Preparation of blood-brain barrier-permeable paclitaxel-carrier conjugate and its chemotherapeutic activity in the mouse glioblastoma model†
Juyoun
Jin‡
b,
Woo Sirl
Lee‡
a,
Kyeung Min
Joo‡
c,
Kaustabh K.
Maiti
a,
Goutam
Biswas
a,
Wanil
Kim
d,
Kyong-Tai
Kim
d,
Se Jeong
Lee
b,
Kang-Ho
Kim
b,
Do-Hyun
Nam
*b and
Sung-Kee
Chung
*a
aDepartment of Chemistry, Pohang University of Science and Technology, Pohang, 790-784, Korea. E-mail: skchung@postech.ac.kr; Fax: +82-54-279-3399
bDepartment of Neurosurgery and Cancer Stem Cell Research Center, Samsung Medical Center and Samsung Biomedical Research Institute, Sungkyunkwan University School of Medicine, Seoul, 135-710, Korea. E-mail: nsnam@skku.edu
cDepartment of Anatomy, Seoul National University College of Medicine, Seoul, 110-799, Korea
dDepartment of Life Science, Pohang University of Science and Technology, Pohang, 790-784, Korea
First published on 11th February 2011
Abstract
The per oral administration of compound 1, prepared by covalently attaching paclitaxel to a molecular carrier, shows good anti-tumor activity in the orthotopic mouse model of glioblastoma. The anti-tumor effects may be attributable to the cytotoxicity as well as the anti-angiogenic activity of the paclitaxel conjugate or paclitaxel itself in the brain parenchyma.
Paclitaxel (sold as Taxol by Bristol-Myers Squibb), a potent anti-tumor agent isolated from the bark of the Pacific yew tree binds to microtubules and inhibits their de-polymerization into tubulin, thus leading to the cell cycle arrest and apoptotic cell death.1,2 Although taxol is currently used for treating a variety of malignances such as ovarian, breast, and non-small-cell lung cancers, its water insolubility, low oral bioavailability, and inability to cross the blood-brain barrier (BBB) limit its clinical application.3 The BBB is a unique and selective barrier formed by the endothelial cells that line cerebral capillaries, together with perivasicular elements such as astrocyte end-foot processes, perivascular neurons and pericytes. Cerebral endothelial cells form complex tight junctions by the interaction of several transmembrane proteins that effectively seal the paracellular pathways in addition to the cell-cell adherens junctions.4 Additionally, induced drug resistance including efflux pumps such as P-glycoprotein (P-gp) expressed on the intestinal epithelial and brain endothelial cells also represent significant hindrance to the therapeutic efficacy of paclitaxel.5
Glioblastoma multiforme (GBM) is a very aggressive brain tumor, and the prognosis for patients with GBMs remains poor despite multidisciplinary treatment approaches, for example surgery, radiotherapy, and chemotherapy. The poor prognosis originates at least in part from the impermeability of the chemotherapeutic agents to BBB; the low permeability is also accountable for the failed or inefficient delivery of paclitaxel to the central nervous system (CNS).3,5,6 Since it is known that GBM cells are sensitive to paclitaxel in vitro,7 an enhanced delivery of paclitaxel to the tumor cells in the CNS would be expected to substantially help the chemotherapeutic prospect.
Various molecular transporters can assist a promising drug candidate to be delivered to the desired tissues. Several cell-penetrating peptides (CPPs) derived from HIV-1 Tat protein, Antennapedia protein of Drosophila, and related peptides have been extensively studied as potential delivery vectors.8 The low-density lipoprotein receptor-related proteins such as lactoferrin and melanotransferrin have been reported to mediate transport across endothelial cells of the BBB.9 However, proteins and peptides as delivery vectors have some real and potential limitations, including instability toward various endogenous peptidases and immunogenic liability. In attempts to improve upon these limitations, we have recently developed several novel classes of synthetic molecular transporters, which possess multiple guanidine residues attached to varying carbohydrate scaffolds (for example, inositol, sorbitol, monosaccharide, and disaccharide) viacarboxylic acid linker chains.10 In particular, a series of G8 molecular transporters based on the sorbitol scaffold and branched chain linkers displayed an excellent ability to mediate the transport across the BBB.10b,11 These observations are quite exciting because of their potential to target the brain. Hence we have investigated the sorbitol-based G8 molecular transporter for the brain delivery of paclitaxel, and herein we report the preparation of the covalent conjugate between the transporter and paclitaxel, and its good in vivo anti-cancer activity in the xenograft mouse model of GBM.
In preparing the covalent conjugates of paclitaxel and the sorbitol-based G8 carrier with and without a fluorophore (compounds 1 and 2 in Fig. 1), we have used 2′-succinyl-paclitaxel, the commonly used side chain derivative.12 Thus, paclitaxel was reacted with succinic anhydride in CH2Cl2 and pyridine to give 2′-succinyl-paclitaxel. The synthetic intermediate 3a, available from the previous study10b was first coupled with 2′-succinyl-paclitaxel in the presence of EDC and DMAP, and then the product was treated with gaseous HCl in EtOAc at rt to provide compound 1 in ca. 45% yield (Scheme 1). For the preparation of the conjugate with FITC fluorophore, we started out with intermediate 3b, also available from the previous study.10b The O-trityl protecting group of 3b was selectively removed by a silica gel column impregnated with CF3COOH.13 The silica gel column was first prepared in hexane containing 1% Et3N. The column was overlaid with a band of sand, and then another layer of silica gel in hexane containing 1% TFA. Compound 3b in CHCl3 was loaded on the column and eluted with methanol–chloroform to give 4, which was then coupled with 2′-succinyl-paclitaxel to yield 5. The N-Cbz protecting group of 5 was removed by hydrogenolysis over Pd/C, and the FITC fluorophore was attached to the free amino group to give 7. The Boc-protecting groups were removed from the guanidines to provide the target compound 2. Compounds 1 and 2 are highly soluble in water as opposed to paclitaxel itself. All key intermediates and the target compounds have been rigorously characterized by HPLC and spectroscopic analyses (Scheme 2).
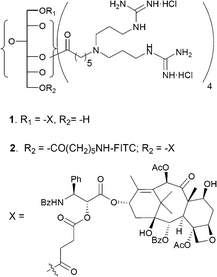 | ||
| Fig. 1 Paclitaxel-carrier conjugates 1 and 2. | ||
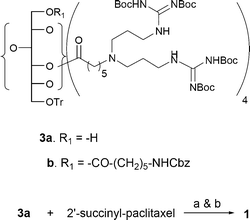 | ||
| Scheme 1 Preparation of taxol-carrier conjugate 1. Reagents and conditions: a) EDC, DMAP, CH2Cl2, rt, 30 h, 69%; b) HCl (g), EtOAc, rt, 24 h, 65%. | ||
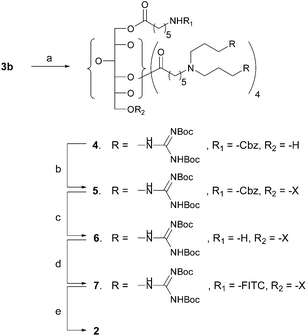 | ||
Scheme 2 Preparation of paclitaxel-carrier conjugate 2 with FITC. Reagents and conditions: a) silica gel in hexane containing 1% TFA → silica gel in hexane containing 1% Et3N, CHCl3–CH3OH = 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 62%; b) 2′-succinyl-paclitaxel, EDC·HCl, DMAP, CH2Cl2, rt, 30 h, 73%; c) H2 (1 atm), 10% Pd/C (10 mol %), CH3OH, rt, 20 h, 76%; d) FITC-I, Et3N, EtOH–THF (5 1, 62%; b) 2′-succinyl-paclitaxel, EDC·HCl, DMAP, CH2Cl2, rt, 30 h, 73%; c) H2 (1 atm), 10% Pd/C (10 mol %), CH3OH, rt, 20 h, 76%; d) FITC-I, Et3N, EtOH–THF (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), rt, dark, 24 h, 56%; e) HCl (g), EtOAc, rt, 24 h, 74%. 2), rt, dark, 24 h, 56%; e) HCl (g), EtOAc, rt, 24 h, 74%. | ||
The cytotoxicity comparison based on the MTT assays indicated that there was no significant difference between paclitaxel and the conjugate 1 (see Table S1 in the ESI†). We then examined the tissue distribution patterns of 2 in mice by i.p. injection (see Fig. S1 in the ESI†). The paclitaxel-carrier conjugate with FITC (2) dissolved in water was found to be well distributed in various tissues including brain. The comparison of the tissue distribution shows that the patterns are very similar between those of 2 and carrier itself.10b The paclitaxel conjugate 2 appears to readily overcome mouse BBB reaching the brain almost as quickly as the unloaded carrier does. Encouraged by these results we decided to study the in vivo anti-cancer activity of the conjugate 1 in mouse models.
In order to examine the BBB permeability by the per oral (po) route as well as the anti-tumor activity of the paclitaxel-carrier conjugate 1, orthotopic brain tumor animal models were produced by injecting 2 × 105U-87MG human glioblastoma (GBM) cells into the basal ganglia of immuno-compromised Balb/c-nu mouse stereotactically.7 Two weeks after the tumor cell implantation, mice were randomized into four groups (n = 7 for each group) and each group was fed via oral administration the PBS solution as control, 7.5 mg kg−1 of paclitaxel, 7.5 mg kg−1, and 3.75 mg kg−1 of the paclitaxel conjugate (1) twice a week for two weeks (total 4 times). Four weeks after the tumor cell implantation, the mice were sacrificed, and the tumor volumes were measured and compared (Fig. 2). Per oral administration of 7.5 mg kg−1 of paclitaxel (equivalent to 8.8 μmol) showed little anti-tumor activity as compared to the control group (PBS; control = 25.0 ± 2.6 mm3), probably reflecting inaccessibility of taxol to tumors in the brain parenchyma (paclitaxel 7.5 mg kg−1 = 19.4 ± 3.5 mm3).14 In contrast, the same weight dose of the taxol conjugate (1, 7.5 mg kg−1, equivalent to 2.6 μmol) significantly reduced the tumor volume (paclitaxel-conjugate 7.5 mg kg−1 = 4.7 ± 0.9 mm3, P < 0.01 against the control group). Even with a half weight dose of the paclitaxel conjugate, similar anti-tumor effects were achieved (paclitaxel-conjugate 3.75 mg kg−1 = 6.1 ± 1.8 mm3, P < 0.01 against the control group).
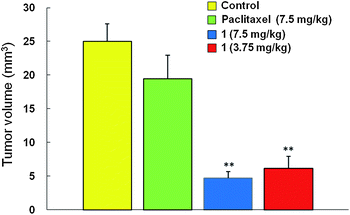 | ||
| Fig. 2 Therapeutic effects of paclitaxel-carrier conjugate (by po) on tumors in the mouse brain. Paclitaxel and paclitaxel-carrier conjugate (compound 1) were injected per oral in tumor-bearing animals. Data are expressed as the mean ± SE. ** P < 0.01. | ||
For possible anti-tumor mechanisms of the paclitaxel conjugate (1), studied were the immunohistochemical responses against PCNA (proliferating cell nuclear antigen) for proliferation of tumor cells, and CD31 for microvessel density, and the TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling) assay.15 Numbers of the PCNA- or TUNEL-positive cells in a unit area, and the density of CD31-positive microvessels were compared (Fig. 3). Proliferation of the tumor cells were substantially reduced and apoptosis of tumor cells increased significantly in the paclitaxel-carrier conjugate (1) groups, when compared with the control and paclitaxel-treated groups (Fig. 3A for proliferation, control = 177.5 ± 23.1; paclitaxel 7.5 mg kg−1 = 157.6 ± 21.4; compound 1 7.5 mg kg−1 = 50.4 ± 7.5; compound 1 3.75 mg kg−1 = 57.2 ± 2.0: Fig. 3B for apoptosis, control = 3.3 ± 0.1; paclitaxel 7.5 mg kg−1 = 6.6 ± 1.1; compound 1 7.5 mg kg−1 = 11.8 ± 1.6; compound 1 3.75 mg kg−1 = 11.6 ± 1.3, P < 0.001). Furthermore, the paclitaxel-carrier conjugate (1) groups also showed significant decreases in microvessel density in the tumor bed (Fig. 3C, control = 24.8 ± 1.5; paclitaxel 7.5 mg kg−1 = 20.7 ± 2.2; paclitaxel conjugate 7.5 mg kg−1 = 14.5 ± 1.9; paclitaxel conjugate 3.75 mg kg−1 = 18.8 ± 0.8, P < 0.01). Taken together, the results indicate that anti-angiogenic effects14 as well as direct cytotoxic anti-tumor effects are likely involved in the overall anti-tumor activity of the paclitaxel conjugate (1).
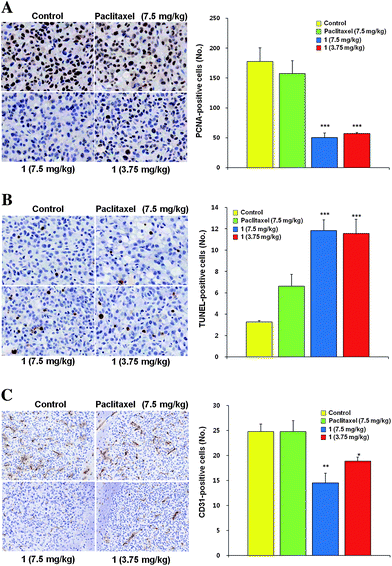 | ||
| Fig. 3 The effects of paclitaxel-carrier conjugate (po) on (A) tumor cell proliferation (brownish stained cells are PCNA-positive), (B) tumor cell apoptosis (brownish stained cells are TUNEL-positive), and (C) microvessel density (CD31-positive cells organized into capillary-like channels) in the mouse brain. Proliferating, apoptotic and endothelial cells were analyzed by anti-PCNA antibody, TUNEL assay and anti-CD31 antibody, respectively, in tumor masses. Numbers of PCNA-, TUNEL-, and CD31-positive cells were calculated and compared (each group, n = 10). Data are expressed as the mean ± SE. * P < 0.05, ** P < 0.01 and *** P < 0.001 vs. control. | ||
U-87MG cells are known to be sensitive to paclitaxel in vitro, where paclitaxel can make direct contact with tumor cells.7 However, clinical data have clearly indicated that the paclitaxel-based chemotherapy has little therapeutic benefit against tumors in the brain, probably because P-gp expressed on brain endothelial cells does not allow paclitaxel accessibility to tumor cells in the brain parenchyma, in addition to the impermeability of taxol into the brain tissue.16 Although the paclitaxel therapy by the po route would be expected to have extra benefits such as superior metronomic effects besides the easier administration, it cannot be clinically employed because P-gp is also expressed on the intestinal epithelium.17 In the present work, the po administration of the paclitaxel conjugate (1) was found to have significant therapeutic effects against GBM mouse model, which suggested that compound (1) was not subjected to the efflux process by P-gp and effectively delivered to tumor cells in the brain parenchyma across both intestinal epithelium and BBB. With regard to the question whether the conjugate (1) is a prodrug or itself an active anti-tumor agent, we are leaning toward the prodrug side because of the activity spectrum that is rather similar to that of paclitaxel. The reason why paclitaxel is not effluxed out may be explainable by the assumption that the compound (1) is permeated deeply into the brain tissue where the P-gp population is not high, and then undergoes hydrolysis.
Several reports have previously shown that metronomic anti-angiogenic treatment could be carried out by frequent low dose paclitaxel administration.18,19 The observed decreases in the numbers of PCNA-positive proliferating tumor cells (Fig. 3A), increases in the number of TUNEL-positive apoptotic tumor cells (Fig. 3B), and a significant decrease in the microvessel density (Fig. 3C) by the paclitaxel conjugate (1) group indicate that the oral delivery of the conjugate 1 can target the tumor cells. Therefore, a successful metronomic chemotherapy with (1) by po administration is expected to be possible against brain tumors, since it can counter P-gp on the intestinal epithelium. In fact, the present observation that the high and the low level dosing of the paclitaxel conjugate (1) showed similar anti-tumor effects (Fig. 2) may be related to this phenomenon. The systemic toxicity of the conjugate (1), based on the body weight changes in experimental groups, has indicated about the same level of toxicity as paclitaxel (see Fig S2 in the ESI†).
Conclusions
In summary, we have prepared a BBB-permeable paclitaxel derivative (1) by covalent attachment of paclitaxel to the sorbitol-based molecular transporter. The po administration of the water-soluble paclitaxel conjugate shows good anti-tumor activity in the orthotopic mouse model of glioblastoma. The anti-tumor effects may be attributable to the cytotoxicity as well as the anti-angiogenic activity of the paclitaxel conjugate or paclitaxel itself. The conjugate 1 may be potentially utilized in the metronomic combination therapy (e.g. with a VEGF receptor antagonist) of glioblastoma.Acknowledgements
Financial support from BK-21, and MOEST (National Frontier Research Program administered through KRICT/CBM) of Korean government to SKC (POSTECH) is gratefully acknowledged.Notes and references
- (a) M. C. Wani, H. L. Taylor, M. E. Wall, P. Coggon and A. T. McPhail, J. Am. Chem. Soc., 1971, 93, 2325 CrossRef; (b) P. B. Schiff, J. Fant and S. B. Horowitz, Nature, 1979, 277, 665 CAS.
- (a) K. C. Nicolaou, W.-M. Dai and R. K. Guy, Angew. Chem., Int. Ed. Engl., 1994, 33, 15 CrossRef; (b) M. Skwarczynski, Y. Hayashi and Y. Kiso, J. Med. Chem., 2006, 49, 1 CrossRef; (c) D. G. I. Kingston, J. Org. Chem., 2008, 73, 3975 CrossRef CAS; (d) I. Ojima, Acc. Chem. Res., 2008, 41, 108 CrossRef CAS.
- (a) A. K. Singla, A. Garg and D. Aggarwal, Int. J. Pharm., 2002, 235, 179 CrossRef CAS; (b) M. S. Lesniak and H. Brem, Nat. Rev. Drug Discovery, 2004, 3, 499 CrossRef CAS.
- (a) S. Tsukita, M. Furuse and M. Itoh, Nat. Rev. Mol. Cell Biol., 2001, 2, 285 CrossRef CAS; (b) W. M. Pardridge, Drug Discovery Today, 2007, 12, 54 CrossRef CAS.
- (a) R. Z. Yusuf, Z. Duan, D. E. Lamendola, R. T. Penson and M. V. Seiden, Curr. Cancer Drug Targets, 2003, 3, 1 Search PubMed; (b) G. Szakacs, J. K. Paterson, J. A. Ludwig, C. Booth-Genthe and M. M. Gottesman, Nat. Rev. Drug Discovery, 2006, 5, 219 CrossRef CAS.
- R. Cecchelli, V. Berezowski, S. Lundquist, M. Culot, M. Renftel, M.-P. Dehouck and L. Fenart, Nat. Rev. Drug Discovery, 2007, 6, 650 CrossRef CAS.
- K. M. Joo, S. Y. Song, K. Park, M. H. Kim, J. Jin, B. G. Kang, M. J. Jang, G. S. Lee, M. S. Kim and D. H. Nam, Int. J. Oncol., 2008, 33, 705 CAS.
- (a) Ü. Langel, Ed. Handbook of Cell-Penetrating Peptides, 2nd Edition, CRC Press, Boca Raton, 2007 Search PubMed; (b) P. A. Wender, W. C. Galliher, E. A. Goun, L. R. Jones and T. H. Pillow, Adv. Drug Delivery Rev., 2008, 60, 452 CrossRef CAS; (c) I. Nakase, T. Takeuchi, G. Tanaka and S. Futaki, Adv. Drug Delivery Rev., 2008, 60, 598 CrossRef CAS.
- (a) W. Pan, A. J. Kastin, T. C. Zankel, P. Van Kerkhof, T. Terasaki and G. Bu, J. Cell Sci., 2004, 117, 5071 CrossRef CAS; (b) S. Ito, S. Ohtsuki and T. Terasaki, Neurosci. Res., 2006, 56, 246 CrossRef CAS.
- (a) K. K. Maiti, O. Y. Jeon, W. S. Lee, D. C. Kim, K. T. Kim, T. Takeuchi, S. Futaki and S. K. Chung, Angew. Chem., Int. Ed., 2006, 45, 2907 CrossRef CAS; (b) K. K. Maiti, W. S. Lee, T. Takeuchi, C. Watkins, M. Fretz, D. C. Kim, S. Futaki, A. Jones, K. T. Kim and S. K. Chung, Angew. Chem., Int. Ed., 2007, 46, 5880 CrossRef CAS; (c) G. Biswas, O. Y. Jeon, W. S. Lee, D. C. Kim, K. T. Kim, S. Lee, S. Chang and S. K. Chung, Chem.–Eur. J., 2008, 14, 9161 CrossRef CAS; (d) W. S. Lee, C. N. Im, Q. Y. Teng, Y. T. Chang, D. C. Kim, K. T. Kim and S. K. Chung, Mol. BioSyst., 2009, 5, 822 RSC.
- J. K. Im, W. Kim, K. T. Kim and S. K. Chung, Chem. Commun., 2009, 4669 RSC.
- (a) N. F. Margi and D. G. I. Kingston, J. Nat. Prod., 1988, 51, 298 CrossRef CAS; (b) H. M. Deutsch, J. A. Glinski, M. Hernandez, R. D. Hauwitz, V. L. Narayanan, M. Suffness and L. H. Zalkow, J. Med. Chem., 1989, 32, 788 CrossRef CAS; (c) T. A. Kirschberg, C. L. VanDeusen, J. B. Rothbard, M. Yang and P. A. Wender, Org. Lett., 2003, 5, 3459 CrossRef CAS; (d) S. Wang, N. Z. Zhelev, S. Duff and P. M. Fischer, Bioorg. Med. Chem. Lett., 2006, 16, 2628 CrossRef CAS.
- A. K. Pathak, V. Pathak, L. E. Seitz, K. N. Tiwari, M. S. Akhtar and R. C. Reynolds, Tetrahedron Lett., 2001, 42, 7755 CrossRef CAS.
- I. B. Paek, H. Y. Ji, M. S. Kim, G. S. Lee and H. S. Lee, J. Sep. Sci., 2006, 29, 628 CrossRef CAS.
- (a) D. S. Grant, T. L. Williams, M. Zahaczewsky and A. P. Dicker, Int. J. Cancer, 2003, 104, 121 CrossRef CAS; (b) D. Belotti, V. Vergani, T. Drudis, P. Borsotti, M. R. Pitelli, G. Viale, R. Giavazzi and G. Taraboletti, Clin. Cancer Res., 1996, 2, 1843 CAS; (c) M. J. Son, H. S. Song, M. H. Kim, J. T. Kim, C. M. Kang, J. W. Jeon, S. Y. Park, Y. J. Kim, M. D. Groves, K. Park, J. H. Kim and D. H. Nam, Int. J. Oncol., 2006, 28, 1385 CAS.
- E. M. Kemper, A. E. van Zandbergen, C. Cleypool, H. A. Mos, W. Boogerd, J. H. Beijnen and O. van Tellingen, Clin. Cancer. Res., 2003, 9, 2849 CAS.
- (a) R. L. Juliano and V. Ling, Biochim. Biophys. Acta, Biomembr., 1976, 455, 152 CrossRef CAS; (b) C. Cordon-Cardo, J. P. O'Brien, J. Boccia, D. Casals, J. R. Bertino and M. R. Melamed, J. Histochem. Cytochem., 1990, 38, 1277 CAS; (c) N. Kartner, J. R. Riordan and V. Ling, Science, 1983, 221, 1285 CrossRef CAS.
- (a) H. Jiang, W. Tao, M. Zhang, S. Pan, J. R. Kanwar and X. Sun, Cancer Invest., 2010, 28, 74 CrossRef CAS; (b) T. Browder, C. E. Butterfield, B. M. Kraling, B. Shi, B. Marshall, M. S. O'Reilly and J. Folkman, Cancer Res., 2000, 60, 1878 CAS.
- D. P. Samuel, P. Y. Wen and M. W. Kieran, Expert Opin. Invest. Drugs, 2009, 18, 973 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details for synthesis and bioassays, Fig. S1, Fig. S2. See DOI: 10.1039/c0md00235f |
| ‡ These authors equally contributed to this work. |
| This journal is © The Royal Society of Chemistry 2011 |
