Effective multi-strain inhibition of influenza virus by anionic gold nanoparticles†
Matias
Sametband
a,
Sourabh
Shukla
a,
Tal
Meningher
bc,
Shira
Hirsh
bc,
Ella
Mendelson
c,
Ronit
Sarid
b,
Aharon
Gedanken
*a and
Michal
Mandelboim
c
aDepartment of Chemistry, Kanabar Laboratory for Nanomaterials, Institute of Nanotechnology and Advanced Materials, Bar-Ilan University, Ramat Gan, Israel 52900. E-mail: gedanken@mail.biu.ac.il; Fax: +972 3 7384053; Tel: +972 3 5318315
bThe Mina and Everard Goodman Faculty of Life Sciences, Bar-Ilan University, Ramat-Gan, 52900, Israel
cCentral Virology Laboratory, Public Health Service, Ministry of health, Sheba Medical Center, Tel Hashmer, 52621, Israel
First published on 10th March 2011
Abstract
The fight against highly contagious influenza infection is often hampered by the appearance of drug resistant strains and the inadequacy of vaccines targeted only against specific variants. In this article, we report a novel route to inhibit influenza using anionic gold nanoparticles, which show effective inhibition properties against several influenza strains. The inhibition of influenza, achieved through gold nanoparticles with different anionic groups, suggested that blocking of viral attachment to cell surface could be the primary mechanism of inhibition, although viral fusion inhibition could not be excluded. At the same time, variation in the degree of inhibition with the anionic groups indicated that the antiviral activity of the nanoparticles is not merely governed by the charge density but the functional group itself has a role to play.
Influenza virus epidemics occur on a seasonal basis, affecting millions of people around the world. The recent outbreak of the swine influenza A virus (H1N1) was declared as a pandemic by the World Health Organization (WHO), and has caused thousands of deaths worldwide.1
Vaccination is the most common approach for preventing the spread of the disease. However, the long period between viral strain appearance and vaccine development, and the rapid virus evolution, through genetic recombinations and mutations,2 call for the development of new methods to prevent the spreading of the influenza virus and to treat already infected individuals.
The influenza infection cycle is divided into several steps including specific viral attachment to the cell surface receptor, entry, fusion with the endosomal membrane, uncoating of the nucleocapsid, multiplication of the genetic material, expression of viral proteins, assembly, budding and release of the new viron.3 In recent years several new viral drugs were developed targeting different steps in the infection cycle. The most common new drugs are Oseltamivir and the recently approved Zanamivir, which inhibit the release of the new virion from the cell's membrane.4 These drugs are effective against influenza A and influenza B, yet some strains became resistant to these drugs.5
One of the critical steps in the infection cycle is the fusion between the viral and the endosomal membrane. Specifically, hemagglutinin (HA), a cell-anchoring viral glycoprotein, which is also essential for the uncoating step, undergoes an acid-catalyzed conformational rearrangement, exposing the fusogenic hydrophobic region of the HA, resulting in the fusion with the membrane.6 In recent years it was discovered that polyanions, mostly polysulfates and polysulfonates, such as sulfated polysaccharides and sulfated polymers, have broad-spectrum of antiviral activity.7 Also, quinone derivatives have shown fusion inhibition.8 It is assumed that these compounds prevent the exposure of the fusion peptide in the HA, which results in fusion inhibition.
In the current manuscript, we present a novel approach for the inhibition of influenza virus infection using anionic gold functionalized groups on their surface, whose end groups may interact with the viruses by multivalent bonds. Their small size enables the gold nanoparticles (Au-NPs) to enter the cell through the endosome vesicle, thus allowing them to possibly interfere with the fusion step as well.9 Several publications demonstrated low or no Au-NPs cytotoxicity, and their toxicity is dependent on the particle's surface charge, size and shape.10 The NPs were composed of an Au core and mercaptoethanesulfonate (MES) molecules bonded to its surface through the thiol group. The Au-NP-MES, 4 ± 1 nm formed a stable aqueous solution having a net negative charge (ζ = −35 mV) originating from the sulfonated end groups of MES. In previous work we showed that these sulfonated nanoparticles inhibit herpes simplex virus 1 (HSV-1) attachment.11 Penades et al. have recently reported that gold nanoparticles capped by sulfate-ended ligands inhibit HIV infection of T-cells.12 However, the influenza virus interacts with the host cell membrane through unique interactions between viral hemagglutinin and host cell terminal sialic acid residues, that differ from previous studied systems.
The effect of Au-NPs-MES on the influenza virus infection was determined by in vitro experiments using an Enzyme Linked Immunosorbent Assay (ELISA) based on the detection of the expressed HA viral protein. In general, different particle concentrations (0.5 to 0.004 mg ml−1) were introduced to a viral suspension (a recombinant of a vaccine H5N1 avian influenza A virus) of known titer (TCID50/ml = 3.95 x 105) and incubated for 1 h. Thereafter, these suspensions were used to infect Madin-Darby Canine Kidney cells (MDCK) while expression of the viral protein was determined 72 h post infection (ESI†). The ELISA results (Fig. 1a) indicated that the Au-NPs-MES inhibited the virus infection effectively. At Au-NPs-MES concentration of 0.5 mg ml−1 the infection level was 11–15% compared to the control (cell cultures without Au-NPs-MES added (0 mg ml−1), regarded as 100% infection).
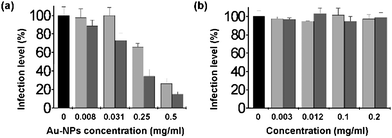 | ||
| Fig. 1 (a) Inhibition of avian influenza A (H5N1) infection by Au-NPs-MES (gray) and Au-NPs-MSA (light gray). (b) Soluble MES (gray) and MSA (light gray) inhibition test. | ||
We studied the importance of the nature of the charged end-group on the Au-NPs inhibition properties. This was done by synthesizing Au-NPs stabilized with mercaptosuccinic acid (Au-NPs-MSA). The product had an average particle size of 4 ± 1 nm with a higher negative charge as compared to the Au-NPs-MES, namely ζ = −50 mV. Similar ELISA assay with Au-NPs-MSA were carried out on the influenza A (H5N1) strain. Comparison between the inhibition properties of Au-NPs-MES and Au-NPs-MSA indicated that both particles inhibited influenza activity in the MDCK tissue culture (Fig. 1a). However, there was a difference in the effectiveness in which these particles inhibited the virus. Au-NPs-MES were more potent inhibitors as compared to the Au-NPs-MSA by ∼50% at high particle concentration. This was surprising, due to the fact that the Au-NPs-MSA has a higher negative charge, and if the mechanism of the NPs inhibition is via a virus-NPs electrostatic non-specific interaction, we expected the Au-NPs-MSA to inhibit the virus activity better than the Au-NPs-MES. To further test the charge effect on the infection inhibition, gold nanoparticles stabilized by mercaptopolyethylene glycol (n = 6) were synthesized (Au-NPs-PEG), with average size of 5 nm. These particles have been used in a previous study, for similar purposes.13 Similar experiments were conducted as with the Au-NPs-MES, in which no inhibition was observed.
This result points out to the importance of the sulfonated group, indicating that a virus-sulfonate interaction may play a unique role in the blocking of the virus activity. This result is consistent with previous reports, which indicated that the negative-charge density is not the sole factor which affects the influenza inhibition properties of polyanionic substances.14
It was important to test if the capping molecules on the Au-NPs (MES, MSA) were able to inhibit the viral infection as free rotating molecules as well, or their orientation on the Au-NPs is crucial for their effectiveness as influenza inhibitors. Similar experiments were conduced, in which pristine MES and MSA were used instead of the Au-NPs. Inductively Coupled Plasma (ICP) measurements indicated that the pristine MES/MSA concentrations tested were higher than their concentration in 0.5 mg ml−1 Au-NPs. No inhibition occurred in both cases (Fig. 1b), indicating that the orientation and multivalency of the capping molecules on the Au-NPs is important to the inhibition process. In previous work we showed that silver and gold nanoparticles, bearing the same capping molecule, inhibited HSV-1 infection.11,15 This indicated that the metal particle serves solely as a carrier agent for the active molecule.
As previously mentioned, the Au-NPs-MES and the viruses were incubated for an hour prior to their introduction with the MDCK cell-lines. A different set of tests were conducted with no pre-incubation of the Au-NPs-MES and the viruses. No difference was noticed at high Au-NPs-MES concentration. At lower concentrations (below 0.031 mg ml−1), a minor effect was detected, in which the Au-NPs-MES were less effective as compared to the pre-incubated case. These current experiments suggest that the interaction between the Au-NPs-MES and the virus precedes the interaction between the virus and the MDCK cell-lines (Fig. S1†).
It was vital to verify that the inhibition was not due to cell mortality resulting from the presence of the Au-NPs. Two methods were applied for this purpose: MTT and crystal violet assays. In both assays there was no reduction in cell viability employing the working concentrations of Au-NPs. In the case of crystal violet, only at a concentration of 2 mg ml−1 the Au-NPs was toxic to the MDCK cells, which is a much higher concentration than used in our experiments (Fig. S2†).
To further characterize the inhibition properties of the Au-NPs, we applied the hemagglutination inhibition (HAI) assay to test if the Au-NPs inhibit the attachment of the H5N1 virus to red-cell membrane (titer of 256 HAU). In this test similar results were achieved, namely: at high Au-NPs-MES concentrations (0.5 and 0.25 mg ml−1; Fig. 2, row a, 1 and 2, respectively), the particles inhibited the virus attachment to the red-cell membrane, resulting in the settling down of the red-cells. This result points out that the Au-NPs-MES inhibited the attachment of the virus to the red-cells. No inhibition occurred when Au-NPs-MSA were applied (Fig. 2, row b), indicating that these particles probably inhibit the viral infection through a different mechanism, and not by attachment inhibition.
 | ||
| Fig. 2 Hemagglutination inhibition assay – viral attachment (H5N1 strain) inhibition by Au-NPs-MES (a) and Au-NPs-MSA (b). (1) = 0.5 mg ml−1 Au-NPs, while (2–8) = consecutive two-fold serial dilutions. | ||
It should be mentioned that sulfonated and carboxylated polymers have been shown to inhibit influenza virus infection by interfering with the fusion step during the viral infection cycle.7 The current experiments suggest that the inhibition obtained by Au-NPs-MES may occur by blocking the attachment of the viruses to the cell's membrane, although fusion inhibition could simultaneously occur.
To determine the effectiveness of our anionic gold nanoparticles against other strains of influenza, we carried out inhibition assays using other common influenza strains, including: swine A/Israel/119/2009 (H1N1), A/Brisbane/10/2007 (H3N2), A/Puerto Rico/8/1934 (H1N1), B/Brisbane/60/2008 and B/Shandong/7/1997 (referred as H1N1, H3N2, PR8, B-Bris and B-Shan, respectively). In the case of the swine H1N1 strain (Fig. 3a), the Au-NPs-MES inhibited the virus infection, and was more effective than Au-NPs-MSA, similar to the H5N1 results (Fig. S3†). For the other types and strains, the results indicated that the Au-NPs-MES inhibit the virus activity in a similar manner as in the H5N1 strain tests (Fig. 3, b-e). At high Au-NPs-MES concentrations (0.5 and 0.25 mg ml−1), the infection levels were between 3–17% compared to the control. This indicates that the Au-NPs-MES represent a versatile inhibitor, thus solving one of the major problems with current influenza treatments.
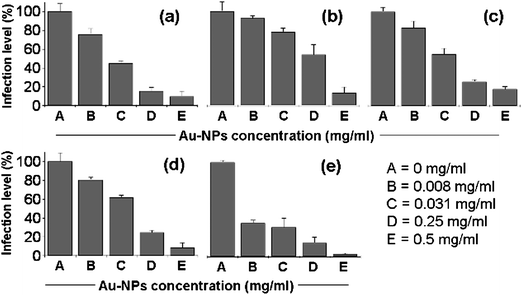 | ||
| Fig. 3 Inhibitory effect of Au-NPs-MES on infection by different influenza strains: (a) H1N1, (b) H3N2, (c) PR8, (d) B-Bris, (e) B-Shan. | ||
In conclusion, we demonstrated that small anionic gold nanoparticles inhibit influenza infection effectively. It was shown that the end group on the particle's surface is important for the interaction between the Au-NPs and the virus. These particles, which exhibited no cytotoxicity towards the MDCK cell, inhibited different influenza virus types and strains (including the recent pandemic swine influenza A (H1N1) strain), indicating that the interaction is not limited to a specific strain. This property is crucial for antiviral compounds, which need to be unaffected by random viral genetic mutations. By using different head groups it may be possible to improve the inhibition efficiency. Although, we believe that the current particles are interfering with the entry or fusion steps, a detailed study to determine the exact mechanism and to determine more efficient head groups is currently being undertaken.
The authors thank New Zealand Pharmaceuticals limited (NZP) for their kind support.
Notes and references
- http://www.who.int/csr/don/2010_02_19/en/index.html .
- W. Fiers, M. De Filett., A. Birkett, S. Neirynck and W. Min Jou, Virus Res., 2004, 103, 173–176 CrossRef CAS.
- M. von Itzstein, Nat. Rev. Drug Discovery, 2007, 6, 967–974 CrossRef CAS.
- E. De Clercq and J. Neyts, Trends Pharmacol. Sci., 2007, 28, 280–285 CrossRef CAS.
- G. A. Poland, R. M. Jacobson and I. G. Ovsyannikova, Clin. Infect. Dis., 2009, 48, 1254–1256 CrossRef.
- J. J. Skehel and D. C. Wiley, Annu. Rev. Biochem., 2000, 69, 531–569 CrossRef CAS.
- (a) M. Hosoya, J. Balzarini, S. Shigeta and E. De Clercq, Antimicrob. Agents Ch., 1991, 35, 2515–2520 Search PubMed; (b) S. Ikeda, J. Neyts, S. Verma, A. Wickramasinghe, P. Mohan and E. De Clercq, Antimicrob. Agents Ch., 1994, 38, 256–259 Search PubMed; (c) M. Lüscher-Mattli, Arch. Virol., 2000, 145, 2233–2248 CrossRef CAS.
- D. L. Bodian, R. B. Yamasaki, R. L. Buswell, J. F. Stearns, J. M. White and I. D. Kuntz, Biochemistry, 1993, 32, 2967–2978 CrossRef CAS.
- A. Verma and F. Stellacci, Small, 2010, 6, 12–21 CrossRef CAS.
- (a) C. A. Simpson, B. J. Huffman, A. E. Gerdon and D. E. Cliffel, Chem. Res. Toxicol., 2010, 23, 1608–1616 CrossRef CAS; (b) Y. S. Chen, Y. C. Hung, I. Liau and G. S. Huang, Nanoscale Res. Lett., 2009, 4, 858–864 CrossRef CAS; (c) N. Lewinski, V. Colvin and R. Drezek, Small, 2008, 4, 26–49 CrossRef CAS.
- D. Baram-Pinto, S. Shukla, A. Gedanken and R. Sarid, Small, 2010, 6, 1044–1050 CAS.
- P. D. Gianvincenzo, M. Marradi, O. M. Martinez-Ávila, L. M. Bedoya, J. Alcami and S. Penades, Bioorg. Med. Chem. Lett., 2010, 20, 2718–2721 CrossRef.
- M. E. Aubin-Tam and K. Hamad-Schifferli, Langmuir, 2005, 21, 12080–12084 CrossRef CAS.
- P. Schoen, J. Cower, D. K. F. Meijer, J. Wilschut and P.-J. Swart, Biochem. Pharmacol., 1997, 53, 995–1003 CrossRef CAS.
- D. Baram-Pinto, S. Shukla, N. Perkas, A. Gedanken and R. Sarid, Bioconjugate Chem., 2009, 20, 1497–1502 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental methods and supporting data, including cytotoxicity assay. See DOI: 10.1039/c0md00229a/ |
| This journal is © The Royal Society of Chemistry 2011 |
