Water soluble flavonol prodrugs that protect against ischaemia-reperfusion injury in rat hindlimb and sheep heart†
Spencer J.
Williams
*a,
Colleen J.
Thomas
be,
Mirna
Boujaoude
c,
Carlie T.
Gannon
a,
Shannon D.
Zanatta
a,
Bevyn
Jarrott
b,
Clive N.
May
*b and
Owen L.
Woodman
*cd
aSchool of Chemistry and Bio21 Molecular Science and Biotechnology Institute, University of Melbourne, 30 Flemington Rd, Parkville, 3010, Australia. E-mail: sjwill@unimelb.edu.au; Fax: +61 3 9347 5180; Tel: +61 3 8344 2422
bHoward Florey Institute, University of Melbourne, Parkville, Victoria 3010, Australia. E-mail: clive.may@florey.edu.au
cDepartment of Pharmacology, University of Melbourne, Parkville, Victoria 3010
dSchool of Medical Sciences, RMIT University, Bundoora, Victoria 3083, Australia. E-mail: owen.woodman@rmit.edu.au
eDepartment of Human Biosciences, Faculty of Health Science, La Trobe University, Bundoora, 3086, Australia
First published on 1st March 2011
Abstract
Phosphate and hemiadipate derivatives of cardioprotective flavonols are water soluble prodrugs that release superoxide scavenging and vasorelaxant parent drugs. These prodrugs protected against ischaemia/reperfusion injury in two distinct animal models, rat hindlimb and sheep heart, and are thus promising small molecule therapeutics for cardiovascular disease.
Introduction
Coronary artery disease leading to acute myocardial infarction is a leading cause of morbidity and mortality worldwide. The primary treatment is to restore blood flow using angioplasty (percutaneous coronary intervention) and drug-eluting stents or thrombolytics to remove the blockage, but the resulting reperfusion causes cellular dysfunction, impaired reflow, and myocyte apoptosis and necrosis.1 Reperfusion injury extends the size of the infarct beyond that present at the end of ischaemia, contributing to long-lasting consequences including ventricular remodeling and a progressive decline in cardiac function, ultimately resulting in heart failure.1,2 At present there are no treatments in clinical use that specifically address myocardial reperfusion injury.A major cause of reperfusion injury is the production of high levels of reactive oxygen species (ROS), at concentrations that overwhelm the endogenous antioxidant defense systems.3 Sources of ROS include mitochondria, NADPH oxidase in invading leukocytes, myeloperoxidase from neutrophils,4 and xanthine oxidase in coronary vascular endothelial cells.1,3 Increased levels of ROS lead to damage to DNA, proteins and lipids, and reaction of superoxide anion (O2−˙) and nitric oxide (NO) to form peroxynitrite (ONOO−), a reactive nitrogen species. Reduction in NO levels leads to impairment of vascular function thereby promoting thrombosis, and peroxynitrite is itself toxic and causes vascular damage and stimulates apoptosis.5 Consequently, antioxidant therapies have been highlighted as a promising approach for the treatment of cardiovascular disease.6
The Zutphen elderly study examined the relationship between dietary flavonoid intake and the risk of coronary artery disease in 805 men aged 65–84 years and showed a significant inverse relation of flavonoid intake and coronary heart disease.7 Structure–activity studies of flavonols have highlighted the importance of B-ring hydroxylation in promoting beneficial cardiovascular actions8,9 and both 4′-hydroxyflavonol 2 and 3′,4′-dihydroxyflavonol 3 (DiOHF) (Fig. 1) have been identified as promising flavonols for the treatment of cardiovascular disease.9 Treatment with DiOHF effectively reduced tissue death (infarct size) after ischaemia and reperfusion in the heart in both conscious and anaesthetized sheep10,11 and goats,12 and in the brain in rats.13 The reduction in infarct size was evident after 1 to 4 weeks of reperfusion, and this was accompanied by preservation of coronary endothelial function and improved reperfusion of the microvasculature. In sheep heart, DiOHF afforded a level of protection similar to that achieved by ischaemic preconditioning,10 currently the most effective method to reduce cardiac reperfusion injury. However, the poor aqueous solubility of DiOHF and related flavonols has required their administration in non-approved solvents such as DMSO, which prevents their further therapeutic development.
We now report the design and synthesis of prodrug derivatives of the most promising flavonols, bearing phosphate or carboxylic acid carrier groups to ensure good water solubility (Fig. 1). These compounds were assessed for in vitro vasorelaxant and superoxide scavenging capacity using isolated rat thoracic aorta. The most promising compounds were evaluated for their ability to protect against ischaemia/reperfusion injury in two animal models, rat hindlimb and sheep heart.
Results and discussion
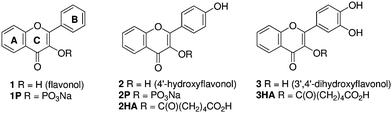
As a 3-hydroxy group is common to the most promising vasoactive flavonols, this represented a promising site for modification to introduce a solubilizing carrier group. We targeted phosphate esters 1P and 2P and carboxylate esters 2HA and 3HA as these functional groups are commonly used in prodrug strategies owing to the widespread occurrence of promiscuous phosphatases and esterases in tissues and blood.14Flavonol 1 was converted to the protected dibenzyl phosphate ester 4 in two steps by treatment with dibenzyl N,N-diisopropylphosphoramidite and 1H-tetrazole, followed by oxidation with mCPBA (Scheme 1). Deprotection to afford 1P was achieved by hydrogenolysis in the presence of palladium on carbon, followed by conversion to the disodium salt using aqueous sodium hydroxide. Phosphate 2P was prepared using a similar approach from 4′-benzyloxyflavonol15 by phosphorylation under identical conditions. Deprotection of the intermediate protected phosphate ester 5 was best achieved by hydrogenolysis in the presence of palladium hydroxide, and conversion to the disodium salt through the intermediacy of the triethylammonium salt followed by ion exchange. In this protocol the use of the weak base triethylamine prevents unwanted deprotonation of the phenol and ensures that only the disodium salt is formed.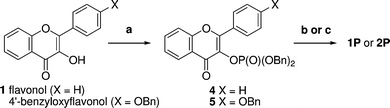 | ||
| Scheme 1 Synthesis of phosphate prodrugs. Reagents and yields: (a) (i) Pri2NP(OBn)2, 1H-tetrazole, CH2Cl2; (ii) mCPBA, 84% for 4, 58% for 5; (b) for 1P: (i) H2, Pd–C, EtOH–H2O; (ii) aq NaOH, 87%; (c) for 2P: (i) H2, Pd(OH)2, THF–H2O (ii) Et3N then Dowex 50 (Na+ form), 36%. | ||
The hemiadipates 2HA and 3HA were prepared in two steps from 4′-benzyloxyflavonol and 3′,4′-dibenzyoxyflavonol (prepared in turn by sequential Claisen-Schmidt condensation and Algar-Flynn-Oyamada reaction of 3,4-dibenzyloxybenzaldehyde and 2-hydroxyacetophenone15) by esterification with monobenzyl adipate using EDC/DMAP to afford benzyl esters 6 and 7, respectively (Scheme 2). Hydrogenolysis using palladium hydroxide furnished the hemiadipates 2HA and 3HA.
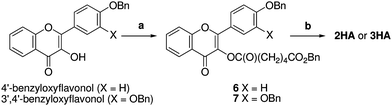 | ||
| Scheme 2 Synthesis of hemiadipate prodrugs. Reagents and conditions: (a) EDC, DMAP, CH2Cl2, 55% for 6, 88% for 7; (b) H2, Pd(OH)2, THF–EtOH–AcOH, 55% for 2HA, 56% for 3HA. | ||
In support of the use of these solubilizing groups to generate prodrugs with improved water solubility, the aqueous solubility of the flavonol phosphates 1P and 2P relative to the parent flavonols was enhanced from <1 mg ml−1 to >200 mg ml−1, and for the flavonol hemiadipates 2HA and 3HA these could be dissolved in an equimolar amount of aqueous sodium carbonate to >50 mg ml−1. Evidence for their facile conversion to the parent drugs was obtained by the intravenous administration of 3HA to rats whereupon the parent 3 could be detected within 2 min of administration upon analysis of isolated blood plasma by LCMS/MS (see Supporting Information).
To model enzymatic processes occuring within the body, we studied the biological activities of the flavonol prodrugsin vitro in the presence of butyrylcholinesterase or phosphatase. It has been shown that the vasorelaxant activity of DiOHF derives, in part, from inhibition of the Rho/RhoA kinase pathway.16 The vasorelaxant activity of the flavonols was determined by cumulative concentration-response curves to Ca2+ in rat isolated thoracic aortic ring segments. Table 1 and Fig. 2 show the effect of varying the concentrations of 1, 1P, 2, 2HA, 2P, 3, and 3HA. Flavonols 1, 2 and 3, bearing a 3-hydroxy group, impaired the response to Ca2+ after a 20 min incubation period (Table 1). The ester-modified prodrugs 2HA and 3HA had no effect on Ca2+-induced contraction in the absence of butyrylcholinesterase. When pre-incubated with butyrylcholinesterase, an enzyme that can cleave the ester side-chain and release the parent flavonol, the activities of 2HA and 3HA were unveiled. Similarly, the phosphate-modified prodrugs 1P and 2P were inactive, but treatment with phosphatase resulted in a concentration-dependent inhibition of Ca2+ contraction. In each case the maximum response for the flavonol prodrugs, after enzyme treatment was similar to that seen for the parent flavonols.
| Calcium inhibition | Superoxide inhibition (%) | ||||
|---|---|---|---|---|---|
| n | pIC50 | R max | n | R max | |
| a Vasorelaxant and superoxide scavenging activities were assayed in the presence of alkaline phosphatase or butyrylcholinesterase. b Prodrugs were pre-treated with butyrylcholinesterase for 1 h prior to measuring vasorelaxant and scavenging activities. | |||||
| 1 | 4 | 5.37 ± 0.57 | 19 ± 1 | 4 | 20 ± 1 |
| 1P a | 5 | 5.33 ± 0.08 | 40 ± 3 | 4 | 36 ± 2 |
| 2 | 5 | 4.93 ± 0.09 | 70 ± 11 | 4 | 62 ± 7 |
| 2HA a , b | 4 | 4.45 ± 0.08 | 70 ± 2 | 4 | 30 ± 15 |
| 2P a | 5 | 5.40 ± 0.08 | 63 ± 10 | 4 | 27 ± 2 |
| 3 | 5 | 5.02 ± 0.26 | 89 ± 3 | 4 | 84 ± 1 |
| 3HA a , b | 5 | 4.47 ± 0.09 | 76 ± 4 | 4 | 70 ± 9 |
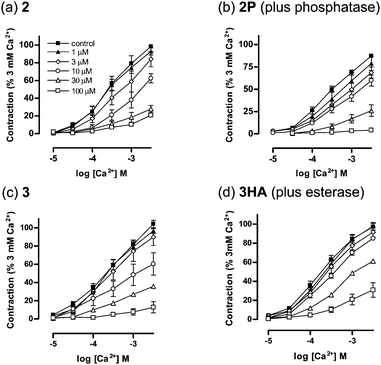 | ||
| Fig. 2 Concentration response curves to Ca2+ in the presence of vehicle (0.1% DMSO) or increasing concentrations of parent flavonols (a) 2 and (c) 3, and prodrugs (b) 2P and (d) 3HA. Responses of rat isolated thoracic aorta to 2P and 3HA were examined in the presence of phosphatase (1 U/mL) and butyrylcholinesterase (1 U/mL), respectively. The contractions are expressed as a percentage of the original response to Ca2+ (3 mM). All values are presented as the mean ± s.e.m (n = 4–5). | ||
Superoxide scavenging activity was assessed by quantifying the ability of compounds to reduce superoxide concentrations formed from aortic tissue in the presence of NADPH by lucigenin-enhanced chemiluminescence.17 All flavonols caused significant reductions in the concentration of superoxide anion (Fig. 3, Table 1). Both 3HA and 2HA were ineffective as superoxide scavengers unless pre-treated with butyrylcholinesterase. Similarly, 1P and 2P were only effective superoxide scavengers when pre-treated with alkaline phosphatase. Control experiments with butyrylcholinesterase or phosphatase alone showed they had no effect upon the level of superoxide anions detected in the presence of NADPH. For 2P and 3HA, upon treatment with phosphatase or butyrylcholinesterase, respectively, the potency and Rmax values were comparable to that found for the corresponding parent flavonols 2 and 3. Together with the ability to inhibit Ca2+-induced contraction, these data demonstrate the potential for the flavonol prodrugs to release the parent drugin vivo.
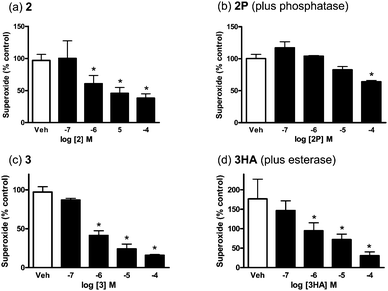 | ||
| Fig. 3 Effect of 2 (n = 5), 2P (n = 3), 3 (n = 5), and 3HA (n = 3) on the level of superoxide anions generated by rat aortic rings in the presence of NADPH. Data are expressed as a percentage of the control, i.e. in the absence of the drug or vehicle (DMSO or Na2CO3). All values are presented as the mean ± s.e.m, * P < 0.05. | ||
We next evaluated the ability of the most promising compounds, 2P and 3HA, to reduce injury following experimental ischaemia-reperfusion in rat hindlimb. Rats were anaesthetized and each hindlimb was occluded with a tourniquet for 2 h, the tourniquet was removed and the hindlimbs were reperfused for 4 h. Skeletal muscle injury was assessed by measuring blood levels of lactate dehydrogenase (LDH). Bilateral hindlimb ischaemia/reperfusion resulted in elevation of circulating levels of LDH after 1, 2, 3 and 4 h of reperfusion (Fig. 4). Administration of 3HA, 5 min prior to reperfusion, prevented any increase in LDH above the level in the sham group. Administration of 2P reduced LDH, in this case to levels below that of sham-treated controls (Fig. 4).
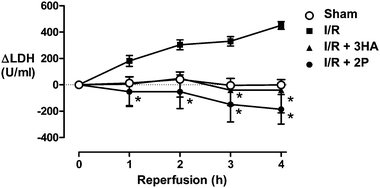 | ||
| Fig. 4 Rats were subjected to bilateral occlusion of the hindlimbs for 2 h followed by 4 h reperfusion. LDH is presented as change from levels observed before ischaemia. LDH levels were measured in sham-operated animals to determine baseline levels. Data are means ± s.e.m (n = 4–5), * P < 0.05 compared to ischaemia and reperfusion, two-way ANOVA followed by Bonferroni's multiple comparison test. | ||
In order to assess the ability of flavonols and flavonol prodrugs to provide cardioprotection, anaesthetized adult female merino sheep were subjected to surgically-induced myocardial ischaemia/reperfusion. The second diagonal (D2) branch of the left anterior descending coronary artery was occluded for 1 h, followed by 3 h of reperfusion. Vehicle or flavonol 3 (5 mg kg−1 iv) was delivered by infusion into the jugular vein over 20 min (beginning after 30 min ischaemia and finishing at 50 min ischaemia, 10 min prior to reperfusion) and flavonol prodrugs 3HA or 2P (at equivalent molar doses) were delivered as bolus intravenous injections 10 min prior to reperfusion. The area of myocardium at risk and infarct size were delineated by Evans blue and triphenyltetrazolium chloride staining as previously described10 (Fig. 5A). The infarct size, as a percentage of the area at risk, was 80 ± 3% after vehicle treatment and was significantly reduced to 50 ± 4% by treatment with 3 (P < 0.05) (Fig. 5B). The hemiadipate prodrug, 3HA, caused a similar reduction in infarct size to 3 following ischaemia-reperfusion (Fig. 5B). The phosphate prodrug, 2P, was less effective, but still produced a significant reduction in infarct size (Fig. 5B).
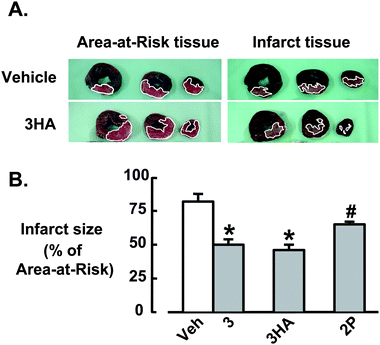 | ||
| Fig. 5 Adult female merino sheep (39–58 kg, 2–3 years old, n = 5–6 per group) were subjected to 1 h occlusion of the 2nd branch of the left anterior descending coronary artery followed by 3 h reperfusion. Vehicle or 3 (5 mg kg−1 iv) was delivered by infusion over 20 min (beginning after 30 min ischaemia and finishing at 50 min ischaemia, 10 min prior to reperfusion). Flavonol prodrugs 2P or 3HA (at equivalent molar doses to 3) were delivered as bolus injections 10 min before reperfusion. Both ischaemic area (area at risk (AAR)) and infarct size were then determined. (A) Representative sheep left ventricle (LV) rings (first 3 of 6 rings from the apex) showing the ability of 3HA to reduce infarct size (lower right panels) relative to the area of myocardium at risk (lower left panels), compared with vehicle treatment (upper panels). The white outlines have been included to improve visualization of AAR and infarct zones in the LV rings. (B) Infarct size as a % of AAR determined 3 h after reperfusion. Data are mean ± s.e.m (n = 5–6 per group); * differs from vehicle by P < 0.05 but not from one another, # differs from vehicle and 3 and 3HA. | ||
To further assess cardiac injury we measured LDH levels in 3HA- and vehicle-treated sheep. All animals had similar baseline LDH levels (280 ± 36 U/L). 3HA treatment resulted in a significantly smaller increase from baseline compared to vehicle-treated sheep after 3 h reperfusion (70 ± 20 U/L versus 152 ± 50 U/L, respectively; P < 0.05). This compares favorably with previous studies in sheep, which showed that after 2 h reperfusion the parent drug 3 reduced the rise in LDH by around one third compared to vehicle treatment (604 ± 58 for vehicle treated versus 405 ± 45 U/L for 3 treated).10 Neither 3, nor prodrugs 3HA and 2P, significantly changed mean arterial pressure or heart rate from resting levels following ischemia and reperfusion compared to vehicle treatment (data not shown).
Conclusions
Most agents that can limit myocardial infarct size in animals have failed to realize any demonstrable benefit when assessed in clinical trials. However, the unsuccessful candidates typically showed only limited pre-clinical efficacy and were often examined only in rodent models.18 The causes for this lack of translation have been discussed,18 and it is widely agreed that it remains an achievable and desirable goal. Various hydroxylated flavonols have been shown to possess a range of promising vascular activities that could contribute to the maintenance of cardiovascular function and have been shown to provide useful cardioprotection and limit post-infarct left ventricular remodelling.10,12 By demonstrating the ability of these flavonol prodrugs to protect against ischaemia/reperfusion injury in both rat hindlimb and sheep heart this work makes a further significant step towards their clinical application by allowing their intravenous or intra-coronary injection concurrent with interventions to reperfuse the ischaemic myocardium. Further studies investigating the timing of administration and the optimization of dosing will be reported in due course. It is noteworthy that post-stroke treatment with the parent flavonol 3 in a rat model of focal stroke has been shown to improve recovery13 and so there is significant potential for the use of these flavonol prodrugs, in particular 3HA, in stroke therapy.Acknowledgements
We acknowledge support from NeuProtect Pty Ltd, the National Heart Foundation of Australia (G03M1072 and G08M3761), the Australian Research Council, and NHMRC Research Fellowships to CNM (350328 and 566819). We thank Tony Dornom and Alan McDonald for expert assistance with the sheep studies. OLW, CNM, and BJ hold financial interests in NeuProtect Pty Ltd.Notes and references
- D. M. Yellon and D. J. Hausenloy, N. Engl. J. Med., 2007, 357, 1121–1135 CrossRef CAS.
- R. J. Burns, R. J. Gibbons, Q. Yi, R. S. Roberts, T. D. Miller, G. L. Schaer, J. L. Anderson and S. Yusuf, J. Am. Coll. Cardiol., 2002, 39, 30–36 CrossRef.
- J. L. Zweier and M. A. Talukder, Cardiovasc. Res., 2006, 70, 181–190 CrossRef CAS.
- S. J. Klebanoff, J. Leukocyte Biol., 2005, 77, 598–625 Search PubMed.
- P. Pacher, J. S. Beckman and L. Liaudet, Physiol. Rev., 2007, 87, 315–424 CrossRef CAS.
- U. Forstermann, Nat. Clin. Pract. Cardiovasc. Med., 2008, 5, 338–349 Search PubMed.
- (a) M. G. Hertog, E. J. Feskens, P. C. Hollman, M. B. Katan and D. Kromhout, Lancet, 1993, 342, 1007–1011 CrossRef CAS; (b) M. G. Hertog, E. J. Feskens and D. Kromhout, Lancet, 1997, 349, 699 CrossRef CAS.
- (a) E. C. Chan, P. Pannangpetch and O. L. Woodman, J. Cardiovasc. Pharmacol., 2000, 35, 326–333 CrossRef CAS; (b) C. X. Qin, X. Chen, R. A. Hughes, S. J. Williams and O. L. Woodman, J. Med. Chem., 2008, 51, 1874–1884 CrossRef CAS; (c) S. Yap, K. J. Loft, O. L. Woodman and S. J. Williams, ChemMedChem, 2008, 3, 1572–1579 CrossRef CAS.
- O. L. Woodman, W. F. Meeker and M. Boujaoude, J. Cardiovasc. Pharmacol., 2005, 46, 302–309 CrossRef CAS.
- S. Wang, G. J. Dusting, C. N. May and O. L. Woodman, Br. J. Pharmacol., 2004, 142, 443–452 CrossRef CAS.
- S. Wang, C. J. Thomas, G. J. Dusting, O. L. Woodman and C. N. May, Eur. J. Pharmacol., 2009, 624, 31–37 CrossRef CAS.
- S. Wang, K. Fei, Y. W. Xu, L. X. Wang and Y. Q. Chen, Chin. Med. J., 2009, 122, 61–67 Search PubMed.
- C. L. Roulston, J. K. Callaway, B. Jarrott, O. L. Woodman and G. J. Dusting, Eur. J. Pharmacol., 2008, 584, 100–110 CrossRef CAS.
- C. G. Wermuth, in The practice of medicinal chemistry, ed. C. G. Wermuth, Elsevier, London, UK, 2003, pp. 617–630 Search PubMed.
- F. A. van Acker, J. A. Hageman, G. R. Haenen, W. J. van Der Vijgh, A. Bast and W. M. Menge, J. Med. Chem., 2000, 43, 3752–3760 CrossRef CAS.
- M. J. Song, I. Baek, M. Seo, S.-H. Kim, K. Suk, O. L. Woodman, S. J. Williams and I.-K. Kim, Clin. Exp. Pharmacol. Physiol., 2010, 37, 803–810 CAS.
- M. P. Skatchkov, D. Sperling, U. Hink, A. Mulsch, D. G. Harrison, I. Sindermann, T. Meinertz and T. Munzel, Biochem. Biophys. Res. Commun., 1999, 254, 319–324 CrossRef CAS.
- (a) J. M. Downey and M. V. Cohen, Circ. J., 2009, 73, 1171–1177 CrossRef; (b) A. Prasad, G. W. Stone, D. R. Holmes and B. Gersh, Circulation, 2009, 120, 2105–2112 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details. See DOI: 10.1039/c0md00240b |
| This journal is © The Royal Society of Chemistry 2011 |