser, D. Wade, T. Kopajtic, J. L. Katz and M. L. Trudell, J. Med. Chem., 2005, 48, 1336 CrossRef CAS.
- P. S. Portoghese, M. Sultana and A. E. Takemor, J. Med. Chem., 1990, 33, 1714 CrossRef CAS.
- T. Cammack and P. C. Reeves, J. Heterocycl. Chem., 1986, 23, 73 CrossRef CAS.
- R. A. Moss, W. Ma, D. C. Merrer and S. Xue, Tetrahedron Lett., 1995, 36, 8761 CrossRef CAS.
- J. T. Gupton, J. E. Gall, S. W. Riesinger, S. Q. Smith, K. M. Bevirt, J. A. Sikorski, M. L. Dahl and A. Arnold, J. Heterocycl. Chem., 1991, 28, 1281 CrossRef CAS.
- J. A. Ragan, R. E. McDermott, B. P. Jones, D. J. am Ende, P. J. Clifford, S. J. McHardy, S. D. Heck, S. Liras and B. E. Segelstein, Synlett, 2000, 8, 1172.
- H. G. Selnick, G. R. Smith and A. J. Tebben, Synth. Commun., 1995, 25, 3255 CAS.
- Experimental conditions for the in vitro affinity and selectivity of the compounds have been described in reference 5. The in vitro values reported represent the average values of 2–6 screening runs. Briefly, frozen cell paste (70-80-mgs per 96 well plate) was homogenized in 50 mM Tris HCl buffer (pH 7.4 @ 4 °C) containing 2.0 mM MgCl2 using a Polytron and spun in a centrifuge at 40,000g for ten minutes. The final pellet was re-suspended in assay buffer (50 mM Tris HCl buffer (pH 7.4) containing 1 mM EDTA, 5 mM MgCl2). Incubations were initiated by the addition of tissue to 96-well plates containing test drugs and 0.6 nM [3H]diprenorphine in a final volume of 250 μL). Non-specific binding was determined by radioligand binding in the presence of a saturating concentration of naltrexone (10 mM). After a one hour incubation period at room temperature, assay samples were rapidly filtered through Whatman GF/B filters and rinsed with ice-cold 50 mM Tris buffer (pH 7.4). Membrane bound [3H]diprenorphine levels were determined by liquid scintillation counting of the filters in BetaScint. The IC50 value (concentration at which 50% inhibition of specific binding occurs) was calculated by linear regression of the concentration-response data. Ki values were calculated according to the Cheng Prusoff equation, Ki = IC50/(1 + (L K−1d)), where L is the concentration of the radioligand used in the experiment and the Kd value is the dissociation constant for the radioligand (determined previously by saturation analysis). Also, see: V. U. K. Dissanayake, J. Hughes and J. C. Hunter, Mol. Pharmacol., 1991, 40(1), 93 Search PubMed.
- Compounds 2, 2a, and 12 were purified by reverse phase chromatography. Purity was determined as 100% by UV and ELSD detection. All compounds gave satisfactory 1H NMR and mass spectral data.
- B. Feng, J. B. Mills, R. E. Davidson, R. J. Mireles, J. S. Janiszewski, M. D. Troutman and S. M. de Morais, Drug Metab. Dispos., 2008, 36, 268 CrossRef CAS.
- K. M. Giacomini, S.-M. Huang, D. J. Tweedie, L. Z. Benet, K. L. R. Brouwer, X. Chu, A. Dahlin, R. Evers, V. Fischer, K. M. Hillgren, K. A. Hoffmaster, T. Ishikawa, D. Keppler, R. Kim, C. A. Lee, M. Niemi, J. W. Polli, Y. Sugiyama, P. W. Swaan, J. A. Ware, S. H. Wright, S. Wah Yee, M. J. Zamek-Gliszczynski and L. Zhang, Nat. Rev. Drug Discovery, 2010, 9, 215 CrossRef CAS.
- R. E. Zelle, M. P. DeNinno, H. G. Selnick and S. J. Danishefsky, J. Org. Chem., 1986, 51, 5032 CrossRef CAS.
- An alternative screening methodology was employed for the initial in vitro evaluation of the R and S enantiomers. 3H-naltrindole in HOPD membranes was used for the delta receptor binding studies and 3H-DAMGO in rat brain was used to determine mu receptor affinity. In those assays the R enantiomer (Compound 2a, CP-646,777) had a delta Ki of 2 nM and a mu Ki of 490 nM. The S enantiomer had a delta Ki of 16 nM and a mu receptor Ki of 165 nM. Based on these studies we focused our attention on the R enantiomer.
- E. C. M. de Lange, P. G. M. Ravenstijn, D. Groenendaal and T. J. van Steeg, AAPS J., 2005, 7(3), 532 Search PubMed.
- A. K. Baker and T. F. Meert, J. Pharmacol. Exp. Ther., 2002, 302, 1253 CrossRef CAS.
- E. J. Bilsky, S. N. Calderon, T. Wang, R. N. Bernstein, P. Davis, V. J. Hruby, R. W. McNutt, R. B. Rothman, K. C. Rice and F. Porreca, J. Pharmacol. Exper. Ther., 1995, 273, 359 CAS.
- The S enantiomer showed significantly reduced activity in the hypothermia reversal assay (18% reversal at 10 mg kg−1).
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c0md00249f/ |
| ‡ Current address; Synthesis and Process Chemistry, Southwest Research Institute, 6220 Culebra Rd, San Antonio, Texas, 78238, USA. |
| This journal is © The Royal Society of Chemistry 2011 |
