Binding of 9-O-(ω-amino) alkyl ether analogues of the plant alkaloid berberine to poly(A): insights into self-structure induction
Md. Maidul
Islam†
,
Anirban
Basu
and
Gopinatha
Suresh Kumar
*
Biophysical Chemistry Laboratory, Indian Institute of Chemical Biology, CSIR, 4, Raja S.C. Mullick Road, Kolkata, 700 032, India. E-mail: gskumar@iicb.res.in; gsk.iicb@gmail.com; Fax: +91 33 2472 3967; Tel: +91 33 2472 4049/2499 5723
First published on 13th May 2011
Abstract
Berberine, the plant alkaloid, was recently shown to induce self-structure in poly(A) by a unique dilution technique. The interaction of berberine and five 9-(ω-amino) alkyl ether analogues with polyadenylic acid [poly (A)] has been studied to understand the role of an additional anchoring module in the formation and/or amplification of self-structure in poly(A). The binding was characterized by absorption and fluorescence titration, Job's plot and isothermal titration calorimetry. All the molecules bound cooperatively to poly(A). Self-structure formation was confirmed by circular dichroic melting, optical melting, and dilution experiments. Energetics of the interaction revealed that as the alkyl chain length increased, the binding was more entropy dominated. The results showed that berberine and all the analogues induced self-structure formation in poly(A). The length of the alkyl chain had a significant influence on the ease of formation of self-structure. New insights in terms of structural and thermodynamic aspects into self-structure formation in poly(A) by berberine analogues are revealed from these studies.
RNA molecules are now known to be involved in several critical life processes occurring in the cell. The role of RNA in the progression of many diseases, particularly viral infections, has led to a growing interest in exploiting RNA as a cellular target for therapeutic drug targeting. The recent discovery of a number of microRNAs and the emerging knowledge of their functions further reiterated the crucial roles played by RNA in cell biology.1,2 Consequently, there is a paradigm shift in interest from DNA binding molecules to RNA targeted molecules for modulating the genomic activity through RNA targeting. Compared to the vast literature on the DNA binding of small molecules, RNA interaction studies are scarce and quantitative elucidations of the binding aspects are of remarkable current interest. RNA molecules have diverse and complex structures compared to the relatively simple double helical DNA structure. All eukaryotic messenger RNAs have a long poly(A) tail at the 3′-end that is attached during the post-transcriptional modification. The addition of adenines as poly(A) tail serves to stabilize the mRNA, prevents its degradation and influences translation. Polyadenylation is functionally linked to mRNA molecules and enhances their splicing. Molecules that can bind to and modulate the polyadenylate tail may stop poly(A) chain elongation, inhibit mRNA function and degrade it, and arrest subsequent protein production in the cell.3,4 It is known that certain viruses target factors for cleavage that either bind the poly(A) tail of mRNA or help connect the 3′-end of poly(A) with the 5′-guanosine cap of mRNA. Neo PAP, a human PAP that catalyzes the post-transcriptional polyadenylation process, has been found to be significantly overexpressed in human cancer cells in comparison to its expression in normal or virally transformed cells. This relates the polyadenylation process to neoplasms5,6 and the presence of enhanced levels of poly(A) tailed mRNA in such cells. Therefore, controlling such overexpression of poly(A) by the binding of small molecules could be a new type of RNA based therapeutic intervention.
Studies on the interaction of small molecules with poly(A) have been reported in the last several years.7–20 But a highly specific binding leading to structural rearrangement was observed for the first time with isoquinoline alkaloids.9,11–15 Xing and coworkers12 showed that the synthetic isoquinoline compound coralyne binds to single stranded poly(A) generating a self-structure involving adenine–adenine base pairing. The benzophenanthridine alkaloid sanguinarine was the first natural product shown to induce self-structure with an intercalative binding geometry.15 More recently europium–L-valine complex and single walled carbon nanotubes have also been shown to induce self-structure in poly(A).21,22 Our laboratory studied the formation of such a secondary structure in poly(A) by a variety of DNA binding molecules using spectroscopic and calorimetric techniques.14–16,18–20 Optical and circular dichroic melting measurements and differential scanning calorimetry were effectively used for detecting self-structure formation. Although coralyne and sanguinarine induced self-structure, the most prominent and widely distributed parent isoquinoline alkaloid berberine (Fig. 1) reportedly failed to induce such structural organization in single stranded poly(A) as inferred from absorption, circular dichroism and differential scanning calorimetry studies. However, employing a dilution technique, Hud's laboratory16 reported that berberine also induced self-structure in poly(A). Because of our long term interest in making quantitative evaluations of specific structural elements of isoquinoline alkaloids in binding to DNA and RNA structures,9–11,13–15,17–20 we planned to effect some modifications in the berberine molecule by introducing a side chain at the 9-position to give a series of 9-(ω-amino) alkyl ether analogues (Fig. 1 and Table 1) that may allow additional contact points for multifunctional interactions with nucleic acids. The significance of 9-substituents on berberine on topoisomerase II interaction is documented in the literature from a comparative study of 9-ethoxycarbonyl berberine, 9-N,N-dimethylcarbamoyl berberine and 12-bromo-berberine (methoxy group at 9-position).23 It was suggested that 12-bromo-berberine was a stronger DNA binder but a weaker enzyme poison compared to berberine both in vitro and in cell-based assays. The results revealed that the proposed drug domain for DNA intercalation is not a major determinant of enzyme inhibition for simple berberine analogues. Rather, the 9-substituent within the domain has a major influence, presumably by facilitating drug interaction with enzyme and/or enzyme–DNA complexes. Due to these revelations various derivatives including those at 9-position were prepared as chemically modified compounds in order to enhance the DNA binding affinities and pharmacological activities.24–30 The recent report29 on the enhanced DNA binding of 9-O-(ω-amino) ethyl substituted berberines compared to other substituents encouraged us to systematically investigate the length of the alkyl chain on nucleic acid binding. It was also observed recently that nanoconjugates of 9-(ω-amino) alkyl berberines were more effective in anti-proliferative activity31 and showed remarkably higher DNA binding affinity compared to the parent berberine.32 In this report, we systematically investigated the ability of five 9-(ω-amino) alkyl berberine analogues (Fig. 1) with alkyl chain length from two to six to induce self-structure in single stranded poly(A) to understand the role of the structural features in small molecules that may be responsible for such self-structure induction.
 | ||
| Fig. 1 Chemical structures of berberine and 9-O-(ω-amino) alkyl ether analogues of berberine investigated in this study. | ||
| Abbreviation of the analogue | Alkyl amino chain |
|---|---|
| BER (berberine) | –CH3 |
| BER1 | –(CH2)2NH2 |
| BER2 | –(CH2)3NH2 |
| BER3 | –(CH2)4NH2 |
| BER4 | –(CH2)5NH2 |
| BER5 | –(CH2)6NH2 |
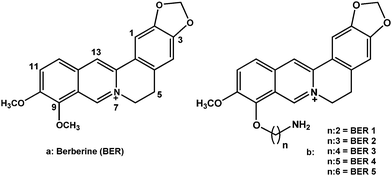
The absorption spectra of all the five berberine analogues were similar to that of berberine with four absorbance maxima centered around 228, 264, 344 and 421 nm, respectively. Absorption spectroscopy is one of the most convenient tools to investigate the interaction between drugs and nucleic acids. The binding to poly(A) was thus studied from absorption spectral titration following changes in the peaks of the alkaloids in the 300–550 nm region.33–36 Poly(A) does not have any absorption in this region. All the analogues exhibited hypochromic changes and bathochromic effects on binding to poly(A) with three well-resolved isosbestic points. The spectral changes in the absorption spectrum of berberine were similar to those reported earlier.9,11 Representative absorption spectral changes in BER and BER5 (BER1 to 5 represent analogues with two to six CH2 groups at the 9-position) are presented in Fig. 2. Such absorbance titrations in each case were used to determine the isosbestic point and the molar extinction coefficient of the fully bound alkaloid analogues. For elucidation of the binding parameters, absorption spectral study was performed by titrating a constant concentration of poly(A) with increasing concentration of each analogue. The data from these titrations were then converted to Scatchard plots of r/Cfversus r. Representative Scatchard plots for BER and BER5 are presented in the inset of Fig. 2. Cooperative binding isotherms evidenced by positive slope at low values of the bound alkaloid (r) were observed in all the cases. Subsequent analysis of Scatchard plots was done by McGhee–von Hippel equation37 to yield binding constants (Ki), the number of occluded binding sites (n), and cooperativity factors (ω). These values for BER and the five analogues are presented in Table 2. It can be seen that the binding affinity increased as the chain length increased. The apparent binding constant (Kiω), which is a product of the cooperative binding affinity and the cooperative factor, gave values of the order of 106 M−1 for the binding of the analogues to poly(A) (Table 2). The affinities of the analogues were several folds higher than that of BER; for BER5 it was about twenty times that of BER.
 | ||
| Fig. 2 Representative absorbance spectra of free (curve 1) and poly(A) bound (curve 2) (a) BER and (b) BER5. Inset: respective cooperative Scatchard plots of binding. | ||
| Alkaloid | Absorbance | Fluorescence | Dilution protocolb | ||||
|---|---|---|---|---|---|---|---|
| K i × 10−4 | ω | K i × ω × 10−6 | K i × 10−4 | ω | K i × ω × 10−6 | K i × 10−5 | |
| a Average of four determinations. Cooperative binding constants (Ki), citrate–phosphate buffer of 10 mM [Na+], pH 7.0 at 20 °C, ω is the cooperativity factor. b As per ref. 17. | |||||||
| BER | 1.21 | 34 | 0.41 | 0.98 | 41 | 0.40 | 1.00 |
| BER1 | 6.41 | 48 | 3.08 | 5.12 | 46 | 2.35 | 2.97 |
| BER2 | 7.23 | 56 | 4.05 | 6.54 | 57 | 3.73 | 3.30 |
| BER3 | 8.15 | 68 | 5.54 | 7.52 | 61 | 4.59 | 4.10 |
| BER4 | 8.62 | 75 | 6.46 | 8.74 | 66 | 5.77 | 5.20 |
| BER5 | 9.24 | 82 | 7.58 | 8.89 | 89 | 7.91 | 5.80 |
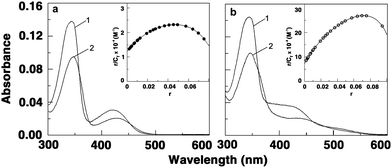
Berberine has a weak fluorescence but the 9-analogues have intense fluorescence with emission spectra in the 450–650 nm range when excited at 350 nm. Therefore one can directly perform a binding experiment by observing the intrinsic fluorescence of these analogues in the absence or presence of poly(A). We found that the fluorescence intensities at the wavelength maximum increased gradually as the poly(A) concentration increased eventually leading to saturation in each case. This phenomenon indicated the interaction between the analogues and poly(A). The stronger enhancement of intensity with the analogues compared to BER may be due to the stronger binding that leads to a decrease of the collisional frequency of the solvent molecules. Large fluorescence enhancement is also suggesting the location of the bound molecules in a hydrophobic environment. Representative fluorescence spectra of free and bound forms of BER and BER5 are shown in Fig. 3. The data from fluorimetric titrations were also analyzed by Scatchard plots. The plots of r/Cfversus ‘r’ (not shown) revealed positive slope at low ‘r’ values as in the case of absorbance indicating cooperativity. The cooperative binding constants (Ki), the number of binding sites (n), and Kiω values for the interaction were estimated and are collated in Table 2. The cooperative binding and apparent binding affinity values obtained from fluorimetric data are close to those obtained from spectrophotometric studies.
 | ||
| Fig. 3 Representative fluorescence spectra of free (curve 1) and poly(A) bound (curve 2) (a) BER and (b) BER5. | ||
Previous results on binding of berberine to DNA and RNA, and of these analogues to double stranded DNA have also shown cooperative binding phenomena.32–36 Positive cooperativity in the binding of small molecules to nucleic acids, in general, has been rationalized as an allosteric effect or as an effect mediated by conformational changes in the nucleic acid on binding of small molecules.38,39 It is not unlikely that the binding of these berberine analogues induces some conformational rearrangements in the poly(A) chain.
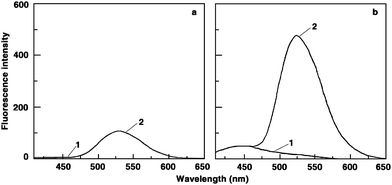
To establish the binding stoichiometry of these molecules to poly(A), continuous variation analysis procedure (Job's plot) was performed in fluorescence as described earlier.34 The Job's plots of the difference fluorescence intensity versus mole fraction of berberine and analogues revealed a single binding mode in each case. Representative plots for BER, BER3 and BER5 are presented in Fig. 4. The mole fraction of berberine and the analogues binding to poly(A) derived from the Job's plot was observed to be in the range 0.18–0.22 in all cases revealing one bound alkaloid molecule per 3–4 adenine bases of poly(A). This value is in excellent agreement with the stoichiometry reported for many drugs that induced self-structure in poly(A)14 and with the number of occluded sites reported for coralyne.12
 | ||
| Fig. 4 Job plots for the binding of (a) BER, (b) BER3, and (c) BER5 to poly(A). | ||
Circular dichroic (CD) and optical melting experiments of poly(A) in the presence of these compounds were performed to ascertain the capability to induce self-structure formation. Cooperative melting profiles were observed for poly(A) complexes in the presence of these five analogues, indicating the formation of self-structure in poly(A). In Fig. 5, the UV absorption and CD melting profiles of poly(A) and its complexes with BER, BER1, BER3 and BER5 are presented. Berberine derivatives induced self-structure of poly(A) with melting temperature values of 58, 59, 58, and 59.5 °C, respectively, for BER, BER1, BER3 and BER5. The melting temperature of poly(A) self-structure induced by BER is similar to that reported for the self-structures induced by coralyne and sanguinarine.12,15 It may be recalled that the binding of all the analogues was cooperative as evident from the Scatchard plots under the conditions of this study. Thus, it appears that cooperativity in binding of small molecules has a direct correlation to self-structure formation in poly(A), a notion that was proposed from our earlier studies.14
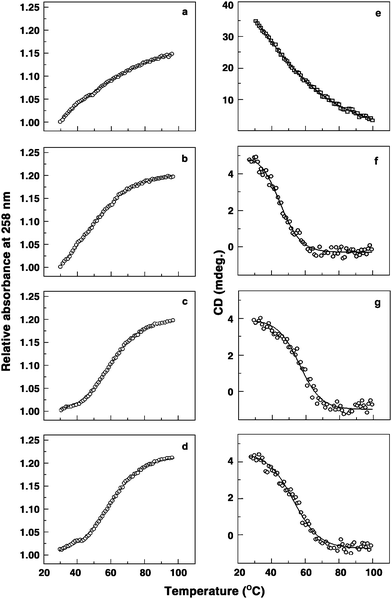 | ||
| Fig. 5 UV melting (a–d) and CD melting (e–h) profiles of free poly(A), and poly(A) bound with BER, BER3 and BER5, respectively. | ||
The CD spectra of poly(A) and of the self-structured poly(A) induced in the presence of BER, BER3 and BER5 are presented in Fig. 6. It can be seen that the ellipticities of both peaks of poly(A) were remarkably altered and reduced in intensity. This indicates that the self-structured poly(A) has similar CD spectral characteristics although the bound alkaloid has some influence on the absolute ellipticity values. The final spectrum in each case resembled the CD spectrum of the poly(A) self-structure induced by sanguinarine,15 coralyne12,18 and europium–amino acid complex.21 The presence of an induced CD band in the visible absorption region on complexation with poly(A) further established the strong environment of the bound molecules inside the poly(A) helix. It may be noted that the decrease of the long wavelength band ellipticity has been correlated to both helix winding angle and base pair twist.40,41 More often, structural change from A-form to B-form and from B-form to C-form in double stranded DNA results in such large decrease of the long wavelength band ellipticity.
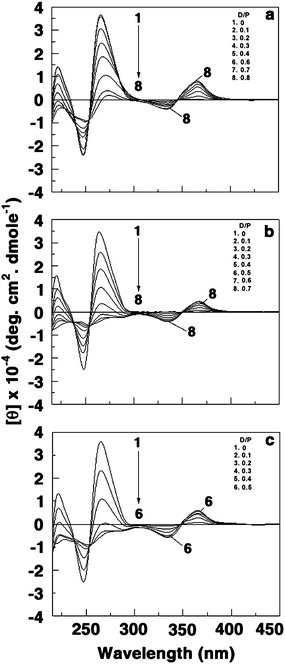 | ||
| Fig. 6 Representative circular dichroic spectra of poly(A) (60 μM) treated with (a) 0, 6, 12, 18, 24, 36, 42 and 48 μM of BER (curves 1–8) (b) 0, 6, 12, 18, 24, 30, 36 and 42 μM of BER3 (curves 1–8) and (c) 0, 6, 12, 18, 24 and 30 μM of BER5 (curves 1–6). | ||
To provide further support for the induction of self-structure in poly(A) by these molecules, we probed the same by the dilution method of Hud and colleagues.17a,b From the binding analysis, the cooperative binding constants for BER and BER1–5 were estimated to be 1.00 × 105, 2.97 × 105, 3.30 × 105, 4.10 × 105, 5.20 × 105 and 5.80 × 105 M−1, and cooperativity factors were obtained as 1.20, 1.31, 1.39, 1.47, 1.78 and 1.86, respectively (Table 2). The binding data obtained from the dilution experiments are in good agreement with the results of other binding analyses. In each case the cooperativity factor was greater than one indicating that the bindings were cooperative. The number of alkaloid molecules assembled on poly(A) for the formation of self-structure was also analyzed by fitting the curve of fraction of bound alkaloids against the concentration of alkaloids (Fig. 7). In each case the number of assembled alkaloid molecules was about four, which was in good agreement with the data reported by Centikol and Hud for berberine.17
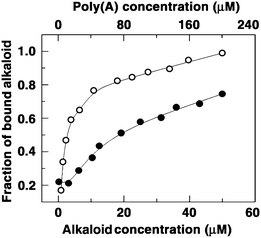 | ||
| Fig. 7 Plot of fraction of bound BER (closed circles) and BER5 (open circles) against their concentration obtained from dilution experiment. | ||
Isothermal titration calorimetry (ITC) was employed to further understand the thermodynamics of the complexation of these molecules with poly(A). Isothermal titration calorimetry has become an effective tool to thermodynamically characterize the binding of small molecules to nucleic acid structures that may offer key insights into the complex formation driven by the molecular forces.42 The advantage of ITC is that it can give a complete thermodynamic profile of the binding such as Gibbs' energy change (ΔG), enthalpy change (ΔH) and entropy change (ΔS) together with stoichiometry (N) and the affinity constant (Ka), and these are independent of the spectroscopic changes that occur during the reactions. The ITC profiles for the binding of berberine analogues BER3 and BER5 to poly(A) are presented in Fig. 8. All the profiles were monophasic and revealed the binding to be exothermic resulting in negative peaks in the plot of power versus time. Each of the heat burst curves in the figure corresponds to a single injection of poly(A) into the alkaloid solution that was corrected by the corresponding dilution heats derived from a titration of identical amounts of poly(A) solution into buffer alone. The resulting corrected heat plotted as a function of the molar ratio is depicted in the lower panel. The data points reflect experimental injection heats while the solid lines reflect the calculated fits of the data. The thermodynamic parameters for the binding of BER and the five analogues to poly(A) are depicted in Table 3. The ITC data yielded an association constant (Ka) of 0.65 × 106 M−1, an enthalpy change of −7.04 kcal mol−1, an entropy change (TΔS) of 0.80 kcal mol−1 and a binding site size (1/N) of 3.33 bases for the binding of berberine to poly(A). As the alkyl amino chain was attached to the berberine chromophore, a dramatic increase in the binding affinity occurred; the Ka was three times higher (1.85 × 106 M−1) for BER1 than for BER. The entropy factor (TΔS) to the Gibbs' energy change was also enhanced considerably from 0.8 for BER to 1.83 for BER1. As the length of the side chain increased, the Gibbs' energy change was enhanced, revealing more favourable contacts to the binding from the side chain. The disruption of the water structure became evident as the enthalpy of the reaction decreased and the entropy contribution became more and more dominant (Table 3). For BER5, the binding affinity was almost 10 times higher (6.10 × 106 M−1) than that of BER with significant entropy contribution to the free energy of binding. The bound BER4 and BER5 molecules spanned about four bases/base pairs of poly(A). It is pertinent to mention here that the binding affinity values obtained from ITC results are in good agreement with the apparent binding values obtained from spectroscopic results. Thus, the addition of the 9-ω-amino alkyl ether side chain dramatically changed the energetics and affinity of berberine–poly(A) interaction.
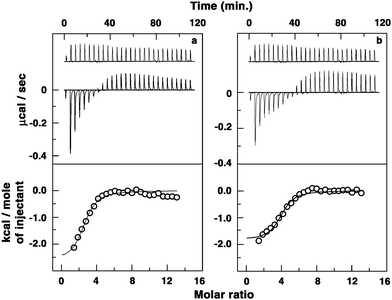 | ||
| Fig. 8 (Top panel) Representative ITC profile for the binding of alkaloids to poly(A) in 10 nm CP buffer pH 7.0 at 20 °C indicating the raw data for sequential injection of 10 μL of poly(A) into (a) BER3 and (b) BER5 solutions (curve at the bottom) and poly(A) dilution control (curve on the top offset for clarity). (Bottom panel) Plot of integrated heat data after correction of heat of dilution of poly(A) against the molar ratio of the alkaloids to poly(A). The data were fitted to a one-site model and the solid curves represent the best fit. | ||
| Alkaloid/analogue | K a × 10−6/M−1 | N | ΔG/kcal mol−1 | ΔH/kcal mol−1 | TΔS/kcal mol−1 |
|---|---|---|---|---|---|
| a All the data in this table are derived from ITC experiments conducted in citrate–phosphate buffer of 10 mM [Na+], pH 7.0 and are average of four determinations. Ka and ΔH values were determined from ITC profiles fitting to Origin 7.0 software as described in the text. The values of ΔG were determined using the equation ΔG = ΔH − TΔS. All the ITC profiles were fit to a model of single binding site. | |||||
| BER | 0.65 | 0.30 | −7.84 | −7.04 | 0.80 |
| BER1 | 1.85 | 0.25 | −8.46 | −6.63 | 1.83 |
| BER2 | 2.56 | 0.30 | −8.65 | −6.41 | 2.24 |
| BER3 | 3.66 | 0.30 | −8.86 | −5.91 | 2.95 |
| BER4 | 5.51 | 0.25 | −9.09 | −5.86 | 3.23 |
| BER5 | 6.10 | 0.28 | −9.16 | −5.64 | 3.52 |
Planar isoquinoline alkaloids like sanguinarine and coralyne have been shown to induce a unique self-structure in poly(A).12,15 We systematically evaluated the ability of berberine and the role of the nature of the 9-ω-amino alkyl ether substitution in berberine in inducing self-structure in poly(A). Our present results clearly indicate that the non-planar conjugated berberine and all the five 9-O-analogues induced self-structure formation in poly(A). All these compounds bound cooperatively to poly(A) as inferred from their Scatchard binding isotherms derived from absorption and fluorescence spectral data. Although all the molecules investigated have similar buckled structure for the isoquinoline chromophore, the affinity remarkably increased as the alkyl chain length of the 9-substituent increased. Especially noteworthy is that between BER and BER5 there is 10 times enhancement in the binding affinity. It appears that flexibility of the alkyl chain confers a better geometry/contact probability for the amino group with poly(A) base pairs and phosphates. Although we have no clear cut correlation between the various structural motifs of a drug in inducing self-structure formation in poly(A) at present, our data suggest that cooperative binding of these molecules and not planarity may be an important, if not indispensable, criterion. It is pertinent to mention here that the DNA binding affinity of these analogues has been found to be enhanced by about thirty times on increasing chain length from one to five carbon atoms.32 This study further confirms the importance of 9-O-substitution in berberine and the role of flexible and long alkyl chain for stronger nucleic acid binding. Further studies are underway to have a clear understanding of the exact role of the side chains in RNA binding by modifying the alkaloid structure at different positions. Since molecular recognition of single stranded RNA by small molecules is an area of current importance in chemical biology and medicinal chemistry, our results open up new avenues for the use of these berberine analogues in modulating the gene expression and development of antiviral agents based on 9-O-substituted berberine molecules.
We thank Dr Srinivas Hotha, Scientist, and Mr Gopalsamy Sureshkumar, Research Fellow, National Chemical Laboratory (CSIR), Pune, India for the gift of berberine analogues used in this work. This work was supported by research grants from the Network project “Comparative genomics and the biology of the noncoding RNA in the human genome” (NWP 0036) of the Council of Scientific and Industrial Research (CSIR), Government of India. Dr M. M. Islam acknowledges the Research Associateship from CSIR; A. Basu acknowledges the Junior Research Fellowship from University Grants Commission, Government of India. The authors thank Prof. Siddhartha Roy, Director, for his patronage, Dr Basudeb Achari, CSIR Emeritus Scientist for critical reading of the manuscript and all colleagues of Biophysical Chemistry Laboratory, Indian Institute of Chemical Biology for their support and cooperation at every stage of this work. The authors are also grateful to the referees for their helpful comments on the manuscript.
Abbreviations
| Poly(A) | polyadenylic acid |
| RNA | ribonucleic acid |
| DNA | deoxyribonucleic acid |
| PAP | polyadenylyl polymerase |
| CD | circular dichroism |
| UV | ultra violet |
| ITC | isothermal titration calorimetry. |
Notes and references
- P. Nelson, M. Kiriakodou, A. Sharma, E. Maniataki and Z. Mourelatos, Trends Biochem. Sci., 2003, 28, 534–540 CrossRef CAS.
- C. C. Esau and B. P. Monia, Adv. Drug Delivery Rev., 2007, 59, 101–114 CrossRef CAS.
- F. W. Alt, A. L. M. Bothwell, M. Knapp, E. Siden, E. Mather, M. Koshland and D. Baltimore, Cell, 1980, 20, 293–301 Search PubMed.
- M. A. McDevitt, R. P. Hart, W. W. Wong and J. R. Nevins, EMBO J., 1986, 5, 2907–2913 CAS.
- S. L. Topalian, S. Kneko, M. I. Gonzales, G. Bond, Y. Ward and J. L. Manley, Mol. Cell. Biol., 2001, 21, 5614–5623 CrossRef CAS.
- S. L. Topalian, M. I. Gonzales, Y. Ward, X. Wang and R. Wang, Cancer Res., 2002, 62, 5505–5509 CAS.
- T. Imae, S. Hayashi, S. Ikeda and T. Sakaki, Int. J. Biol. Macromol., 1981, 3, 259–266 CrossRef CAS.
- I. B. Comparini, E. Gaggelli and G. Valensin, Biophys. Chem., 1981, 14, 217–220 Search PubMed.
- R. Nandi, D. Debnath and M. Maiti, Biochim. Biophys. Acta, 1990, 1049, 339–342 Search PubMed.
- T. Antony, M. Atreyi and M. V. R. Rao, J. Biomol. Struct. Dyn., 1993, 11, 67–81 Search PubMed.
- R. C. Yadav, G. Suresh Kumar, K. Bhadra, P. Giri, R. Sinha, S. Pal and M. Maiti, Bioorg. Med. Chem., 2005, 13, 165–174 CrossRef CAS.
- F. Xing, G. Song, J. Ren, J. B. Chaires and X. Qu, FEBS Lett., 2005, 579, 5035–5039 CrossRef CAS.
- P. Giri, M. Hossain and G. Suresh Kumar, Bioorg. Med. Chem. Lett., 2006, 16, 2364–2368 CrossRef CAS.
- P. Giri and G. Suresh Kumar, Arch. Biochem. Biophys., 2008, 474, 183–192 CrossRef CAS.
- P. Giri and G. Suresh Kumar, Biochim. Biophys. Acta, 2007, 1770, 1419–1426 CrossRef CAS.
- P. Giri and G. Suresh Kumar, Curr. Med. Chem., 2009, 16, 965–987 CrossRef CAS.
- (a) P. Centikol and N. V. Hud, Nucleic Acids Res., 2009, 37, 611–621; (b) A fixed concentration of poly(A)–alkaloid complex [200 μM poly(A) + 50 μM alkaloid] was taken in the spectrophotometer cuvette. Then the solution was half diluted. If the pattern of spectra remained the same then the alkaloid is considered to be fully bound. The solution was diluted gradually and the absorbance at the λmax (344 nm) was noted. The absorbance of free alkaloids and free poly(A) at λmax of alkaloids was measured and their values added. This value was taken as Af. The absorbance of fully bound complex was taken as Ab and the value of absorbance at each dilution was taken as A. The molar extinction coefficient was determined in each case by dividing with the concentration. If the molar extinction coefficients for free, bound and intermediate bound alkaloids were εf, εb, and ε, respectively, the fraction of bound alkaloidθ = (εf − ε)/(εf − εb). For berberine, the spectra of the fully bound state could not be obtained even at the highest sample concentration investigated. Therefore, the amount of bound ligand could not be determined by the same two-spectra fitting procedure. As an alternative mentioned by Hud and coworkers, the ratio of average absorbance between 458 nm and 478 nm (representing bound alkaloid) to average absorbance between 398 nm and 418 nm (representing unbound alkaloid) was taken as fraction of bound alkaloid. The spectra were corrected for background using the average absorbance between 510 nm and 530 nm. To determine the binding constant, θ was also fitted to the Hill equation, θ = n[D]/[D] + Kd where n, [D], and Kd were the cooperativity factor, alkaloids concentration and dissociation constant, respectively. The binding constant is 1/Kd.
- P. Giri and G. Suresh Kumar, Mol. BioSyst., 2010, 6, 81–88 RSC.
- P. Giri and G. Suresh Kumar, Mini-Rev. Med. Chem., 2010, 7, 568–577 Search PubMed.
- K. Bhadra and G. Suresh Kumar, Med. Res. Rev., DOI:10.1002/med20202, in press.
- H. Zhang, H. Yu, J. Ren and X. Qu, FEBS Lett., 2006, 580, 3726–3730 CrossRef CAS.
- C. Zhao, Y. Peng, Y. Song, J. Ren and X. Qu, Small, 2008, 4, 656–661 CrossRef CAS.
- P. Krishnan and K. Bastow, Anti-Cancer Drug Des., 2000, 15, 255–264 CAS.
- W. J. Zhang, T. M. Ou, Y. J. Lu, Y. Y. Huang, W. B. Wu, Z. S. Huang, J. L. Zhou, K. Y. Wong and L. Q. Gu, Bioorg. Med. Chem., 2007, 15, 5493–5501 CrossRef CAS.
- Y. Ma, T. M. Ou, J. H. Tan, J. Q. Hou, S. L. Huang, L. Q. Gu and Z. S. Huang, Bioorg. Med. Chem. Lett., 2009, 19, 3414–3417 CrossRef CAS.
- Y. Ma, T. M. Ou, J. Q. Hou, Y. J. Lu, J. H. Tan, L. Q. Gu and Z. S. Huang, Bioorg. Med. Chem., 2008, 16, 7582–7591 Search PubMed.
- Y. H. Long, L. P. Bai, Y. Qin, J. Y. Pang, W. H. Chen, Z. Cai, Z. L. Xu and Z. H. Jiang, Bioorg. Med. Chem., 2006, 14, 4670–4676 Search PubMed.
- K. Isawa, M. Kamigauchi, M. Ukei and M. Tanigauchi, Eur. J. Med. Chem., 1996, 31, 469–478 CrossRef.
- J. Y. Pang, Y. Qin, W. H. Chen, G. A. Luo and Z. H. Jiang, Bioorg. Med. Chem., 2005, 13, 5835–5840 Search PubMed.
- K. Iwasa, M. Moriyasu, T. Yamori, T. Turuo, D. U. Lee and W. Wiegrebe, J. Nat. Prod., 2001, 64, 896–898 CrossRef CAS.
- M. Halimani, S. P. Chandran, S. Kashyap, V. M. Jadhav, B. L. V. Prasad, S. Hotha and S. Maiti, Langmuir, 2009, 25, 2339–2347 Search PubMed.
- M. M. Islam, A. Basu, M. Hossain, G. Sureshkumar, S. Hotha and G. Suresh Kumar, DNA Cell Biol., 2011, 30, 123–133 Search PubMed.
- K. Bhadra, G. Suresh Kumar and M. Maiti, Biochim. Biophys. Acta, 2007, 1770, 1071–1080 CrossRef CAS.
- M. M. Islam, S. Roy Chowdhury and G. Suresh Kumar, J. Phys. Chem. B, 2009, 113, 1210–1224 CrossRef CAS.
- J. B. Chaires, N. Dattagupta and D. M. Crothers, Biochemistry, 1982, 21, 3933–3940 CrossRef CAS.
- J. D. McGhee and P. H. von Hippel, J. Mol. Biol., 1974, 86, 469–489 CrossRef CAS.
- K. Bhadra, M. Maiti and G. Suresh Kumar, Biochim. Biophys. Acta, 2008, 1780, 1054–1061 Search PubMed.
- L. S. Rosenberg, M. J. Carvlin and T. R. Krugh, Biochemistry, 1986, 25, 1002–1008 CrossRef CAS.
- R. H. Elmore, R. M. Wadkins and D. E. Graves, Nucleic Acids Res., 1988, 16, 9707–9719 CrossRef CAS.
- B. B. Johnson, K. S. Dahl, I. Tinoco, Jr, V. I. Ivanov and V. B. Zhurkin, Biochemistry, 1981, 20, 73–78 Search PubMed.
- M. R. Dufff, Jr, V. K. Mudhivarthi and C. V. Kumar, J. Phys. Chem. B, 2009, 113, 1710–1721 Search PubMed.
- R. O'Brien and I. Haq, Applications of Biocalorimetry: Binding, Stability and Enzyme Kinetics, in Biocalorimetry 2, ed. J. E. Ladbury and M. Doyle, John Wiley and Sons Ltd., 2004, pp. 1–34 Search PubMed.
Footnote |
| † Current address: Department of Chemistry, Aliah University, DN-41, Sector V, Salt lake City, Kolkata 700 091, India. |
| This journal is © The Royal Society of Chemistry 2011 |
