20-O-IngenolEZ, a catalytic topoisomerase II inhibitor, specifically inhibits cell proliferation and induces double-strand DNA breaks in BLM-/-cells
Manami
Watanabe
a,
Yuta
Kamada
a,
Khosuke
Miyazaki
a,
Shoko
Mizoguchi
a,
Keiichi
Matsuzaki
b,
Susumu
Kitanaka
b and
Shohei
Miyata
*a
aDepartment of Chemistry, College of Humanities and Sciences, Nihon University, Sakurajosui, Setagaya-ku, Tokyo 156-8550, Japan. E-mail: miyata@chs.nihon-u. ac.jp; Fax: +81 3 5317 9433; Tel: +81 3 5317 9735
bSchool of Pharmacy, Nihon University, 7-7-1 Narasinodai, Funabashi, Chiba 274-8555, Japan
First published on 7th July 2011
Abstract
Bis-dioxopiperazines have been termed catalytic inhibitors to distinguish them from topoisomerase poisons that induce DNA double-strand breaks (DSBs). However, it has been reported that ICRF-193 acts as a poison in cells containing mutated genes related to checkpoint mechanisms. We also showed previously that 20-O-ingenolEZ acts as a catalytic inhibitor to inhibit the ATPase activity of topoisomerase IIα, inducing the G2 arrest of mouse mammary tumor (MMT) cells. In this study, we observed the effects of 20-O-ingenolEZ on cells containing a mutation in the RecQ helicase gene. 20-O-IngenolEZ completely inhibited the proliferation of BLM-/-cells in BLM-/- and WRN-/-DT40 cells and wild-type DT40 cells. This inhibition induced the phosphorylation of H2AX in response to agents that introduce topoisomerase II-mediated DSBs. Following DNA damage, the induction of apoptosis in the BLM-/-cells by 20-O-ingenolEZ showed the characteristics of a topoisomerase II poison.
Introduction
The activity of topoisomerase II is essential for the survival of proliferating cells and is involved in recombination, chromosome condensation, and the decatenation of sister chromatids prior to the anaphase of mitosis.1 In cancer chemotherapy, topoisomerase II is a major target for a variety of anti-cancer drugs. Two well-characterized modes of action have been described for inhibitors of eukaryotic topoisomerase II. Topoisomerase II poisons, such as adriamycin and etoposide, induce DNA double-strand breaks (DSBs) and trigger the DNA damage checkpoint and apoptosis.2,3 In contrast, catalytic inhibitors of the other agent class, bis-dioxopiperazine (ICRF-193) do not induce extensive DNA breaks but instead induce the decatenation checkpoint and G2 arrest.4,5 The term “catalytic inhibitor” is used to distinguish these agents from topoisomerase poisons, and catalytic inhibitors do not induce DNA cleavage. We previously reported that some ingenol compounds inhibit topoisomerase II and/or cellular proliferation and showed that the presence of 20-O-ingenolEZ in these compounds inhibits the ATPase activity of topoisomerase II and induces G2 arrest in mouse mammary tumor (MMT) cells by inhibiting DNA decatenation.6–8 20-O-IngenolEZ treatment did not induce any changes in the levels of the well-recognized DNA damage marker, γ-H2AX.The decatenation checkpoint is activated when cells are treated with a catalytic inhibitor, such as ICRF-193 or 187, and is distinct from the DNA damage checkpoint activated by etoposide.9–11 However, recent studies have suggested that ICRF-193 induces DSBs by acting as a topoisomerase II poison.12,13 These findings are consistent with previous results showing that cells with an impairment in the decatenation checkpoint were highly sensitive to the ICRF-193-induced inhibition of proliferation and exhibited apoptosis.14 Rec Q helicase functions during DNA replication and is essential for the repair of DNA lesions.15–18 In human cells, mutations of the BLM and WRN genes give rise to cancer predispositions known as Bloom syndrome (BS) and Werner syndrome (WS), respectively. When WS cells were treated for long periods at a high concentration of ICRF-187, DSBs formed in the cells and led to the activation of DNA repair and ultimately to apoptotic cell death, which was markedly elevated in the absence of active WRN protein.19 BLM-impaired cells were also hypersensitive to ICRF-193.20 These results revealed that cells containing mutated genes related to checkpoint reactions comprising WRN-impaired and BLM-impaired genes may be hypersensitive to ICRF-187 or 193. To determine whether a new catalytic inhibitor, 20-O-ingenolEZ, induces the DNA damage checkpoint in BS cell and/or WS cells, we examined the effects on proliferation in BLM-/- and WRN-/-cells. 20-O-IngenolEZ completely inhibited the proliferation of BLM-/-cells in BLM-/- and WRN-/-DT40 cells and wild-type DT40 cells, and the induction of DNA damage and apoptosis in BLM-/-cells was observed.
Materials and methods
Cell lines and cell proliferation
BLM -/- and WRN-/-DT40 cells and wild-type DT40 cells were provided by the RIKEN BRC through the National Bio-Resource Project of the MEXT, Japan. BLM-/- and WRN-/-DT40 cells and DT40 cells were grown in RPMI 1640 supplemented with 50 μM of mercaptoethanol, 10% fetal calf serum and 1% chicken serum, then transplanted onto microculture plates (1–2 × 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per 0.1 ml). The diterpene compound, 20-O-(2′(E),4′(Z)-decadienoyl) ingenol (20-O-ingenolEZ) was dissolved in dimethylsulfoxide (DMSO). After appropriate dilution, the drug was added to 100 μl of medium and the cells were incubated for 2 days at 37 °C in an atmosphere of 95% air and 5% CO2. Cell growth in the presence or absence of the compound was determined using the MTT (3-(4,5-dimethylthiazol-2-yl)-2-5-diphenyltetrazolium bromide)21 assay using the Cell Proliferation Kit I (Roche Applied Science). The optical density of each well was measured at 620 nm using a plate reader (Amersham).
000 cells per 0.1 ml). The diterpene compound, 20-O-(2′(E),4′(Z)-decadienoyl) ingenol (20-O-ingenolEZ) was dissolved in dimethylsulfoxide (DMSO). After appropriate dilution, the drug was added to 100 μl of medium and the cells were incubated for 2 days at 37 °C in an atmosphere of 95% air and 5% CO2. Cell growth in the presence or absence of the compound was determined using the MTT (3-(4,5-dimethylthiazol-2-yl)-2-5-diphenyltetrazolium bromide)21 assay using the Cell Proliferation Kit I (Roche Applied Science). The optical density of each well was measured at 620 nm using a plate reader (Amersham).
Immunoblotting
BLM -/- DT40 cells were cultured for 24 h in the presence of 200 μM of 20-O-ingenolEZ or 0.9 μM of adriamycin, washed in phosphate-buffered saline (PBS), suspended in a lysis buffer (20 mM HEPES (pH 7.8), 10 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.1% Triton-X100, 20% glycerol and 1 mM DTT, supplemented with protease inhibitors) and lysed. After centrifugation at 2100 rpm for 4 min, the precipitate was resuspended in a lysis buffer containing 500 mM NaCl instead of 10 mM NaCl and placed on ice for 30 min. The suspension was centrifuged at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min at 4 °C. The protein concentrations were determined using the Bradford reagent for protein assays from Bio-Rad Laboratories, and 20 μg of nuclear protein was resolved on 15% SDS-polyacrylamide gels and transferred to a polyvinylidene difluoride membrane. The blots were made using anti-γ-H2AX antibody (Millipore, Billerica, MA, USA)22 followed by detection using an enhanced chemiluminescence system.
000 rpm for 15 min at 4 °C. The protein concentrations were determined using the Bradford reagent for protein assays from Bio-Rad Laboratories, and 20 μg of nuclear protein was resolved on 15% SDS-polyacrylamide gels and transferred to a polyvinylidene difluoride membrane. The blots were made using anti-γ-H2AX antibody (Millipore, Billerica, MA, USA)22 followed by detection using an enhanced chemiluminescence system.
Immunofluorescence assay
BLM -/- DT40 cells were cultured for 24 hours in the presence of 200 μM of 20-O-ingenolEZ or 0.9 μM of adriamycin, washed three times with PBS for 5 min, fixed with ethanol–acetic acid (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for 10 min and coverslipped. Apoptotic cells and nuclei were stained for 10 min at room temperature with 4,6-diamino-2-phenyl indole (DAPI, at 1 μg ml−1PBS) and detected using fluorescence microscopy.
1) for 10 min and coverslipped. Apoptotic cells and nuclei were stained for 10 min at room temperature with 4,6-diamino-2-phenyl indole (DAPI, at 1 μg ml−1PBS) and detected using fluorescence microscopy.
Results and discussion
To investigate the inhibitory effects of 20-O-ingenolEZ on the proliferation of BLM-/- and WRN-/-cells, a concentration–response range (2–100 μM) was established using an exposure time of 2 days (Fig. 1A). The cellular proliferation of WRN-/-cells was not inhibited by 20-O-ingenolEZ at a concentration range of 2–100 μM. The proliferation of BLM-/-DT40 cells was not inhibited at concentrations of 2–60 μM, but was immediately inhibited at a concentration of 100 μM (Fig. 1A). The anti-proliferation activity was compared between WRN-/- and BLM-/-DT40 cells and wild-type cells exposed to a high concentration of 20-O-ingenolEZ, and the effects were compared with those of ICRF-193 (Fig. 1B). When the WRN-/-cells were treated with a concentration of 200 μM of 20-O-ingenolEZ, no effects on cellular proliferation were observed, whereas the proliferation of BLM-/-DT40 cells was completely inhibited after treatment with a concentration of 200 μM of 20-O-ingenolEZ. The proliferation of wild-type cells was not affected at a concentration range of 2–100 μM of 20-O-ingenolEZ but was inhibited by about 50% at a concentration of 200 μM of 20-O-ingenolEZ, and the inhibition of BLM-/-DT40 cellular proliferation was more sensitive to 20-O-ingenolEZ than the inhibition of wild-type DT40 cellular proliferation (Fig. 1B). The anti-proliferation activity was also compared between WRN-/- and BLM-/-DT40 cells and wild-type cells treated with 200 μM of ICRF-193. The effects of ICRF-193 on WRN-/-DT40 cells differed completely from those of 20-O-ingenolEZ, resulting in inhibition (Fig. 1B). ICRF-193 inhibited the proliferation of the three cancer cell lines to almost the same degree.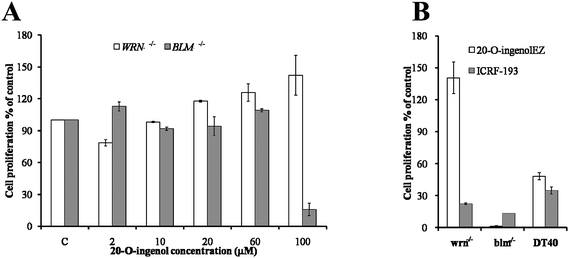 | ||
| Fig. 1 Effects of 20-O-ingenolEZ on cell proliferative activity of BLM-/- and WRN-/-DT40 cells and DT40 cells were cultured in microplates at 37 °C for 2 days at varying 20-O-ingenolEZ or ICRF-193 concentrations. Relative cell growth was determined using the MTT (3-(4,5-dimethylthiazol-2-yl)-2-5-diphenyltetrazolium bromide) assay. The cell growth in untreated cells was defined as 100%, and the cell growth of the cells treated with varying 20-O-ingenolEZ or ICRF-193 concentrations was expressed relative to the level in untreated cells (100%). The expressions were assessed in triplicate and the data were shown as the means ± SD. (A) Effect of varying 20-O-ingenolEZ concentrations on mutant cells; (B) effect of 200 μM of 20-O-ingenolEZ and ICRF-193 on mutant and wild type cells. | ||
Since the cellular proliferation of WRN-/-DT40 cells was not inhibited by 20-O-ingenolEZ, the phosphorylation of H2AX and apoptosis were observed using BLM-/-cells to study the mechanism of anti-proliferation.22γ-H2AX was visualized as a band stained with anti-γ-H2AX in the nuclei of 20-O-ingenolEZ-treated BLM-/-cells (Fig. 2, lane 2). We observed the induction of DSBs in 20-O-ingenolEZ treated BLM-/-cells similar to the effects of adriamycin (Fig. 2, lane 3). The morphological characteristics of the apoptotic cells in the topoisomerase inhibitor-treated sample were determined based on staining with 4,6-diamino-2-phenyl indole (DAPI). After 24 hours of treatment with 0.9 μM of adriamycin, the BLM-/-cells were stained with DAPI (Fig. 3). Apoptosis was induced in the cells after 24 h of continuous treatment with adriamycin (Fig. 3A). The apoptosis of about 60% of the cells was induced (Fig. 3B). 20-O-IngenolEZ-treated BLM-/-cells also exhibited the morphological characteristics of apoptosis, similar to the effects of adriamycin (Fig. 3A and B). Although catalytic inhibitors can be distinguished from the other class of agents that act by stabilizing the covalent complex, several reports have suggested that ICRF-193 can also induce DNA damage,12–14,19 similar to 20-O-ingenolEZ. We demonstrated that 20-O-ingenolEZ was a novel anti-proliferative agent that induces DNA DSBs in BLM-/-cells. Further biological experimentation using 20-O-ingenolEZ may provide insight into damage response pathways involving BLM in DNA damage recognition and the arrest of the cell cycle or topoisomerase II inhibitor-induced apoptosis in addition to the useful information for the development of potential anti-cancer agents.
 | ||
| Fig. 2 Influence of 20-O-ingenolEZ on the phosphorylation of H2AX in BLM-/-DT40 cells. For the immunoblotting of γ-H2AX in the BLM-/-DT40 cells, the cells were cultured in the presence of 200 μM of 20-O-ingenolEZ and 0.9 μM of adriamycin or a control for 24 h. The nuclear protein fraction (20 μg) was resolved using SDS-PAGE followed by western-blot analysis and chemiluminescence detection. γ-H2AX was detected using a specific antibody against γ-H2AX. Lane 1, control; lane 2, 20-O-ingenolEZ; lane 3, adriamycin. | ||
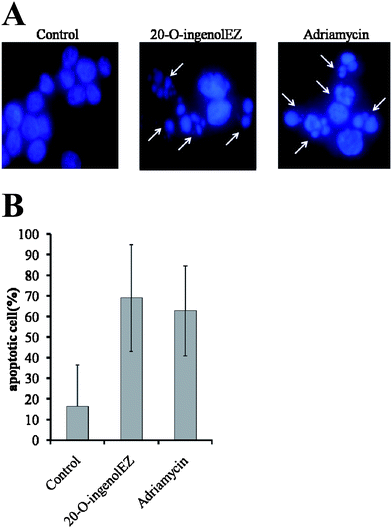 | ||
| Fig. 3 Effects of 20-O-ingenolEZ on BLM-/-DT40 cell apoptosis. BLM-/-DT40 cells were treated at 37 °C with 200 μM of 20-O-ingenolEZ and 0.9 μM of adriamycin or a control for 24 h. Apoptosis of the cells was detected by DAPI staining, and the arrows in the photograph indicate examples of the apoptotic cells after 24 h treatment (Fig. 3A). The percentage of apoptotic BLM-/-cells at each concentration of 20-O-ingenolEZ, adriamycin and the control treatment is shown (Fig. 3B). | ||
Conclusions
In the present study, the anti-proliferative effects of the catalytic topoisomerase inhibitor 20-O-ingenolEZ were studied using cells containing mutations in the BLM or WRN helicase genes and wild-type cells. 20-O-IngenolEZ, which induced the inhibition of the ATPase activity of topoisomerase II in MMT cells, did not inhibit the proliferation of WRN-/-DT40 but showed a greater sensitivity for the inhibition of the proliferation of BLM-/-DT40 cells and induced DNA DSBs in the BLM-/-DT40 cells. The inhibition of the proliferation of BLM-/-DT40 cells by 20-O-ingenolEZ was more sensitive than the inhibition of wild-type DT40 cellular proliferation. Thus, 20-O-ingenolEZ appears to be a potential anti-proliferative agent against specific cell types, and more studies using cells from a BS patient may provide useful information for the development of potential anti-cancer agents targeting BS.Acknowledgements
This investigation was supported in part by a grant from Nihon University to S. Miyata.Notes and references
- J. C. Wang, Nat. Rev. Mol. Cell Biol., 2002, 6, 430–440 CrossRef.
- S. J. Froelich-Ammon and N. Osheroff, J. Biol. Chem., 1995, 270, 21429–21432 CrossRef CAS.
- D. A. Burden and N. Osheroff, Biochim. Biophys. Acta, 1998, 1400, 139–154 CAS.
- T. Andoh and R. Ishida, Biochim. Biophys. Acta, 1998, 1400, 155–171 CAS.
- D. A. Skoufias, F. B. Lacroix, P. R. Andreassen, L. Wilson and R. L. Margolis, Mol. Cell, 2004, 15, 977–990 CrossRef CAS.
- L. Y. Wang, N. L. Wang, X. S. Yao, S. Miyata and S. Kitanaka, J. Nat. Prod., 2002, 65, 1246–1251 CrossRef CAS.
- S. Miyata, L. Y. Wang, C. Yoshida and S. Kitanaka, Bioorg. Med. Chem., 2006, 14, 2048–2051 CrossRef CAS.
- C. Yoshida, K. Hishiyama, K. Miyazaki, M. Watanabe, M. Kanbe, Y. Yamada, K. Matsuzaki, K. Miyashita, S. Kitanaka and S. Miyata, Cancer Sci., 2010, 101, 374–378 CrossRef CAS.
- P. B. Deming, C. A. Cistulli, H. Zhao, P. R. Graves, H. Piwnica-Worms, R. S. Paules, C. S. Downes and W. K. Kaufmann, Proc. Natl. Acad. Sci. U. S. A., 2001, 21, 12044–12049 CrossRef.
- D. J. Clarke, R. T. Johnson and C. S. Downes, J. Cell Sci., 1993, 105, 563–569 CAS.
- C. S. Downes, D. J. Clarke, A. M. Mullinger, J. F. Giménez-Abián, A. M. Creighton and R. T. Johnson, Nature, 1994, 6505, 467–470 CrossRef.
- I. Park and H. K. Avraham, Exp. Cell Res., 2006, 312, 1996–2008 CrossRef CAS.
- H. M. Robinson, S. Bratlie-Thoresen, R. Brown and D. A. Gillespie, Cell Cycle, 2007, 15, 1265–1267 CrossRef.
- T. Nakagawa, Y. Hayashita, K. Maeno, A. Masuda, N. Sugito, H. Osada, K. Yanagisawa, H. Ebi, K. Shimokata and T. Takahashi, Cancer Res., 2004, 64, 4826–4832 CrossRef CAS.
- G. Villani and N. Tanguy Le Gac, J. Biol. Chem., 2000, 275, 33185–33188 CrossRef CAS.
- K. Hanada, T. Ukita, Y. Kohno, K. Saito, J. Kato and H. Ikeda, Proc. Natl. Acad. Sci. U. S. A., 1997, 94, 3860–3865 CrossRef CAS.
- F. G. Harmon and S. C. Kowalczykowski, Genes Dev., 1998, 12, 1134–1144 CrossRef CAS.
- O. Bischof, S. H. Kim, J. Irving, S. Beresten, N. A. Ellis and J. Campisi, J. Cell Biol., 2001, 153, 367–380 CrossRef CAS.
- A. Franchitto, J. Oshima and P. Pichierri, Cancer Res., 2003, 63, 3289–3295 CAS.
- T. Marple, T. M. Kim and P. Hasty, Mutat. Res., 2006, 602, 110–120 CrossRef CAS.
- M. C. Alley, D. A. Scudiero, A. Monks, M. L. Hursey, M. J. Czerwinski, D. L. Fine, B. J. Abbott, J. G. Mayo, R. H. Shoemaker and M. R. Boyd, Cancer Res., 1988, 48, 589–601 CAS.
- E. P. Rogakou, D. R. Pilch, A. H. Orr, V. S. Ivanova and W. M. Bonner, J. Biol. Chem., 1998, 273, 5858–5868 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
