DOI:
10.1039/C0MD00211A
(Concise Article)
Med. Chem. Commun., 2011,
2, 274-277
Received
10th November 2010
, Accepted 14th December 2010
First published on 24th January 2011
Abstract
The syntheses, characterisation and biological activities (IC50; DNA binding) of four mononuclear Pt oxazoline complexes are reported. These materials are the compounds cis-[PtCl2(NH3)(Etox-κ1N)] (1: Etox = COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound2-ethyl-2-oxazoline), [PtCl2(anilox-κ2N,N′)] (2: anilox = COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound4,4-dimethyl-2-[o-anilinyl]-2-oxazoline), cis-[PtCl2(Etox-κ1N)2] (3) and [PtCl(pyox-κ3N,N′,N′)] (4: pyoxH = pyridine-2-carboxyanil-[o-{4,4-dimethyl-2-oxazolinyl}-ide]) and all four are shown to have slightly lower cytotoxicities in vitro when compared to cisplatin against the A2780 ovarian cancer cell line. These new materials all appear to be bio-active via the formation of DNA adducts. Complexes 1 and 2 have been further characterised in the solid-state by X-ray diffraction methods.
Introduction
Platinum compounds containing N-donor ligands have played a unique role in the development of both coordination and medicinal chemistry. The serendipitous discovery (circa 1965) and later clinical application of the simple inorganic complex cisplatin (i.e., cis-[PtCl2(NH3)2]: Fig. 1) as an anti-cancer agent marks1 an important milestone in the development of inorganic chemotherapy agents.1,2 Since that time, a number of 2nd and 3rd generation Pt-containing reagents have been designed, tested and/or entered clinical use.3,4 Modifications of the original cisplatin scaffold have been undertaken to increase clinical efficacy and/or to reduce the (toxic) side-effect profile. Synthetically, this has focused on three main areas: (i) application of Pt complexes with higher formal oxidation state(s) at the metal centre (primarily Pt4+) such as Satraplatin (a.k.a. JM216: Fig. 1);5 (ii) the use of non-halide formal anions within the metal coordination sphere (e.g., Carboplatin; Fig. 1)3,4 and/or (iii) replacement of one or more ammine ligands with other N-donor fragments, such as the substituted pyridine ligand found in Picoplatin (i.e., ZD0473: Fig. 1).3 These modifications come from the very early establishment of the observed Structure–Activity Relationship (SAR) within the general class of bio-active Pt species.1,4
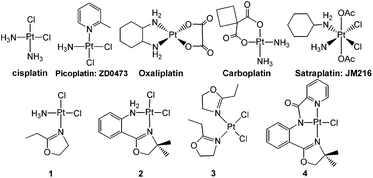 |
| | Fig. 1 Some current clinically used Pt-based drugs and the compounds (1–4) used in this study. | |
Our particular interests lie in the arena of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundoxazole (and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound4,5-dihydro-1,3-oxazole: i.e, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound2-oxazoline)6,7 coordination chemistry and in ligand design strategies involving these heterocyclic frameworks. Oxazoles (ox) are found in a wide variety of both natural and synthetic products and have found a number of diverse applications (e.g., coordination chemistry, catalysis, etc.).6 Despite these advances, relatively few transition metal oxazol(in)e compounds have been investigated as anti-cancer agents and even fewer contain Pt as the metal centre.8 The work described herein details the synthesis of representatives of four classes of Pt-ox compounds: (a) ox analogues (1) of ZD0473 (i.e., cis-[PtCl2(NH3)(2-picoline)]: Fig. 1); (b) relatives of Oxaliplatin which contain an N,N′-chelating ox ligand (2); (c) bis-ox Pt-compounds devoid of an N–H bond (3) and finally (d) a Pt complex containing a tridentate ligand of our own design which incorporates both a pyridine and an ox fragment (4).9 These four compounds are shown schematically in Fig. 1. Hence, the work described herein addresses the latter of the three main areas of Pt chemotherapy design, namely the replacement of one or more ammine ligands (vide supra). These four model compounds will serve as indicators as to the efficacy of the four general molecular designs (Fig. 1).
Results and discussion
Syntheses and structural characterisation
Compound 1 is obtained by employing previously established protocols8 in which cisplatin is first converted in situ to Pt(NH3)2(NO3)2, followed by ligand metathesis with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound2-ethyl-2-oxazoline (Etox) to give Pt(Etox)(NH3)(NO3)2, and finally treatment with excess KCl to yield 1.10,11 Compound 2 can be obtained by the addition of solid 4,4-dimethyl-2-(2′-anilinyl)-2-oxazoline7b,9 to a stoichiometric aq. solution of K2PtCl4 containing a trace of c. H2SO4 (as ligand transfer agent). Subsequent stirring yields 2 in the form of a yellow-coloured powder.12,13 The treatment of a methanolic suspension of the inorganic polymer [PtCl2]n with excess Etox and stirring overnight at room temperature (RT) yielded grey-coloured 3,14,15 and 4 was prepared as reported previously.9
Compounds 1 and 2 have been further characterised in the solid-state by single crystal X-ray diffraction methods.11,13,15 Both materials are found to be mononuclear Pt complexes as expected and exhibit the desired cisoidal oriented chlorido ligands. Molecular representations of a molecule of 1 or 2 that is found in the unit cell can be found below in Fig. 2 and 3, respectively.
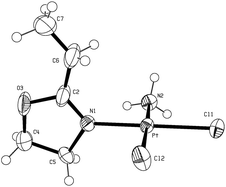 |
| | Fig. 2
ORTEP
16 representation of a molecule of complex 1 showing the atomic numbering scheme (thermal ellipsoids are shown at the 50% probability level). | |
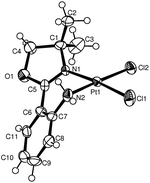 |
| | Fig. 3
ORTEP
16 representation of a molecule of complex 2 showing the atomic numbering scheme (thermal ellipsoids are shown at the 50% probability level). | |
These two materials contain a Pt atom that is in essentially a square planar coordination environment.11,13 The Pt–Cl and Pt–N bond lengths are typical for a formally Pt(II) metal centre and are otherwise unsurprising.17
Biological screening
The results of the biological screening of complexes 1–4 can be found in Table 1; the results for cisplatin are also given.18 The latter four materials show slightly lower cytotoxicity values when compared with cisplatin. However, all are active within an order of magnitude. These results suggest that the four representatives of the potential classes of 2nd generation cisplatin analogues (i.e., Fig. 1) are all worthy of further investigations. Hence, the syntheses, screening and establishment of more detailed Structure–Activity Relationships for a larger series of each class represented by 1–4 is warranted.
Table 1 Cytotoxicity of 1–4 and cisplatinvs. the A2780 cancer cell line (72 h incubation) and rb values with pUC19 plasmid DNA
| Compounds |
Inhibition IC50, μMa |
Reference |
r
b values |
|
Values are mean for data from at least three independent experiments with quadruplicate readings in each experiment.
cis-isomer; Meox = 2-methyl-2-oxazoline-κ1N.
n.b
., 96 h exposure.
|
|
cisplatin
|
1.3 (± 0.1) |
this work |
0.076 |
|
1
|
4.1 (± 0.3) |
this work |
0.083 |
|
2
|
16.9 (± 0.5) |
this work |
0.41 |
|
3
|
9.9 (± 1.0) |
this work |
0.20 |
|
4
|
20.2 (± 1.7) |
this work |
0.81 |
|
[PtCl
2
(NH
3
)(Meox)]
b
,
c
|
0.65 (± 0.1) |
8
|
— |
|
cisplatin
c
|
0.2 ± (0.05) |
8
|
— |
DNA interaction studies
The cytotoxicity associated with cisplatin and its analogues is generally believed to arise from direct interactions with DNA. Intracellular formation of monoaquated platinum species leads to monofunctional adducts with DNA primarily through attachment to guanineN7 residues. Upon further coordination to DNA, such adducts cyclise to form guanine-COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundguanine and adenine-guanine cross-links. This class of modification of the DNA tertiary structure by Pt compounds can be followed by an electrophoretic mobility shift assay, whereby the unwinding of covalently-closed, supercoiled plasmid DNA is monitored.20 It has been shown previously that cisplatin unwinds supercoiled (Form I) DNA by 13° with a bound drug-to-nucleotide ratio (rb) of 0.076, where rb corresponds to the amount of platinum complex that is necessary for complete removal of all supercoils from DNA.21 Experimentally, rb is measured as the coalescence point in a titration of plasmid DNA with a platinum complex, where the rate of migration of Form I decreases until it comigrates with relaxed, nicked DNA (Form II). Fig. 4 shows the DNA unwinding assay for 1 with pUC19 plasmid DNA. The coalescence point in a titration of 120 μM pUC19 occurs with 10 μM 1, corresponding to an rb value of 0.083, which is only slightly larger than that measured for cisplatin. The degree of distortion in the DNA helix induced by 1 is especially evident at higher concentrations (Lane 7), where the gel bands disappear because the intercalating dye used for imaging can no longer penetrate the helix. In general, the magnitude of rb for 1–4 parallels the cytotoxicity observed toward the A2780 cell line, with smaller values of rb translating to smaller IC50 values. As expected, platinum complexes that interact more strongly with DNA are the most cytotoxic. An exception to this trend occurs in the case of 3, underscoring the importance of additional factors in predicting anticancer activity. Together these results indicate that the Pt-oxazoline compounds interact with DNA much like cisplatin but may offer certain advantages, including reduced toxicity toward normal cells or higher selectivity toward cancer cells.
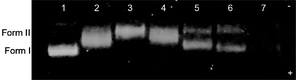 |
| | Fig. 4
DNA unwinding assay for 1 with 800 ng pUC19 plasmid (electrophoresed for 50 min on a 1% agarose gel in 1X TAE at 80 V). Lane 1, DNA only; Lane 2, 5 μM 1; Lane 3, 10 μM 1; Lane 4, 50 μM 1; Lane 5, 100 μM 1; Lane 6, 200 μM 1; and Lane 7, 400 μM 1. | |
Acknowledgements
This work has been generously supported by funding from Research Corporation (RAG: Cottrell College Science Award). The authors are also indebted to the support of The Canadian Breast Cancer Foundation (RAG: Research Project Grant), NSERC Canada (Discovery Grants: JB, SAM and RAG; USRA: MJH, JAL and MWC), in addition to the support of Acadia and Ryerson Universities. RAG was the recipient of a 2006 J. W. T. Jones Travelling Fellowship, generously supplied by the Royal Society of Chemistry, which enabled this author to carry out some of this research during a sabbatical leave. The technical assistance of Annette Hooper (SAM) is also acknowledged.
References
-
(a) N. J. Wheate, S. Walker, G. E. Craig and R. Oun, Dalton Trans., 2010, 39, 8113 RSC;
(b)
B. Lippert (Ed.), Cisplatin: Chemistry and Biochemistry of a Leading Anticancer Drug, Wiley-VCH: Oxford, 1999 Search PubMed.
-
(a) T. W. Hambley, Dalton Trans., 2007, 4929 RSC;
(b) R. A. Alderden, M. D. Hall and T. W. Hambley, J. Chem. Educ., 2006, 83, 728 CrossRef CAS.
-
M. Gielen and E. R. T. Tiekink (ed.), Metallotherapeutic Drugs and Metal-Based Diagnostic Agents: the Use of Metals in Medicine, Wiley-VCH: Toronto, 2005 Search PubMed.
-
(a) A. M. Montaña and C. Batalla, Curr. Med. Chem., 2009, 16, 2235 CrossRef CAS;
(b) E. Wong and C. M. Giandomenico, Chem. Rev., 1999, 99, 2451 CrossRef CAS;
(c) B. W. Harper, A. M. Krause-Heuer, M. P. Grant, M. Manohar, K. B. Garbutcheon-Singh and J. R. Aldrich-Wright, Chem. Eur. J., 2010, 16, 7064.
- M. D. Hall, H. R. Mellor, R. Callaghan and T. W. Hambley, J. Med. Chem., 2007, 50, 3403 CrossRef CAS.
-
T. Eicher and S. Hauptmann, The Chemistry of Heterocycles, 2nd Ed., Wiley-VCH: Weinheim, 2003, Ch. 5.23-5.25 Search PubMed.
-
(a) For example: R. A. Gossage, P. N. Yadav, T. D. MacInnis, J. W. Quail and A. Decken, Can. J. Chem., 2009, 87, 368 Search PubMed;
(b) F. J. Baerlocher, R. Bucur, A. Decken, C. R. Eisnor, R. A. Gossage, S. M. Jackson, L. Jolly, S. L. Wheaton and R. S. Wylie, Aust. J. Chem., 2010, 63, 47 CrossRef CAS.
- F. P. Intini, A. Boccarelli, V. C. Francia, C. Pacifico, M. F. Sivo, G. Natile, D. Giordano, P. De Rinaldis and M. Coluccia, J. Biol. Inorg. Chem., 2004, 9, 768 CAS.
- A. Decken, R. A. Gossage and P. N. Yadav, Can. J. Chem., 2005, 83, 1185 CrossRef CAS.
- Synthesis of 1. An analogous procedure was used as previously reported8 with the substitution of Etoxin lieu of Meox (Yield: 36%). Mp 194 °C (decomp.). 1H NMR (dmso-d6): δ = 1.20 (t, 3H, J = 7.6 Hz, –C H3), 2.83 (q, 2H, J = 7.6 Hz, –CC H2), 3.78 (t, 2H, J = 9.6 Hz, –NC H2), 4.14 (s [br], 3H, N H3), 4.45 (t, 2H, J = 9.6 Hz, –OC H2). Anal. Calc. (Found): C 15.71 (15.73); H 3.16 (3.08); N 7.33 (7.22)%. MS (EI, 70 eV): m/z = 382.0 (M+).
-
X-Ray diffraction data of 1. Formula: C5H12N2OCl2Pt, Mr: 382.16 g mol−1, crystal size: 0.425 × 0.10 × 0.025 mm3, space group: C2/c, a = 18.924(2), b = 10.8634(7), c = 11.9925(10) Å;, β = 127.540(2)°, V = 1954.8(3) Å3, T = 173(1) K, Z = 8, ρc = 2.597 g mL−1, Mo-Kα = 0.71073 Å, F(000): 1408, hkl range: –23/+22, –13/+14, –15/+14, refl. measured: 6568, refl. unique: 2194, Rint = 0.0591, parameters refined: 102, R(F)/w R(F2) (all refl.): 0.0484/0.1109, GoF (F2): 1.024, Δρfin (max./min.): 2.709/-2.530 eÅ−3. Selected bond lengths (Å) and angles (°): Pt–Cl1 2.305(1), Pt–Cl2 2.299(2), Pt–N1 1.995(4), Pt–N2 2.042(7), N1–C2 1.282(9); Cl1–Pt–Cl2 91.79(6), Cl1–Pt–N1 179.0(2), Cl1–Pt–N2 90.3(2), N1–Pt–N2 88.9(2) Cl1–N2–C7 121.1(6).
- Synthesis of 2. A sample of K2PtCl4 (0.3760 g: 0.9058 mmol) was dissolved in 7 mL of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundwater in the presence of 8 drops of c. H2SO4. To this mixture was added a sample of 2-(2′anilinyl)-4,4-dimethyl-2-oxazoline7b,9, 22 (0.1733 g: 0.9109 mmol) in the form of a solid. The mixture was then stirred rapidly at RT (overnight). The resulting precipitate was isolated by filtration and washed with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundwater (2 × 2 mL), 95% aq. COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundEtOH (1 mL) and Et2O (2 × 2 mL). The yield of yellow-orange powder was 0.2111 g (52%). Mp >200 °C. 1H NMR (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundacetone-d6): δ = 1.47 (s, 6H, –C H3), 4.42 (s, 2H, –OC H2), 6.65 (m, 1H, Ar H), 6.82 (m, 1H, Ar H), 7.28 (m, 1H, Ar H), 7.63 (m, 1H, Ar H). Anal. Calc. (Found): C 28.96 (28.44); H 3.09 (2.97); N 6.14 (6.13)%. Crystals suitable for X-ray diffraction work were obtained by recrystallisation of 2 from aq. 95% COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundEtOH at RT.
-
X-ray diffraction data of 2. Formula: C11H14N2OCl2Pt, Mr = 456.22 g mol−1, space groupP21/n, a = 6.525(2), b = 11.816(3), c = 17.129(4) Å, β = 99.99(4)°, V = 1300.6(6) Å3, T = 193(2) K, Z = 4, ρc = 2.330 g mL−1, Mo-Kα = 0.71073 Å, F(000): 856, hkl range: ±7, ±14, ±20, refl. unique = 2230, Rint = 0.0454, parameters refined = 160, R(F)/w R(F2) (all refl.) = 0.0454/0.1335, Δρfin (max./min.) = 0.428/0.313 eÅ−3. Selected bond lengths (Å) and angles (°): Pt–Cl1 2.294(3), Pt–Cl2 2.324(3), Pt–N1 2.043(7), Pt–N2 2.06(1), N1–C5 1.28(1); Cl1–Pt–Cl2 88.3(1), Cl1–Pt–N1 174.1(2), Cl1–Pt–N2 90.6(3), N1–Pt–N2 84.3(3) Pt1–N2–C7 110.0(7).
- Synthesis of 3. A sample of PtCl2 (0.12 g: 0.45 mmol) was suspended in 7 mL of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundMeOH. To this mixture was added COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound2-ethyl-2-oxazoline (0.22 g: 2.2 mmol) and the mixture stirred rapidly at RT overnight. The resulting precipitate was isolated by filtration and washed with Et2O (3 × 5 mL). The yield of cream coloured powder was 0.15 g (72%). Mp >200 °C. 1H NMR (MeOH-d4): δ = 1.29 (t, 3H, J = 7.6 Hz, –C H3), 3.00 (q, 2H, J = 7.6 Hz, –CC H2), 3.90 (t, 2H, J = 9.6 Hz, –NC H2), 4.54 (t, 2H, J = 9.6 Hz, –OC H2). Anal. Calc. (Found): C 25.87 (25.65); H 3.91 (3.51); N 6.03 (6.01)%.
- Full details on the X-ray structural characterisation of complex 3 will be published separately: Gossage, R. A.; Jenkins, H. A. unpublished results..
- L. J. Farrugia, J. Appl. Crystallogr., 1997, 30, 565 CrossRef CAS.
-
(a) For example: P. N. Yadav, R. A. Gossage and A. Decken, Anal. Sci., 2008, 24, x301 Search PubMed;
(b) A. Albinati, H. Moriyama, H. Rüegger, P. S. Pregosin and A. Togni, Inorg. Chem., 1985, 24, 4430 CrossRef CAS;
(c) R. A. Michelin, R. Bertani, M. Mozzon, G. Bombieri, F. Benetollo and R. J. Angelici, Organometallics, 1991, 10, 1751 CrossRef CAS;
(d) U. Belluco, R. Bertani, F. Meneghetti, R. A. Michelin, M. Mozzon, G. Bandoli and A. Dolmella, Inorg. Chim. Acta, 2000, 300, 912 CrossRef;
(e) Y. Chen, Z. Guo, S. Parsons and P. J. Sadler, Chem.–Eur. J., 1998, 4, 672 CrossRef CAS.
- Human ovarian carcinoma cell lines A2780 were maintained in exponential growth as monolayers in Advanced DMEM supplemented with 2.5 mM COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundglutamine and 2% fetal calf serum at 37 °C in 5% CO2. Complexes tested were prepared as 250 μM aq. solutions containing 1% DMF immediately prior to the assay. Cytotoxicity was determined using the MTT assay as previously described.19 Single cell suspensions were obtained by trypsinisation of monolayer cultures, with cell counts performed using a heamocytometer counter (Weber). Approximately 2 × 104cells in 100 μL culture medium were seeded onto each well of flat bottomed 96-well plates (Becton Dickinson) and allowed to attach overnight. Platinum complex solutions were diluted in culture medium such that 5 to 40 μL of each drug solution added to quadruplicate wells produced the final desired concentrations spanning a 4-log range (final DMF concentrations were limited to 0.3%). Following incubation of the cells for 72 h, MTT (1.0 mM) was added to each well and they were incubated for a further 4 h. The culture medium was then removed from each well and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO (150μL) (Sigma) was added, the plate shaken for 5 s and the absorbance measured immediately at 600 nm in a Victor3V microplate reader (Perkin Elmer). IC50 values were determined as the drug concentration that reduced the absorbance to 50% of that in untreated control wells.
- J. Carmichael, W. G. DeGraff, A. F. Gazdar, J. D. Minna and J. B. Mitchell, Cancer Res., 1987, 47, 936 CAS.
- M. V. Keck and S. J. Lippard, J. Am. Chem. Soc., 1992, 114, 3386 CrossRef CAS.
- S. F. Bellon, J. H. Coleman and S. J. Lippard, Biochemistry, 1991, 30, 8026 CrossRef CAS.
-
R. A. Gossage in Experiments in Green and Sustainable Chemistry (ed.: H. W. Roesky and D. K. Kennepohl), Wiley-VCH, Weinheim, 2009, Ch. 4 Search PubMed.
Footnotes |
| † Oxazoles XXVI |
| ‡ CCDC reference numbers 800341 and 800342. For crystallographic data in CIF or other electronic format see DOI: 10.1039/c0md00211a |
| § Current address: School of Chemistry, Institute of Science & Technology, Tribhuvan University, Kirtipur, Kathmandu, Nepal. |
| ¶ Undergraduate research participant; these authors contributed equally to this research. |
| || Author to whom correspondence should be addressed concerning the crystallographic characterisation of complex 1. E-mail: adecken@unb.ca |
| ** Author to whom correspondence should be addressed concerning the crystallographic characterisation of complex 2. E-mail: michael.gardiner@chem.utas.au |
|
| This journal is © The Royal Society of Chemistry 2011 |
Click here to see how this site uses Cookies. View our privacy policy here. 



