Quantification of the orientations of pyrrolidine-based oxypeptide nucleic acid–DNA hybrid duplexes†
Mizuki
Kitamatsu
* and
Masahiko
Sisido
Department of Medical and Bioengineering, Graduate School of Natural Science and Technology, Okayama University, 3-1-1 Tsushimanaka, kita-ku, Okayama, 700-0082, Japan. E-mail: kitamatu@cc.okayama-u.ac.jp; Fax: +81-86-251-8219; Tel: +81-86-251-8219
First published on 28th April 2011
Abstract
We describe the fluorescence quenching-based quantification of complementary parallel and antiparallel hybrids of DNAs with pyrrolidine-based oxypeptide nucleic acids (POPNAs). When BODIPY-modified DNAs as fluorescent probe formed hybrids with complementary POPNAs, fluorescence of the BODIPY was effectively quenched by the guanine unit and the Lys unit on the POPNAs. The orientations of hybrids of POPNA with DNA were estimated by the quenching efficiencies of two BODIPY-modified DNAs. As a result, we clarified that configurations of POPNAs affect the extent of orientation of the hybrid duplexes.
Oxypeptide nucleic acids (OPNAs)1 and the related conformationally restricted pyrrolidine-based OPNAs (POPNAs)2 are DNA surrogates that bind to complementary oligonucleotides with moderate affinity and sequence specificity (Fig. 1). When hybridized to complementary oligonucleotides, the OPNAs and POPNAs have very sharp melting curves. The OPNAs and POPNAs also have high water solubility relative to Nielsen-type peptide nucleic acids (PNAs, Fig. 1),3 and this is due to the ether linkages in the main chains. These characteristics of OPNAs and POPNAs are important properties that enable the identification of single mismatches in oligonucleotide sequences in aqueous media and as such, provide useful biomedical tools and medicine based on the peptide nucleic acids.
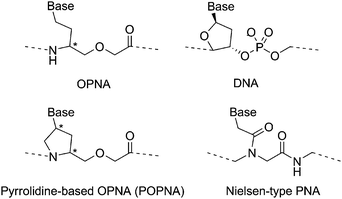 | ||
| Fig. 1 Chemical structures of OPNA, POPNA, DNA, and Nielsen-type PNA. | ||
In addition to these properties, the orientation of hybrids between peptide nucleic acids and oligonucleotides (i.e., whether the N-terminus of a hybridized peptide nucleic acid is facing the 5′-end [parallel orientation] or the 3′-end [antiparallel orientation] of an oligonucleotide) is also an important factor to be considered in the discussions of hybrid structures and in the design of optimal base sequences for hybridization to various oligonucleotide sequences. In the nucleic acid medicine without side effect, the orientation is especially important to design the base sequences. The preferred orientations of peptide nucleic acid/oligonucleotide pairs are generally evaluated by comparison of melting temperatures (Tms) of the duplex formed with complementary oligonucleotides in the parallel orientation and the antiparallel orientation.4 However, the preferred orientation of the hybrids estimated by this method is qualitative and does not enable quantification of the individual content of the two orientations of a given hybrid. Furthermore, this method cannot be used to evaluate the preferred orientation of hybrids involving biologically important palindromic DNA sequences.
In this study, we quantified the fraction of orientation of the hybrids of POPNAs with DNAs by using quenching of fluorescence tag-modified DNAs induced by the presence of a guanine moiety linked to the POPNAs. The POPNAs contain pyrrolidine rings in the main chain and the pyrrolidine ring has 2 chiral centers, which provide a total of 4 configurations (cis-L-, trans-L-, cis-D-, and trans-D-). The POPNAs form hybrids with DNA and RNA depending on the configurations of the pyrrolidine rings. The different configurations of POPNAs are expected to influence the content of the 2 orientations of the DNA hybrids.
To quantify the fraction of orientation of hybrids of POPNAs with complementary DNAs, we selected a BODIPY as the fluorophore (hereafter Bdp; 4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-5-indacene-3-propionyl group). First, Bdp moieties linked at the 5′- or 3′-end of 9-mer thymine DNAs with an intervening cytosine unit (Bdp-dCT9 and dT9C-Bdp, respectively) were purchased from Greiner BioOne (Germany). We prepared a 9-mer of adenine trans-D-POPNA with an N-terminal Lys unit and a C-terminal acetylated guanine POPNA residue (G-tD-A9-K, Fig. 2). The latter is provided as a quencher of Bdp.5 The sequence is Ac-G-AAAAAAAAA-Lys-NH2, where G, A, and Lys indicate a guanine unit of OPNA, an adenine unit of trans-D-POPNA, and lysine, respectively. The N-terminal Lys is a primary amine and the C-terminal guanine is acetylated. We also prepared a 9-mer adenine trans-D-POPNA modified with a Lys unit at the C-terminus (tD-A9-K, sequence: Ac-AAAAAAAAA-Lys-NH2, Fig. 2) as a control (without the guanine quencher). For example, if G-tD-A9-K effectively quenches fluorescence of dT9C-Bdp, but not of Bdp-dCT9, an antiparallel orientation is expected. Fluorescence spectra of G-tD-A9-K/dT9C-Bdp and G-tD-A9-K/Bdp-dCT9 before and after hybridization are shown in Fig. 3A. In both the cases, the fluorescence arising from Bdp (50 °C) is decreased when the hybrids are formed (5 °C).‡ However, fluorescence of the G-tD-A9-K/dT9C-Bdp pair appears to be more effectively quenched than that of the G-tD-A9-K/Bdp-dCT9 pair. This indicates a preference of antiparallel orientation for the G-tD-A9-K/dT9 hybrid duplex. Furthermore, the temperature dependence of the fluorescence intensity at 514 nm emitted from Bdp of these mixtures was measured (Fig. 3B).
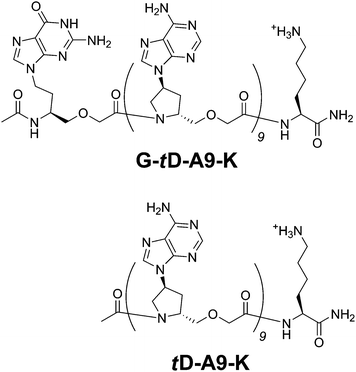 | ||
| Fig. 2 Chemical structures of G-tD-A9-K and tD-A9-K. | ||
![(A) Fluorescence spectra of the mixtures of G-td-A9-K with the complementary Bdp-dCT9 and dT9C-Bdp, (B) temperature dependence of fluorescence intensity of the mixtures of G-td-A9-K with the complementary Bdp-dCT9 (open circles) and dT9C-Bdp (closed circles), (C) fluorescence spectra of the mixtures of td-A9-K with the complementary Bdp-dCT9 and dT9C-Bdp, and (D) temperature dependence of the fluorescence intensity of the mixtures of td-A9-K with the complementary Bdp-dCT9 (open circles) and dT9C-Bdp (closed circles). Conditions: λex = 495 nm. In aqueous buffer (100 mM NaCl, 10 mM NaH2PO4, and 0.1 mM EDTA, pH 7.0). [G-td-A9-K] = [Bdp-dCT9] = [dT9C-Bdp] = 1 μM. These spectra and melting curves were recorded upon cooling of the solution. Essentially the same curves were obtained during the heating process.](/image/article/2011/MD/c0md00223b/c0md00223b-f3.gif) | ||
| Fig. 3 (A) Fluorescence spectra of the mixtures of G-tD-A9-K with the complementary Bdp-dCT9 and dT9C-Bdp, (B) temperature dependence of fluorescence intensity of the mixtures of G-tD-A9-K with the complementary Bdp-dCT9 (open circles) and dT9C-Bdp (closed circles), (C) fluorescence spectra of the mixtures of tD-A9-K with the complementary Bdp-dCT9 and dT9C-Bdp, and (D) temperature dependence of the fluorescence intensity of the mixtures of tD-A9-K with the complementary Bdp-dCT9 (open circles) and dT9C-Bdp (closed circles). Conditions: λex = 495 nm. In aqueous buffer (100 mM NaCl, 10 mM NaH2PO4, and 0.1 mM EDTA, pH 7.0). [G-tD-A9-K] = [Bdp-dCT9] = [dT9C-Bdp] = 1 μM. These spectra and melting curves were recorded upon cooling of the solution. Essentially the same curves were obtained during the heating process. | ||
The melting curve of G-tD-A9-K/Bdp-dCT9 mixture is in accord with that of the G-tD-A9-K/dT9C-Bdp mixture. Each hybrid resulted in well-defined sigmoidal melting curves similar to that of 9-mer adenine trans-D-POPNA/dT9 mixture (see Fig. S1†). These results clearly indicate that the quenching behavior is due to formation of the hybrid duplexes.§ These results are also supported by the 1/1 duplex formation obtained in the titration experiments of the mixtures (see Fig. S2†).
However, the quantitative evaluation of the content of the 2 orientations was complicated by the observation that tD-A9-K also quenches the fluorescence of Bdp-dCT9 and dT9C-Bdp to some extent with the quenching caused by the C-terminal Lys unit of the tD-A9-K. Indeed, tD-A9-K quenches the fluorescence of Bdp-dCT9 more effectively than dT9C-Bdp, due to the close proximity of the Lys unit to the Bdp of the Bdp-dCT9 in an antiparallel hybrid (Fig. 3C). The temperature dependence of the fluorescence intensity from Bdp of these mixtures was also in fair agreement with the melting curves of the G-tD-A9-K/dT9 mixtures shown in Fig. 3B (Fig. 3D). Consequently, we must evaluate the extent of orientation of the hybrids by considering the effect of quenching of Bdp by the Lys unit.
To evaluate the fractions of parallel and antiparallel orientations of G-tD-A9-K/dT9 pairs (fp′ and fap′, respectively), and those of tD-A9-K/dT9 pairs (fp and fap, respectively), the following relationships were derived. For quenching of Bdp-dCT9 and dT9C-Bdp by hybridization with G-tD-A9-K, the quenching efficiency of the G-tD-A9-K/Bdp-dCT9 pair (Q1) and the G-tD-A9-K/dT9C-Bdp pair (Q2) are expressed as follows:
| Q1 = qGfp′ + qKfap′ | (1) |
| Q2 = qKfp′ + qGfap′ | (2) |
Also, for quenching of Bdp-dCT9 and dT9C-Bdp by hybridization with tD-A9-K, the quenching efficiencies of the tD-A9-K/Bdp-dCT9 pair (Q3) and the tD-A9-K/dT9C-Bdp pair (Q4) are expressed as follows:
| Q3 = qKfap | (3) |
| Q4 = qKfp | (4) |
In the above equations, qG denotes the quenching efficiency for a pair of Bdp units and a guanine unit located on the same side of a hybrid. Similarly, qK denotes the quenching efficiency for a pair of Bdp units and Lys unit. Since Tms of tD-A9-K/dT9 and G-tD-A9-K/dT9 pairs are well above the temperature required for fluorescence measurements (5 °C), the sum of the parallel and antiparallel fractions must be unity.
| fap′ + fp′ = 1 | (5) |
| fap + fp = 1 | (6) |
By inserting the experimental quenching efficiencies into the above equations, the 6 variables (fp, fap, fp′, fap′, qG, and qK) were determined as listed in Table 1. As expected from the qualitative discussion above, the antiparallel orientation (62%) is preferred for the G-tD-A9-K/dT9 pair, where, qG = 0.91, whereas qK = 0.29.¶ The value quantified from the tD-A9-K/dT9 pair also showed that the antiparallel orientation (71%) is preferred.
| POPNA and DNA as quenchers | Quenching efficiency for | T m a/°C | f p | f ap | f ap/(fp + fap) | |
|---|---|---|---|---|---|---|
| Bdp-dCT9 | dT9C-Bdp | |||||
| a These values were estimated from melting curves recorded upon heating the solution at 0.5 °C/0.5 min. Essentially the same curves were obtained in the cooling process. Conditions: fluorescence was monitored at 514 nm with duplexes in aqueous buffer with 100 mM NaCl, 10 mM NaH2PO4, and 0.1 mM EDTA, pH 7.0). [POPNA] = [DNA] = 1 μM. b Calculated from eqn (1)–(6). The qG and qK values were 0.91 and 0.29, respectively. c Calculated using qK = 0.29, without constraints on the (fp + fap) value. d Calculated using qG = 0.91, without constraints on the (fp + fap) value. | ||||||
| G-tD-A9-K | 0.52 | 0.68 | 26 | 0.38b | 0.62b | 0.62 |
| t D-A9-K | 0.21 | 0.08 | 23 | 0.29c | 0.71c | 0.71 |
| c D-A9-K | 0.20 | 0.08 | 22 | 0.29c | 0.68c | 0.70 |
| t L-A9-K | 0.06 | 0.01 | 19 | 0.01c | 0.20c | 0.95 |
| c L-A9-K | 0.26 | 0.03 | 26 | 0.12c | 0.87c | 0.88 |
| d(A9G) | 0.88 | 0.03 | 14 | 0.03d | 0.97d | 0.97 |
| d(GA9) | 0.07 | 0.84 | 12 | 0.08d | 0.92d | 0.92 |
Since Bdp is quenched by the Lys, for the remaining configurations of POPNA, the preferred orientation was quantified from the quenching of Bdp by the Lys unit at the C-terminus of the control POPNAs (without the guanine unit) by using qK = 0.29 as determined for their hybrids. We prepared cis-D-, trans-L-, and cis-L-configured 9-mer adenine POPNA with the C-terminal Lys (cD-A9-K, tL-A9-K, and cL-A9-K, respectively) and quantified the fractions of orientation of these hybrid duplexes with dT9 by the aforementioned protocols. The results are shown in Table 1. As references, the dA9/dT9 (hybrid duplexes of d(GA9) or d(A9G) with Bdp-dCT9 or dT9C-Bdp) were also used to quantify the fractions of the 2 orientations (see Fig. S3†). These results are also listed in Table 1. All POPNAs favored the antiparallel orientation with the DNA but the parallel orientation is also allowed to some extent. In contrast, nearly complete antiparallel orientation of DNA/DNA pairs is observed (the fraction of antiparallel orientation of the dA9/dT9 hybrid is 92–97%). The L-configured POPNAs (cis-L: 88%, trans-L: 95%||) preferred antiparallel orientation to a greater extent than the D-configured POPNAs (cis-D: 70%, trans-D: 71%). These results indicate that configurations of POPNAs do not only affect the hybrid stability of POPNAs with oligonucleotides, but also affect the extent of orientation of the hybrid duplexes.
Conclusions
We successfully quantified the fractional orientations of hybrid duplexes of POPNAs with DNAs by using fluorescence quenching of the Bdp fluorophore, which occurs upon formation of the POPNA–DNA hybrid duplexes. The preferred orientation of these hybrids is the same antiparallel orientation as the DNA/DNA hybrid and the PNA/DNA hybrid. However, among POPNAs with different configurations, the content of the 2 different orientations was different. L-Configured POPNA/DNA hybrids prefer the parallel orientation over the antiparallel orientation to a greater extent than the D-configured POPNA/DNA hybrid duplexes. The method for quantification of the orientation of hybrids can be also used for other artificial nucleic acids. This method will be useful to design more effective nucleic acid medicine.Notes and references
- M. Kuwahara, M. Arimitsu and M. Sisido, J. Am. Chem. Soc., 1999, 121, 256 CrossRef CAS; M. Kuwahara, M. Arimitsu, M. Shigeyasu, N. Saeki and M. Sisido, J. Am. Chem. Soc., 2001, 123, 4653 CrossRef CAS.
- M. Kitamatsu, M. Shigeyasu, T. Okada and M. Sisido, Chem. Commun., 2004, 1208 RSC; M. Kitamatsu, M. Shigeyasu and M. Sisido, Chem. Lett., 2005, 34, 1216 Search PubMed; M. Kitamatsu, M. Shigeyasu, M. Saitoh and M. Sisido, Biopolymers, 2006, 84, 267 Search PubMed; M. Kitamatsu, T. Kashiwagi, R. Matsuzaki and M. Sisido, Chem. Lett., 2006, 35, 300 Search PubMed; M. Kitamatsu, A. Takahashi, T. Ohtsuki and M. Sisido, Tetrahedron, 2010, 66, 9659 Search PubMed; M. Kitamatsu, S. Kurami, T. Ohtsuki and M. Sisido, Bioorg. Med. Chem. Lett., 2011, 21, 225 Search PubMed; K.-H. Altmann, D. Hüsken, B. Cuenoud and C. Garcia-Echeverria, Bioorg. Med. Chem. Lett., 2000, 10, 929 CrossRef CAS.
- P. E. Nielsen, Peptide Nucleic Acids—Protocols and Applications, Horizon Bioscience, Norfolk, England, 2004 Search PubMed.
- K. Siriwong, P. Chuichay, S. Saen-oon, C. Suparpprom, T. Vilaivan and S. Hannongbua, Biochem. Biophys. Res. Commun., 2008, 372, 765 Search PubMed; T. Vilaivan and C. Srisuwannaket, Org. Lett., 2006, 8, 1897 CrossRef CAS; M. Hollenstein and C. J. Leumann, J. Org. Chem., 2005, 70, 3205 CrossRef CAS; V. A. Kumar and K. N. Ganesh, Acc. Chem. Res., 2005, 38, 404 CrossRef CAS; T. Wada, N. Minamimoto, Y. Inaki and Y. Inoue, J. Am. Chem. Soc., 2000, 122, 6900 CrossRef CAS; B. Hyrup, M. Egholm, P. E. Nielsen, P. Wittung, B. Nordén and O. Buchardt, J. Am. Chem. Soc., 1994, 116, 7964 CrossRef CAS.
- S. Kurata, T. Kanagawa, K. Yamada, M. Torimura, T. Yokomaku, Y. Kamagata and R. Kurane, Nucleic Acids Res., 2001, 29, e34 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Melting curves of POPNA/DNA pairs, fluorescence titration curves of G-tD-A9-K/Bdp-dCT9 and G-tD-A9-K/dT9C-Bdp, and fluorescence spectra of DNA/DNA pairs. See DOI: 10.1039/c0md00223b |
| ‡ The Tm of the hybrid of G-tD-A9-K and dT9 was 25 °C. See Table 1. |
| § Since Bdp of DNA adjoins a guanine unit of POPNA in order to form a hybrid between DNA and POPNA, the Bdp is quenched by the guanine unit. |
| ¶ It is assumed that qG and qK are independent of the hybrid structures. These values indicate that Bdp is quenched by G and Lys enough in the hybrid. |
| || In this case, however, (fp + fap) was not set to unity, because the Tm (19 °C) of the trans-L-POPNAs/DNA pair is not always much higher than the temperature used for the fluorescence measurements (5 °C). |
| This journal is © The Royal Society of Chemistry 2011 |
