DOI:
10.1039/C1MD00134E
(Review Article)
Med. Chem. Commun., 2011,
2, 1135-1161
Minisci reactions: Versatile CH-functionalizations for medicinal chemists
Received
24th May 2011
, Accepted 3rd July 2011
First published on 22nd August 2011
Abstract
The addition of a radical to a heteroaromatic base is commonly referred to as a Minsici reaction. Such reactions constitute a broad-set of selective CH-functionalization processes. This review describes some of the major applications of Minisci reactions and related processes to medicinal or biological chemistry, and highlights some potential developments within this area.
Introduction
The aim of this review is to summarize the use of Minisci reactions within medicinal chemistry, and to highlight some future opportunities to continue progression of this chemistry. As such, it is not an aim that detailed mechanistic information, or a comprehensive list of examples be described. For this, the reader is directed to excellent articles from Minisci, Harrowven and Bowman.1–3 Rather, the review is written to show that Minisci reactions are extremely valuable CH-functionalization processes within medicinal chemistry. However, their use has been somewhat under-utilized when compared with other well-known selective transformations (e.g. COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundpalladium-catalysed cross-couplings). Therefore, it is hoped that in the future, Minisci chemistry will continue to develop, such that the reactions become a staple-set of methods for medicinal and biological chemists alike.
To aid discussion, the review is divided in to several sections. First, some historical perspective is given. This is followed by a discussion of scope and limitations. The main-body of the review describes some specific examples of Minisci reactions and related processes, with a focus on their use within medicinal, or biological chemistry. Finally, brief mention is given to potential future applications, some of which may be beneficial in providing ‘high-content’ diverse libraries for screening.
1 Historical background
Additions of radicals to aromatic systems have an impressive history dating to an earlier era of organic chemistry. For example, the Gomberg-Bachmann reaction is a well-studied biaryl synthesis, involving the addition of aryl radicals to an aromatic system, such as COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundbenzene, that dates from the early 1920's.4 However, as early as the 1890's, chemists had also been investigating similar reactions involving the addition of aryl radicals to heteroaromatic systems, such as COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundpyridine.2a,5 Over the ensuing half century or more, modest progress was made in developing this methodology as a synthetically-useful transformation for mainstream organic chemists. For example, by the early 1960's, a number of sources for producing phenyl radical had been uncovered, but the efficiency of the reaction with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundpyridine remained low, and multiple regioisomers were produced in the addition process (Scheme 1).6 However, throughout the 1960's much progress began to be made in understanding and controlling the regioisomeric ratio encountered in the radical addition process. For example, in 1961 it was found that additions of phenyl radical to COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundpyridine-N-oxide occurred in both good yield, and with enhanced regioselectivity (for the 2-position of the pyridine ring), than the analogous reaction with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundpyridine (Scheme 2).7
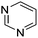
A bigger breakthrough in understanding the rate of reaction, and in controlling the regiochemistry of aryl additions to heteroaromatic bases, emerged in 1964 from the work of Lynch and Chang,8,9 and later, from Lynch and Dou.10–13 For example, Lynch and Chang proposed that the rate of addition of phenyl radical to pyridinium (and imizolium) ions was greatly enhanced when compared to that of the neutral heteroaromatic base.9 By considering calculated localization energies for radical substitution of pyridinium and imidazolium ions, which strongly favored high reactivity at the 2-position,14 Lynch and Chang also proposed that ionic species could dominate the orientation of radical substitution in these heteroaromatic systems. This led to the proposal of investigating the reactivity and selectivity of conjugate acids of heteroaromatic compounds with phenyl radicals, in order to test the above predicitions.9 A short-time later, in 1965, Lynch and Dou published the first in a series of papers, in which they gained experimental support for this conjugate acid hypothesis.10–13 As well as radical phenylation undertaken with pyridines (Scheme 3), COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundN-methylimidazole and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundthiazole, Dou and Lynch also found that protonation enhanced reaction rates and positional selectivity for phenyl radical addition to other heteroaromatic bases such as COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundquinoline, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundisoquinoline, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundbenzothiazole, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundpyrazine, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundpyrimidine, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundquinoxaline and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundN-methylbenzimidazole.10 These results led Dou and Lynch to conclude that the use of acidic conditions would make radical additions to heteroaromatic bases a synthetically-viable transformation.10
 |
| | Scheme 3 Use of conjugate acids to enhamce regio-selectivity for radical addition to heteroaromatic bases (1964–1968)8–15 | |
Later, Minisci would also demonstrate the benefit of acidic conditions to reaction rate and positional selectivity for radical additions to heteroaromatic bases.1,15 Through a series of developments and refinements that has persisted to the present day, Minisci and other organic chemists, have refined such reactions to the point where they constitute a very effective set of distinct transformations. For this reason, radical additions to heteroaromatic bases are sometimes referred to as “Minisci reactions” in the chemical literature (this description is used throughout this review). Formally, Minisci reactions represent a powerful method of CH-functionalization for heteroaromatic bases. The reactions are considered complementary to other CH-functionalization processes such as carbo-cation mediated reactions. For example, Punta and Minisci have desscribed the radical-based methods as, ‘a Friedel–Crafts-type process with opposite reactivity and selectivity’.1a
In the ensuing sections we attempt to demonstrate that Minisci reactions are attractive methods for medicinal chemists. We will detail some select examples of Minisci transformations within medicinal chemistry, and will consider some recent developments along the way, including the potential of such reactions for preparing diverse libraries from a common scaffold.
2 Scope and limitations
Minisci reactions are versatile transformations because they represent a diverse set of complementary and selective processes, making them ideal candidates for medicinal chemists looking to functionalize substances with pharmacological activity, or intermediates en-route to target compounds. In order to clearly illustrate the diversity and potential of Minisci chemistry, we will consider the heterocyclic base and radical partner in-turn.
2.1 The heterocyclic base
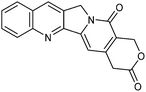
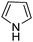
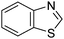
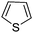
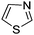
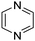
A large percentage of commerical drugs are based upon a heterocyclic core.16 Therefore, methodology to selectively functionalize a CH-bond within a heteroaromatic system should be very valuable. For Minisci chemistry, the range of heterocyclic core that has been utilized is vast. As can be seen in Table 1, 5-membered, 6-membered, 6,5-bicyclic, 6,6-bicyclic and other polycyclic heteroaromatics have all been successfully employed in Minisci transformations. Radicals can also add to non-basic heteroaromatics such as COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundpyrrole, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundthiophene and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundfuran, together with annulated versions, such as indoles. We will not dwell too much on these variants, except for a small number of choice examples.
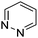
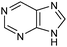
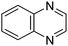
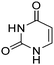
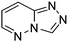
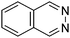
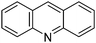
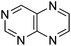
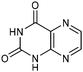
Table 1 Common heterocyclic partners encountered in Minisci reactions
| 5-Membered rings |
6-Membered rings |
6,5-Bicyclics |
6,6-Bicyclics |
Polycyclic systems |
Non-basic systems |
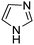
|
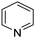
|
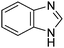
|
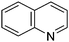
|
|
|
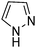
|
|
|

|
|
|
|
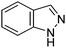
|

|
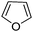
|
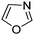
|
|
|
|
|

|
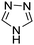
|
|
|
|
|
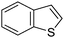
|
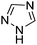
|
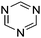
|
|

|
|
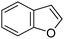
|
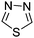
|
|
|
|
|
|
|
|
|
|
|
|
|
2.2 The radical partner
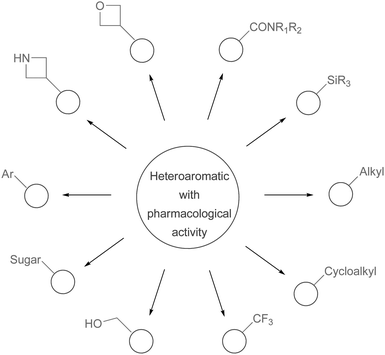
As is the case with the heteroaromatic base, a wide-array of radical groups can be utilized in Minisci reactions. Some of the more important examples for medicinal chemists are detailed in Table 2, together with some of the more common radical precursors from which they are derived.
Table 2 Representative groups added to heteroaromatics in Minisci chemistry
When the examples in Table 1 and Table 2 are considered in combination, it is apparent that there is considerable scope to these reactions; essentially, any heteroaromatic system can be open to addition of a radical, providing that a suitable position for its attack is free. Likewise, the choice of radical coupling partner is huge and varied. Since radicals are involved as reactive intermediates, protection and deprotection strategies are rarely needed, as the rate of side-reactions arising from free functionalization, is slower than the rate of attack on the heteroaromatic system. This feature of Minisci chemistry makes the reactions especially appealing for industrial applications, since time is an important economic factor.
2.3 Limitations
Although Minisci reactions are very versatile, there are limitations. In the experience of this author, there are three major drawbacks with Minisci chemistry. First, there may be the possibility of obtaining regioisomeric products if more than one appropriate position of the heteroaromatic base is open to substitution. For example, reaction of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundquinoline with methyl radical yields a mixture of regiosiomeric products (Scheme 4).17 Of course, a reaction providing more than one product can be an ‘opportunity’ for medicinal chemists, since more than one compound can be submitted for biological testing! None-the-less, the possibility of regioisomers may be a significant obstacle in some instances.
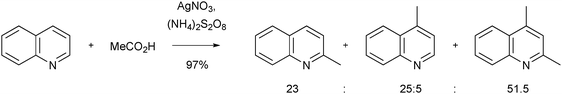 |
| | Scheme 4 Regio-isomers encountred in a Minisci reaction17 | |
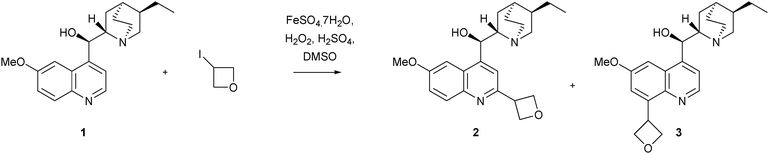
The second issue with Minisci chemistry concerns the yields. In many cases, the isolated yields can be modest (<50%), even when an excess of the less valuable reacting partner (heteroaromatic base or radical precursor) is used. It is also our experience that reactions can progress to a certain point then stop - the addition or further reagents or heating making little, if any, difference. For example, in our studies on the addition of the oxetan-3-yl or azetidin-3-yl groups to heteroaromatic bases,18 the vast majority of reactions did not go to completion and starting material, along with desired product, was usually isolated. A neat solution to reactions that progress only so-far, then stop, can be found in the synthesis of CGS 19755 (section 3.7).
The final drawback is really a manifestation of the above two limitations. Since reactions do not always completely consume the starting material, and since more than one regioisomeric product can be obtained, purifying individual target compounds to a level suitable for biological testing can sometimes be challenging. As an example, consider the addition of the oxetan-3-yl group to COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundhydroquinine 1 (Scheme 5).18 Although it was found that this reaction was very facile, and most of the starting material appeared to convert to addition products by LC/MS analysis, obtaining those products in a pure form required extensive purification by high-performance liquid chromatography. The result, was that a 27% yield of the 2-substituted product 2, together with a small quantity of the presumed 2,8-disubstituted isomer 3 were obtained, even though the reaction appeared to work better than the isolated yields would suggest.
On balance, even with some genuine limitations in less favorable examples, Minisci reactions are extremely useful. It should also be remembered that this chemistry is used to undertake some very selective CH-functionalizations (vide infra), and that protection and deprotection sequences are not always required, potentially saving time. Additionally, a vast array of diverse functionalization processes can be undertaken, meaning that more ‘chemical space’ can be investigated by judicious choice of coupling partners. Taken together, the benefits of Minisci chemistry certainly outweigh the limitations.
In the next section the utility of Minisci methodology for medicinal chemists in illustrated by examining specific cases that have been used in medicinal or biological applications.
3 Medicinal or biological chemistry applications of Minisci chemistry
3.1 Introduction of alkyl and cycloalkyl groups
For the potential of Minisci reactions in medicinal and biological chemistry, one need look no further than their use in the synthesis of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcamptothecin anti-cancer analogues. Such compounds are very important to medical science, due to their use as treatments for a variety of cancers, which is a consequence of their inhibition of Topoisomerase I.19 The synthesis of compound 5, an intermediate en-route to the marketed COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcamptothecin analogue Irinotecan 6, by Sawada and colleagues, provides a stunning example of this chemistry. Thus, reaction of unprotected 20(S)-camptothecin 4 with an ethyl radical (generated from an aldehyde precursor), gave 7-ethyl camptothecin in 77% yield (Scheme 6). Compound 5 could be elaborated to Irinotecan in 3 steps.20 Additionally, the same research group used this selective functionalization reaction to investigate structure–activity relationships about the 7-position of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcamptothecin.21 Since these pioneering reports, Minisci reactions have been used extensively to prepare a multitude of other COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcamptothecin analogues, many of which are either marketed, or are being examined in clinical trials. It is beyond the scope of this review to detail every example of such reactions from the extensive patent and research literature. However, an illustration of some representative examples can be found in Scheme 7.22–26 Overall, the use of Minisci reactions in the COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcamptothecin arena provides a beautiful illustration of the utility and potential of this chemistry; one might describe this work as a text-book example of the functional group compatibility and selectivity of CH-functionalizations using radical processes in general, and Minisci chemistry in particular.
Subsequent to the very impressive Minisci chemistry used to prepare COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcamptothecin derivatives, there have been other noteworthy uses of the reaction to introduce alkyl groups to give compounds with pharmacological activity. For example, Jain has used Minisci reactions to effectively introduce alkyl and cycloalkyl groups in to histidines and histamines, in an unparalleled regio-specific manner.27–33 Similar reactions have also been used to introduce alkyl groups in to 1,2,4-triazoles.34 One application of this work is in the synthesis of selective Thyrotropin-Releasing Hormone (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundTRH) Receptor 2 agonists with in vivo activity.30,32,33 Thus, compound 7 was synthesized using a Minisci reaction of a protected COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundhistidine with a cyclopropyl radical (derived from COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcyclopropyl carboxylic acid) as the key step (Scheme 8).31 Compound 7 was shown to be a potent agonist for THR-2 receptors (EC50 = 0.41 μM), with >250-fold selectivity over related THR-1 receptors.31 Additionally, 7 was active in an in vivo model of pentobarbitol-induced sleeping time at a dose of 10 μmol/kg. Interestingly, a close analogue 8, which was also prepared using a similar Minisci reaction as a key step (Scheme 8), showed an increase in pentobarbitol sleeping time.33
Jain and colleagues have continued with their studies on the application of Minisci chemistry within a medicinal setting by preparing new COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundquinoline compounds with anti-tuberculosis activity.35–38 Such agents are sorely needed as TB kills many millions of individuals each year.39 Jain's work has led to the identification of several new anti-TB agents, such as compounds 9–11. These molecules are believed to exert their biological effect through a novel mechanism of action, since they do not inhibit M-tuberculosis-purified DNA-gyrase (a target of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundquinolone anti-TB compounds). Some features of the Minisci reactions used to synthesize these analogues is worthy of discussion. As mentioned previously (Section 2.3), it is sometimes possible to obtain more than one addition product in certain instances. In the present work this ‘limitation’ of Minisci chemistry had a fortuitous outcome. Thus, radical alkylation of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundlepidine under traditional Minisci conditions (a COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcarboxylic acid serving as the radical precursor), led to the isolation of mono- and di-alkylated products (Scheme 9). The di-alkylated product 10, was shown to have a more favorable biological profile than its mono-alkylated congener 9. Similarly, compounds 12 and 13 were shown to be particularly interesting analogues, from another set of Minisci reactions; other leading anti-TB compounds were also synthesized in an analogous manner (Scheme 10). Overall, the results from this work may open another avenue of opportunity for finding new treatments for TB patients.
Another excellent example of the utility of Minisci chemistry for the introduction of alkyl or cycloalkyl groups, concerns the synthesis of selective GABAA α2/α3 selective agonists from Carling, Street, Castro and their colleagues at Merck.40 Such α2/α3 agonists were of interest as they were shown to retain anxiolytic properties, but were non-sedating in animal models. The synthesis of a development candidate TPA023 14 is shown in Scheme 11. It is noteworthy that the tert-butyl group could be introduced by Minisci chemistry at a late- or early-stage of the synthesis (radical precursor tBuCO2H), thus allowing for some flexibility in examing structure–activity relationships (SAR). Cycloalkyl groups were also introduced using the same methodology. However, the biological profile for these compounds was not as favorable to that of TPA023. A somewhat similar example concerns the investigation of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundHistamine H2-Receptor antagonists as detailed by Lipinski, LaMattina and Hohnke (Scheme 12).41
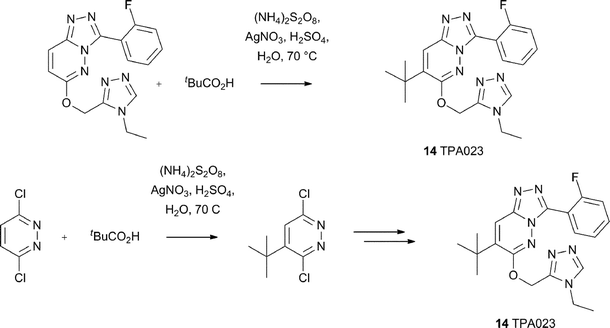 |
| | Scheme 11 Synthesis of TPA023, an α2/α3 selective GABAA agonist40 | |
The application of Minisci chemistry has also found novel uses in biological chemistry. For instance, Minisci reactions have been used to prepare functional organo-receptors via a tri-directional coupling of cis-1,3,5-cyclohexane-tri-COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcarboxylic acid and a heteroaromatic building block (Scheme 13). The resulting tripodal adducts functioned as carbohydrate receptors, with particular sensitivy for α-octyl-glucopyranoside. For example, receptor 15 displayed the highest binding affinity for noncovalent binding of an α-glucopyranoside in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundchloroform, reported by 2008. Significantly, receptor 15 could also bind monosaccharides in polar solvents such as COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundmenthanol.42
As a final offering in this section, it has been shown that Barton esters and alkyl xanthates may be used as radical precursors for Minisci reactions.43–45 For example, Barton and co-workers showed that reactions may be used to introduce alkyl groups in to biologically-active molecules such as COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcaffeine, and other purines (Scheme 14). Jarman and colleagues have used this particular variant of Minisci chemistry to synthesize steroid 16, a relative of the marketed compound, Abiraterone 17, a recently approved treatment for prostate cancer (Scheme 15). Compound 16 was shown to be a reversible inhibitor of human cytochrome P45017α, (IC50 = 27nM), compared to the irreversible inhibition observed with Abiraterone (IC50 = 5nM).46
3.2 Introduction of trifluoromethyl, difluoromethyl and perfluoroalkyl groups
The trifluoromethyl group is a very important substructural element within many drug molecules.47 A reason behind its success lies with the unique qualities of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundfluorine in modulating numerous physicochemical and biological characteristics in an advantagous manner. For example, beneficial effects upon biological activity and metabolic stability are well-documented for many compounds.48 Recently, Yamakawa and co-workers have introduced some impressive trifluoromethylation procedures based upon a Minisci reaction with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundiodotrifluoromethane (ICF3).49–51 For example, a 50kg-scale preparation of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound5-trifluoromethyluracil (5-TFU) 19 from COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compounduracil COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound18 has been devised (Scheme 16).49 5-TFU is a valuable intermediate in both the medicinal and agricultural chemistry disciplines. The method could also be extended to other COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compounduracil analogues, including nucleosides. For example, the mechanism-based thymidylate synthase inhibitor, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundTrifluridine COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound20, could be synthesized using this methodology. Interestingly, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundiron sources such as COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundferrocene (Cp2Fe) could also be used in the reaction, and sometimes gave superior yields of trifluoromethylated product (Scheme 16).
Yamakawa has also broadened the scope of this trifluoromethylation procedure, so that other heteroaromatic bases may be used as starting materials.50,51 For instance, pyridines, pyrimidines, pyrazines, thiazoles, triazoles, thiadiazoles, pyrazoles, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundpyrazolone and oxazoles, amongst others, have all been successfully employed (Scheme 17). One important difference for the trifluoromethylation reactions, when compared to their traditional Minisci counterparts, concerns the nature of the radical intermediate. The introduction of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundfluorine changes the nature of the COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcarbon-centered radical to an electrophilic species, rather than a nucleophilic radical encountered in most Minisci transformations.52 Thus, activating groups such as amino substituents were sometimes needed in order to increase the reactivity of the heteroaromatic base,51 although the role of these substituents may be somewhat more complicated (vide infra). The scope of such reactions has also been extended further to encompass the synthesis of ethoxycarbonyldifluoromethyl analogues. For example, a fluorinated COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundazaindolone derivative 21 could be prepared by reaction of a difluorocarboxylate radical with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound2-aminopyridine (Scheme 18).53 As to be expected for an electrophilic process, reaction took place at the position ortho- to the amine substituent, in this case, the 3-position of a pyridine ring.
The work from the above studies will undoubedly be useful, and will complement other methodology to introduce a trifluormethyl group based upon well-known cuprate and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundpalladium methodology.47 A couple of extensions for this chemistry may also be worthy of investigation. For example, it may be interesting to investigate the use of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundtrifluoroacetic acid rather than COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundiodotrifluoromethane as the radical precursor in Schemes 16 and 17. Second, extending the reaction to prepare COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundpentafluorosulfur analogues could also be considered, as COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundbromopentafluorosulfane (F5SBr) and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundchloropentafluorosulfane (F5SCl) are known to be precursors of the F5S radical (F5S˙).54 Therefore, it may be possible to substitue CF3I for BrSF5, or ClSF5, in a Minisci-type reaction, to enable facile introduction of SF5 in to heteroaromatic systems. Since there has been increasing interest in the biological properties of SF5-containing molecules,55 a one-step process to introduce this group could present an interesting opportunity.
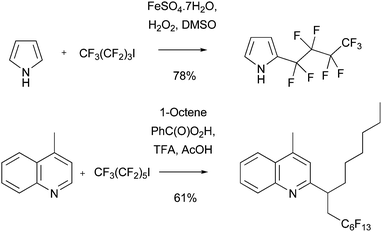
Related radical processes to selectively introduce perfluoroalkyl substituents in to electron-rich heteroaromatic systems such as COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundpyrrole are also known (Scheme 19).56 In contrast, perfluoroalkylation of electron-deficient heteroaromatic bases can result in poor chemo- and regio-selectivity. For example, perfluoroalkylation of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundquinoline results in the formation of eight addition products by GC-MS analysis.57a This result was ascribed to enthalpic-effects acting as the driving-force for the reaction with the electrophilic perfluoroalkyl radical, as opposed to the usual situation in Minisci transformations, where kinetic polar-effects dominate, and selective reaction with nucleophilic radicals occurs.57a This suggested a method for controling the selectivity in the addition of perfluoroalkyl radicals to heteroaromatic bases, by undertaking the reaction in the presence of an alkene. The perfluoralkyl radical reacts faster with an electron-rich alkene, than with an electron-poor protonated heteroaromatic base (because both enthalpic and polar effects are favorable for reactions with alkenes). The new radical intermediate from addition of the perfluoroalkyl group to the alkene posesses sufficient nucleophilic character, such that kinetic polar-effects can control a further selective addition to a protonated heteroaromatic base, resulting in formation of a single perfluoralkylated addition adduct (Scheme 19).57a In a further demonstration of how consideration of enthalpic and polar effects can be utilized to control reactivity and selectivity with perfluoroalkyl radicals, Minisci and colleagues have also undertaken selective reactions with quinones.57b Since perfluoroalkyl groups are also useful in medicinal chemistry settings, and have also been used for separations by fluorous technology,58 perfluoroalkylation methodology will also be of interest to a broad chemistry audience. Presumably, consideration of enthalpic and polar effects for the transformations outlined in Schemes 16–18 may similarly account for the reactivity and selectivity observed in these processes.
 |
| | Scheme 19 Perfluoroalkylation using Minsci chemistry56,57 | |
3.3 Introduction of aryl groups
As can be seen in Section 1 of this review, additons of aryl radicals to heteroaromatic bases have a long history. Some applications of this methodology to medicinal or biological chemistry can be seen in Scheme 20.59 For example, addition of aryl groups to COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundpteridine heteroaromatic cores gives a range of substituted products in ca. 15–65% yield. In the examples detailed in Scheme 20, the radical precursor is an arenediazonium salt, the radical being generated under traditional Meerwein conditions (base).
Recently, other methods for generating an aryl radical have been developed. For example, mild conditions to undertake radical formation from aryl iodides using COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundpotassium tert-butoxide have been reported.60 The resulting radical could be added to heteroaromatic bases to give the coupled products in 56–98% yield, although the heteroaromatic base was used in a 40-fold excess (Scheme 21).
At this juncture, it would also be appropriate to discuss some new developments using COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundboron species as radical precursors. Boranes, boronic acids or boronic esters are well-known radical precursors, although radical reactions with boranes or boronic esters are more common than other COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundboron species.61 Minisci-type chemistry with trialkylboranes has also been known for sometime. For instance, reaction of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundtriethylborane with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundlepidine furnished a 62% yield of the addition adduct 22 using an aqueous acidic system (Scheme 22).62 More recently, a number of research groups have began to examine the use of boronic acids in Minisci-type reactions. Formally, such processes represent a change of the radical precursor from the commonly-encountered COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcarboxylic acid, to a COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundboronic acid. Demir, Reis and Emrullahoglu were the first to investigate these processes.63 They found that reaction of a heteroaromatic base with an aryl radical, generated from a COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundboronic acid precursor and 3 equivalents of Mn(OAc)3, furnished the expected biaryl products in moderate yield (Scheme 23). Similar results have been observed by Guchhait et al.,64 and again by Demir et al.,65 using a microwave-assisted variant of the reaction (Scheme 23). However, there are some drawbacks to the Mn(OAc)3 reactions as currently practised. For example, the heteroaromatic base tends to be used in large excess with respect to the COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundboronic acid radical precursor. Additionally, high temperatures are frequently used for the reactions. These limitations may restrict the above processes in medicinal chemistry applications.
Considerably more mild conditions to effect a Minisci-style arylation with boronic acids have recently been described by Baran and co-workers.66 The reactions employ 1.5 equivalents of an arylboronic acid, 1.0 equivalent of a protonated heteroaromatic base, and utilize Minisci's AgNO3 and K2S2O8 oxidation conditions at room temperature. Some impressive examples were undertaken. For instance, similar to our own experience of Minisci reactions with cinchona alkaloids,18 Baran found that unprotected substrates could participate in such reactions. For example, the anti-malarial natural product COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundquinine was cleanly arylated in 40% yield with 4-phenoxyboronic acid (Scheme 24).
The use of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundboron species in Minisci reactions will likely prove to be a valuable addition for medicinal chemists, since many departments carry large collections of these building blocks, due to their widespread use in Suzuki reactions. This area of Minisci chemistry is also ripe for further development. For example, it would be interesting to see whether other boronic acids such as alkyl-, cycloalkyl- (e.g. cyclopropyl), or heteroaryl-boronic acids can be used as radical precursors in Minisci reactions.67 Additionally, the possibility of using COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundN-methyl iminodiacetic acid (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundMIDA) boronates, and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundpotassium trifluoroborates, as alternatives to boronic acids would also be worthwhile, as these compounds benefit from increased bench-top stability compared to their COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundboronic acid counterparts.68,69 On this theme, Molander has recently reported the use of alkyl trifluoroborates as radical precursors in Minisci reactions, the processes employing Mn(OAc)3 to promote radical formation (Scheme 25).70a Importantly, the trifluoroborates and heteroaromatic bases are used in equimolar quantities, and proceed under relatively mild conditions (AcOH, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundH2O, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundTFA, 50 °C).70a These features may render the Molander conditions more attractive for medicinal chemists than the alternative Mn(OAc)3 reactions described above. It will also be interesting to see whether Molander's conditions can be transferred to arylboronic species, be they trifluoroborates, boronic acids, or boronic esters (including COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundMIDA esters).
3.4 Introduction of oxetan-3-yl and azetidin-3-yl groups
The oxetan-3-yl substituent has recently been characterized as a privileged motif within medicinal chemistry.71 For example, introduction of an COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundoxetane module can result in changes to drug-like properties, resulting in improvements to characteristics such as metabolic stability and hERG activity by modulating log P, basicity and amphiphilicity.72 The oxetan-3-yl group has also been found to be acceptable when model compounds containing this group were screened for the formation of reactive metabolites.73 Azetidin-3-yl substituents are also prominent motifs within medicinal chemistry, being a common substructural feature of numerous agents that act through the central nervous system (CNS).74
Recently, our own research group has described the use of a Minisci reaction to introduce both oxetan-3-yl and azetidin-3-yl groups, using the appropriate iodides as the radical precursor (Scheme 26).18 The reactions could be undertaken on some highly functionalized substrates such as the anti-malarial, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundhydroquinine 1 (Scheme 5), and the marketed anti-cancer compound, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundGefitinib COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound23 (Scheme 26). The product from the addition to COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundGefitinib, compound 24, has been tested in biological screens and results will be disclosed soon. In addition to the reactions above, a wide-range of heteroaromatic bases could also be utilized, with isolated yields of the oxetan-3-yl, or azetidin-3-yl products ranging from 5–50%.
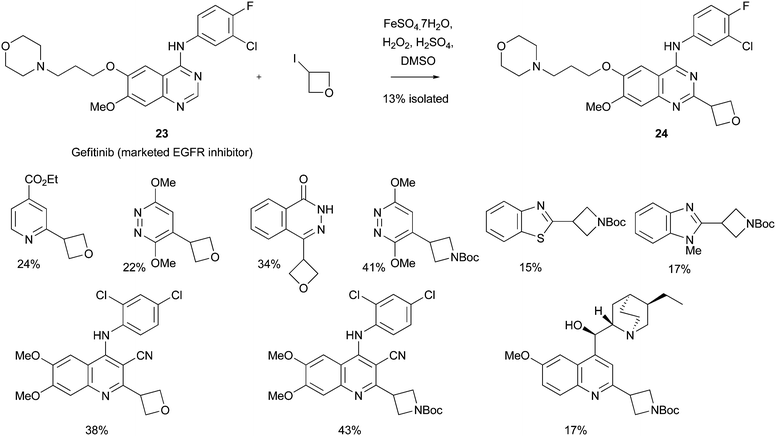 |
| | Scheme 26 Introduction of the oxetan-3-yl and azetidin-3-yl groups18 | |
Read more about this on ChemSpider
Download mol file of compoundfuran, or furanose module, may be efficiently introduced in to a heteroaromatic base using a radical approach.75–77 The reaction may also be extended to hexoses. Thus, this methodology provides a unique synthesis of C-glycosides (Scheme 27), which are of interest for their potent anti-viral and anti-tumor activity, amongst others.78 For future applications, it would also be interesting to see whether di-saccharides and poly-saccharides could also be added to heteroaromatic bases using this methodology, since complex sugars are encountered in many biological processes.
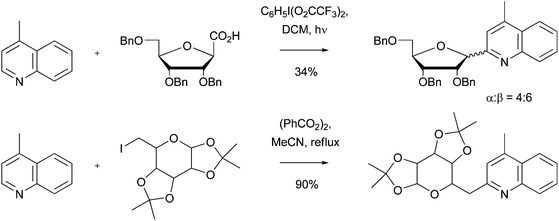 |
| | Scheme 27 Introduction of furanoses and hexoses to provide anti-viral compounds75–78 | |
Read more about this on ChemSpider
Download mol file of compoundbromopyrimidine COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound25 afforded the COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundbenzoyl adduct 26 in 61% yield on a 42g-scale (Scheme 30).82a Compound 26 could be elaborated to a potent COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundtriazole anticonvulsant 27, which was shown to possess activity superior to COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundvalproic acid (Depakene®) in two in vivo anticonvulsant screens. Similar reactions could yield COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundpyrimido[1,4]diazepin-2-one anticonvulsants.82b Acyl radicals have also been utilized to provide a number of acyl pteridines (Scheme 30).59b,83 Compounds containing the COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundpteridine structure have many biological functions, being present in important co-factors such as COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundtetrahydrofolic acid.84 Additionally, derivatives of acyl pteridines are known to possess a multitude of medicinal properties.85
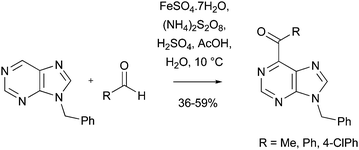 |
| | Scheme 28 Synthesis of acylpurines using Minisci chemistry80 | |
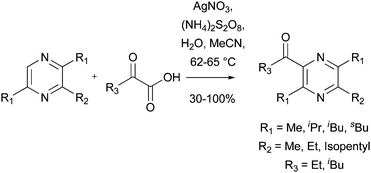 |
| | Scheme 29 Preparation of ant phermones81 | |
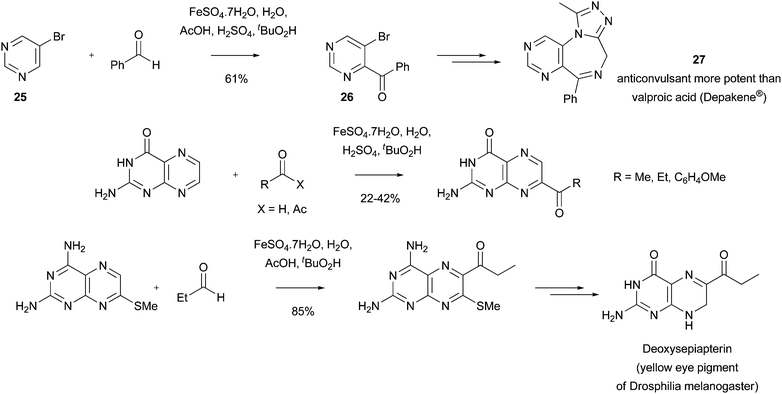 |
| | Scheme 30 Preparation of an anti-convulsant and a naturally-occurring pigment using Minisci acylations82,83 | |
Radical-based formylation reactions have also been developed.86 Although there are few examples on the use of these reactions in medicinal chemistry, the aldehyde (or masked aldehyde) products are useful for reductive-amination chemistry, and other functionalizations (e.g. reaction with Grignard reagents and other organometallics).
Primary, secondary and tertiary amides, as well as esters, can all be introduced using Minisci chemistry.1a,87 One of the first examples of an amide reaction being used in a medicinal setting, concerns a large-scale synthesis of the COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundN-methyl-D-aspartate (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundNMDA) antagonist, CGS 19755 30.88 Thus, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethyl isonicotinate COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound28 (25g) was amidated to give 29 in 30–40% yield (Scheme 31). As mentioned in Section 2.3, one of the limitations with Minisci chemistry is that the reactions have a tendancy to progress so far, then stop. The authors of this present study partly overcame this limitation by working-up the reaction, then repeating the same transformation on the crude mixture (consisting of ca. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 starting material COMPOUND LINKS
1 starting material COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound28 and desired product 29). In this way, a 60% yield (19.3g) of desired amide 29 could be obtained after crystallization. The tactic employed above, of repeating a reaction after workup, may be a useful method for improving yields of other Minisci transformations that proceed in modest conversion.
Another important example for the introduction of an amide group concerns the synthesis of the marketed multi-kinase inhibitor, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundSorafenib COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound31, which is approved to treat advanced renal cell carcinoma and advanced primary hepatocellular carcinoma.89 COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundSorafenib, which is classified as a multi-kinase inhibitor, can be prepared using Minisci chemistry on an early precursor, albeit in a very low 5% yield (Scheme 32).90 In an interesting modification, it has recently been shown that the primary amide can be introduced on a large-scale, the product also serving as a precursor to Sorafeninib (Scheme 32).91 Similar industrial utilities of Minisci chemistry to introduce amides have appeared frequently in both the patent and primary literature. For example, BAY 50-9193 33, an amide analogue of the clinical VEGFR-2 inhibitor, PTK787 32, was prepared in modest yield using Minisci chemistry (Scheme 33).92 The introduction of an amide group in to the pyridine ring of PTK787, to give compound 33, may have beneficial effects upon CYP3A4 inhibitory activity. In other examples, researchers from Roche have used an amidation reaction to prepare compound 34 (possibly, an intermediate to endothelin receptor antagonists) on a 132g scale (Scheme 33),93 and a group from BASF have used a Minisci amidation, on a 42kg-scale, in a synthesis of a highly potent thromin inhibitor 35 (Scheme 33).94
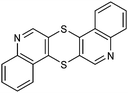
Amidation reactions have also been useful to prepare natural products with pharmacological activity. For example, Göksh and colleagues have used an amidation process in their studies of Vertilecanin natural products, which have shown antibacterial activity against Bacilus subtilis (ATCC 6051), amongst other biological properties.95 Thus, amide 37 was synthesized in 50% yield from precursor 36 (Scheme 34). The amide product could be elaborated to Vertilecanin C 38 in one step. The methodology was short and flexible, which allowed for the synthesis of alternative analogues related to the Vertilecanin family. In a similar manner, amidation reactions can also be undertaken with the thioquinanthrene core (Scheme 34).96
Finally, in this section, Minisci reactions can be used to introduce both esters and amides (Scheme 35).83a,92 Such reactions may be valuable for gaining access to COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundpteridine analogues that function as Ricin A chain inhibitors.97
The introduction of hydroxymethyl groups can be undertaken efficiently using Minisci chemistry. COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundHydrogen abstraction from COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundMeOH yields the CH2OH radical. This radical is highly nucleophilic, and reacts with heteroaromatic bases very readily to give the expected substitution product(s).1a,98 However, the reaction may suffer from some drawbacks. For example, Minisci has found that an aldehyde by-product may also be formed in significant quantities, even when a large excess of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundmethanol is used.1a,99 Poly-hydroxymethylation can also occur when more than one free position is available for reaction. For example, in their studies of inhibitors of gastric acid secretion, Mitchell and co-workers found that mono-hydroxymethylation of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound4-chloropyridine COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound39 proceeded in 20–30% yield.100 One problem was the formation of a bis-hydromethylated product 41, together with unreacted starting material (Scheme 36). In order to improve the yield of desired product 40, an ingenious solution was devised. This involved using an N-methoxypyridinium salt starting material 42, to provide an electrophilic COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundpyridine that would react under neutral conditions. The thinking behind the use of this species was that if the pyridinium species lost the nitrogen substituent during the course of the hydroxymethylation, the product should be considerable less electrophilic than the starting material, thus allowing for the selective synthesis of compound 40. Further consideration of the reaction mechanism indicated that the process could be made catalytic in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundammonium persulfate. The results of the optimization were dramatic; hydroxymethylation of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound4-chloro-N-methoxypyridinium tetrafluoroborate 42 (made from COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound4-chloropyridine-N-oxide), with sub-stoichiometric amounts of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundammonium persulfate in refluxing COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundMeOH, gave the desired mono-hydroxymethylated product 40 in 71% isolated yield (Scheme 36). Other N-methoxypyridinium tetrafluoroborates could also be reacted efficiently.100 This general strategy of increasing the reactivity of heteroaromatic bases by using pyridinium salts (similar results can also be observed with N-oxides),101 may be worth remembering when other difficult Minisci procedures are encountered.
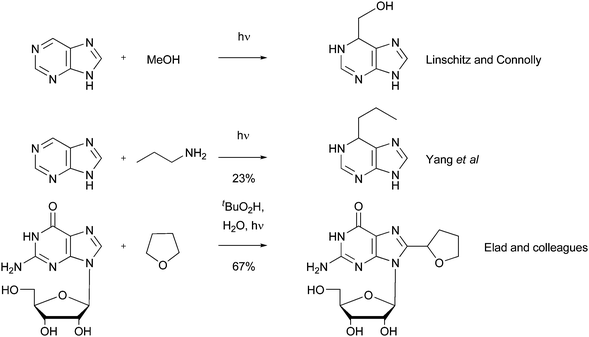
Although bis-hydroxylation may be observed in some circumstances, it is not always encountered. For instance, an interesting example of a radical mono-hydroxymethylation was provided in the synthesis of a transition state analogue inhibitor of adenosine deaminase.102 Addition adducts 44–46 were synthesized by a UV-induced reaction of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundmethanol with purine ribonucleoside 43, a known reversible inhibitor of adenosine deaminase (Scheme 37). Similar to early results with radical additions to DNA-bases (Section 3.12), the products from the reaction were shown to be a diasteromeric mixture of dihydro-derivatives in a combined yield of 54% (25% diastereomer A COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound44 and 29% diastereomer B 45), together with an oxidized addition product 46 (yield not disclosed). The diastereomers COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound44 and 45 could be separated, and one diastereomer (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound44) was shown to be an exceptionally potent inhibitor of adenosine deaminase from calf duodenum (Ki = 0.76 × 10−6).102
Another application of the hydroxymethylation reaction has also been provided in the preparation of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundtetrahydro-1,8-naphthyridol analogues of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundα-tocopherol that function as antioxidants of lipid peroxidation. Since lipid peroxidation has been associated with pathologies such as cardiovascular disease, neurodegeneration and cancer,103 such compounds may be very valuable. For example, in the synthesis of a key compound known as N-TOH 49, a COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundnaphthyridine starting material COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound47 was selectively hydroxymethylated to provide addition adduct 48 in 88% yield (Scheme 38).104
Introduction of aminomethyl groups has also been undertaken successfully. A particularly useful procedure was developed by Cowden at Merck and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundCo.105 Thus, reaction of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compounddichloropyrazine with a protected amino acid affords the aminomethylated product 50 in good yield (Scheme 39). Minisci hydroxymethylation and aminomethylation reactions have been used extensively in COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcamptothecin, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundpteridine and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundnicotine chemisty (see also Sections 3.1, 3.6 and 3.7).21a,106–111 For instance, such reactions are used as key steps in the synthesis of gimatecan 51 and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundwater soluble aminomethyl camptothecin analogue 52 (Scheme 39). Gimatecan is currently being examined in clinical trials. Analogous reactions can also provide analogues of the natural COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundpterin, asperopterin-B, as well as intermediates en-route to COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundfolic acid, and “aza-COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundnicotine” analogues 53 (Scheme 39). A further illustration of such chemistry, with many similarities to the chemistry outlined in Scheme 37, concerns the hydroxymethylation and aminomethylation of the natural nucleoside, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundnebularine, protected as a tri-O-benzoate ester (compound 54).112 The benzoate esters were removed after the Minisci reactions to provide C-alkylated derivatives of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundnebularine, such as COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound55 and 56 (Scheme 39).
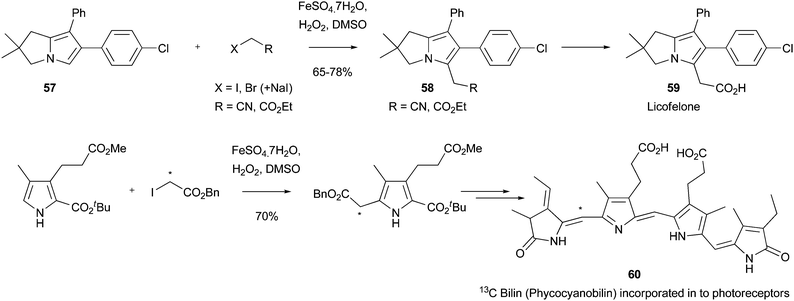
Other useful applications of Minisci chemistry to introduce functionalized methyl groups include the synthesis of licofelone 59 (ML3000), a compound that recently completed Phase III clinical trials as an analgesic and anti-inflammatory agent.113 Thus, advanced intermediate 57 was alkylated under radical conditions using COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundiodoacetonitrile, or a haloacetate as the radical precursor, and a COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO/FeSO4.7H2O system (Fenton conditions) to provide addition adduct 58 (Scheme 40).114,115 Other sulfoxides were also investigated for an ability to promote the reaction of 57 to 58, but none proved as effective as COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundDMSO. The use of Minisci chemistry was especially noteworthy in the above synthesis, as previous transformations of 57 to licofelone 59 used COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compounddiazoacetate reagents, or COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundoxalyl chloride.113
Another particularly interesting application of a similar heteroaryl COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundacetate functionalization has been undertaken to provide 13C-labelled COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundbilin compounds, e.g.60 (Scheme 40).116 The labelled bilins synthesized in this study could be incorporated in to biological photoreceptors such as phytochromes, and may be useful for following conformational changes induced by photoisomerization of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundbilin chromophores.116
3.9 Introduction of ketyl groups (Emmert reaction)
Related to the introduction of hydroxymethyl groups outlined in Section 3.8, and acyl groups outlined in Section 3.6, is the reaction of aldehydes or ketones with heteroaromatic bases, in the presence of aluminium or COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundmagnesium, and mercuric salts (COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundiodine sometimes being used as an initiator). Such processes are known as Emmert reactions, and pre-date modern Minisci chemistry, as they were described in the late-1930's.117 Modern variants of the reaction can also be undertaken with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundlithium or COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundsamarium(II) iodide.118,119
An example from 1948 details the use of an Emmert reaction to prepare substituted pyridines as histamine antagonists.120 A key intermediate 61 was prepared from COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundpyridine and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundbenzaldehyde in 39% yield. Intermediate 61 could be elaborated to the desired target histamine antagonist 62 (Scheme 41). Similarly, α-methyl congener 63, which was also a potent histamine antagonist, was synthesized starting from COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundpyridine and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundacetophenone, although a slightly modified procedure was used for the key radical-addition step. A similar use of an Emmert reaction has recently provided COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound2-pyridyl-benzopyran derviatives, such as COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound64, as potassium channel openers (Scheme 41).121
The use of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundsilicon within medicinal chemistry has been an active area of research, especially within recent years.122 An example of the advantageous use of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundsilicon within biologically-active scaffolds, is provided by the ‘Silatecans’ which constitute an important class of hydrophobic COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcamptothecin derivatives. The increased hydrophobicity of silatecans offers advantages over other COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcamptothecin derivatives, such as superior plasma stability, and an improved pharmacokinetic profile.26,123 A lead member of this group is DB-67 65, which is currently in Phase II clinical development.124 In a large-scale and practical synthesis of DB-67, and a series of closely-related analogues, Curran and colleagues developed a COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundsilicon-variant of standard Minisci chemistry in order to functionalize COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcamptothecin COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound44 or a COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compounddiacetate protected COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcamptothecin analogue 66 (Scheme 42).125 The method uses a silane as the radical precursor and a thiol/peroxide to facilitate H-abstraction, providing the R3Si radical (R3Si˙). The use of this protocol could be undertaken on a >50g scale. The reaction also provided small quantities of 12-substituted COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcamptothecin derivatives as minor by-products. Curran and colleagues concluded that this radical-addition method was shorter and higher-yielding than a previous total synthesis, even though the yields for the addition of R3Si radical were relatively modest. With this well-tried and trusted method in-hand, future studies may examine the generality of the process for introducting R3Si groups in to heteroaromatic systems other than the COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcamptothecin core. Another Silatecan in clinical studies is Karenitecan (BNP1350) 67 (Scheme 42), which is prepared in a similar manner to the chemistry originally utilized by Sawada and colleagues outlined in Section 3.1.126 A related analogue, which contains a novel “flipped” lactone E-ring, and a COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundgermanium side-chain, can also be synthesized using similar methodology (Scheme 42).127
3.11 Intramolecular reactions
Intramolecular variants of Minisci's chemistry have been used extensively, most frequently as key-steps in total synthesis of natural products. The reader is directed towards reviews from Harrowven2a and Bowman3 for detailed discussion within this area; some representative examples of intramolecular reactions directly applicable to medicinal or biological chemistry are detailed below.
One of the earlier examples of an intramolecular Minisci reaction, in the synthesis of a biologically-important scaffold, was provided by the synthesis of an aza-COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundergoline ring structure COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound70, available in three-steps from commercially-available COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundindole-4-boronic acid COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound68; the key cyclization step used a Minisci reaction of an acyl radical (Scheme 43).128 Of note, was the absence of an COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundindole protecting group in this short reaction sequence, clearly supporting the idea that Minisci chemistry does not necessarily benefit from a protecting group. Similar intramolecular radical reactions have very recently been described by Bennasar and Roca to obtain COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundergoline-related COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundindole-fused isoquinolines (Scheme 43).129
Intramolecular Minisci chemistry is also useful for undertaking multi-step “tandem” syntheses. For example, González and Molina-Nazarro have described a beautiful cascade-sequence, terminating in a Minisci-type reaction, in their attempted synthesis of Spongidines (e.g. Spongidine A 74), which are a group of anti-inflammatory pyridinium alkaloids.130 Thus, reaction of keto-ester precuror 71, with Mn(OAc)3 in AcOH gave the tetracyclic product 72, in a very respectable 40% isolated yield (Scheme 44). Tetracycle 72 was isomeric with the Spongidine natural product, as the final cyclization had occurred exclusively at the 2-position of the pyridine ring, rather than the desired 4-position. The authors proceeded to prepare compound 73, an isomeric relative of Spongidine A, and are currently trying to prepare an appropriate derivative of 73 to measure its biological activity.
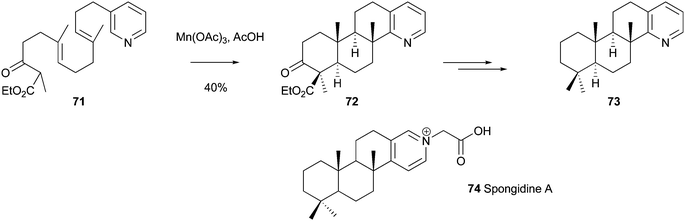 |
| | Scheme 44 A cascade approach to a relative of the anti-inflammatory alkaloid, Spongidine A130 | |
In a similar vein to the work in Scheme 44, Rouden and colleagues have used an intramolecular Minisci cyclization to synthesize a COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcytisine/COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundepibatidine hybrid, for in vivo imaging of nicotinic acetylcholine receptors (nAChRs), particularly those of the α4β2 subtype (Scheme 45). The desired cyclized compounds 75 and 76 were tested as racemic mixtures for inhibition of α4β2 nAChRs and showed Ki values of 3.5 nM and 0.5 nM respectively.131
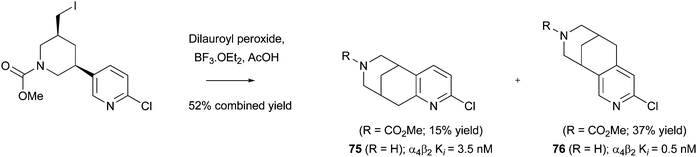 |
| | Scheme 45 Intramolecular Minisci reactions to provide ihibitors of nicotinic acetylcholine receptors131 | |
In a further demonstration of intramolecular Minisci reactions to prepare compounds of biological significance, Zard and Gagosz have provided compounds related to the anti-tumor agent PB1 77 (Scheme 46),132 and Bennesar et al. have synthesized the potent anti-malarial/anti-tumor COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundquinone Calothrixin B COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compound78,133 and ellipticine quinones, such as 79 (Scheme 46), which may also be useful as anti-tumor agents, or as a precursor of ellipticine itself.134
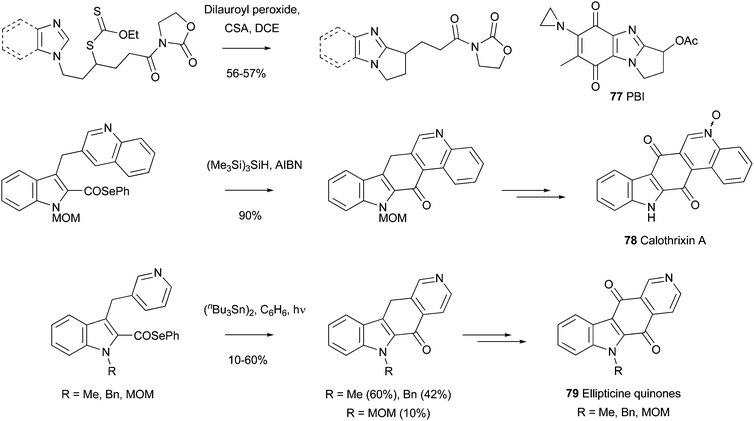 |
| | Scheme 46 Preparation of anti-tumor and anti-malarial agents by intramolecular radical additions132–134 | |
As mentioned in Section 2.1, radicals can add to heteroaromatic systems that are not basic. Intramolecular variants of this reaction have provided some of the earliest examples on the use of Minisci reactions to prepare cyclic structures. For example, a number of cyclized products, including the ant pheromone (-)-Monomorine 80 were prepared using this method (Scheme 47).135–137 Similar reactions could also be extended to the cyclization of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundquinone and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundpyridone derivatives (Scheme 47).138,139 As another illustration of this chemistry, albeit using traditional radical-generating conditions, potent tricyclic 5-HT2C agonists, such as 81, were synthesized from N-substituted indoles (Scheme 47).140 The cyclization conditions were based upon prior work by Moody and Norton.141 More recently, similar chemistry has been used to prepare other bicyclic COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundazole derivatives, such as Withasomnine 82, which is used in ayurvedic alternative medicine (Scheme 47).142
3.12 Reaction with DNA or RNA-bases
Finally, in section 3, we change track and consider the reaction of a class of heteroaromatics with COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcarbon-centered radicals, rather than the introduction of a specific group.
The reaction of nucleic heteroaromatics with alkyl radicals is a huge and diverse field. A full discussion of this chemistry, which is important in biological systems, is well-beyond the scope of this review, and readers are directed to a selection of other articles for a more in-depth account of these processes.143 However, a few selective examples are shared here for illustrative purposes.
The addition of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcarbon-centered radicals to DNA or RNA bases has been known for over 40 years. For example, in 1968 Linschitz and Connolly reported the addition of alkoxymethyl radicals to COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundpurine, to give COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compounddihydro-purine products (Scheme 48).144 Contemporaneous studies were also reported by Yang et al., and by Elad and colleagues, who examined the reaction of purines, or purine nucleosides, with amines and alcohols respectively, under the influence of UV-radiation. The products from these reactions were also shown to be addition adducts resulting from radical attack on the heteroaromatic base (Scheme 48).145,146 Subsequently, modifications of nucleic acid bases with other alkyl and aryl radicals have been investigated by those in the chemical toxicology and biological chemistry fields. For example, in 1974 Kawazae and co-workers detailed the addition of methyl and amide groups to various nucleic acid bases, using Minisci's conditions for radical-formation, as a model of DNA-damage caused by carcinogenic peroxides (Scheme 49).147,148 Further studies have elucidated the site of radical attack for all four DNA-heteroaromatics (Scheme 50).149 The relevance of these radical reactions to in vivo biology is an area of current interest.143 Thus, the study of Minisci-type reactions in biological systems provides a nice illustration of how a one-time nineteenth century chemical curiosity, rarely encountered in simply-equipped organic chemistry laboratories, has blossomed, to encompass the modern-day study of such processes in highly-sophisticated cellular systems (complex chemistry laboratories!).
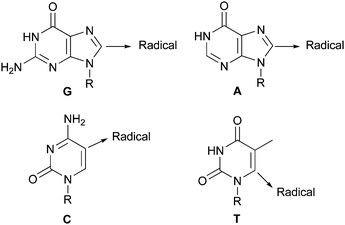 |
| | Scheme 50 Site of radical reactions for bases found in DNA143,149 | |
4 Future developments
4.1 ‘High Content’ libraries
An under-utilized, yet potentially powerful application of Minisci chemistry, is in the production of chemical libraries. There are at least two themes that could be developed further. The first, involves the use of a single Minisci reaction, undertaken in a parallel manner, to prepare small arrays. Such an approach was recently used by Baran and colleagues to prepare a 17-membered 2-aryl-4-tert-butylpyridine library (Scheme 51).66
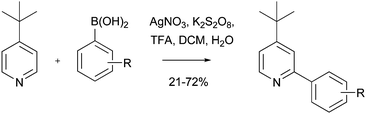 |
| | Scheme 51 Library synthesis using Baran's version of a Minisci reaction66 | |
The second approach, and one highlighted by our own research group, is to use different variations of Minisci functionalization to introduce diverse substituents within a pharmacologically-active scaffold.18 Thus, one could introduce diverse substituents such as alkyl, cycloalkyl (e.g. cyclopropyl), fluoroalkyl (e.g. CF3), aryl, carbamoyl, oxetan-3-yl, azetidin-3-yl, trialkylsilyl, hydroxymethyl, C-glycoside, and other groups, from a common starting material (Scheme 52). One possible application of such a strategy would be the functionalization of approved drugs containing appropriate heteroaromatic cores. Since it has been said that the best way to find a new drug is to start from an existing one,150 such an exercise may give-rise to a ‘high-content’ library for screening against multiple biological targets, with an aim of unearthing new pharmacology, or optimizing against a known biological target. The adoption of Minisci chemistry to introduce diverse substituents is also attractive from a synthetic perspective. As has recently been discussed, medicinal chemists tend to use a relatively small variety of reactions in their syntheses.151 Minisci chemistry provides a formidible set of transformations which would make a fine addition to the compendium of routine organic reactions employed by medicinal chemists.
 |
| | Scheme 52 Potential synthesis of a ‘high content’ library by functionalization of pharmacologically-active heteroaromatics using Minisci chemistry to introduce multiple and diverse substituents18 | |
Conclusions
In conclusion, this review describes the addition of radicals to heteroaromatic bases, which are commonly referred to as Minisci reactions, and details specific examples of these methods to the fields of medicinal and biological chemistry. Rather than describing one particular transformation, Minisci chemistry encompasses a whole set of related CH-functionalization processes for the introduction of diverse functional groups in to heteroaromatic systems. In many cases, these processes are highly chemo- and regio-selective, which makes them ideal for industrial applications, since complicating factors like protecting group strategies can be avoided. Like any synthetic procedure, there are limitations; the most frequently encountered with Minisci chemistry being a modest yield of product after purification, which itself may sometimes be cumbersome. The versatility of Minisci chemistry is exemplified by the successful use of these reactions to prepare marketed COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcamptothecin analogues, as key-steps in the synthesis of compounds undergoing clinical evaluation, and for the introduction of privileged motifs such as trifluoromethyl, COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundoxetane and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundazetidine groups, amongst others. Although there is ample evidence that Minisci chemistry is particularly suited for medicinal and biological applications, its widespread use by chemists working in these areas is far from routine. New developments, such as the use of boronic acids and COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundpotassium trifluoroborates as radical precursors may be helpful in promoting these reactions to a wider audience. Similarly, an increased use in library synthesis may also assist in the utilization of this methodology. Looking ahead, there are many more new developments and uses of Minisci reactions within medicinal and biological chemistry settings. In order to realize the potential of these reactions, medicinal chemists are encouraged to use this chemistry fequently. Indeed, it is not hard to envisage a time when Minisci reactions become a routine set of transformations for chemists looking to functionalize a heteroaromatic compound with biological activity.
Acknowledgements
We thank Amelia Cervantes and Rigel Pharmaceuticals, Inc. for obtaining a small selection of references used in this review. Jing Zhang is thanked for translation of a Chinese patent application (ref. 91). We also thank a referee for highlighting the relative contributions of enthalpic and polar effects in perfluoroalkylation reactions of heteroaromatic bases, and recommending ref. 57a and 57b for further reading.
Notes and references
-
(a) C. Punta and F. Minisci, Trends Het. Chem., 2008, 13, 1–68 CAS
 ;
(b) F. Minisci, F. Fontana and E. Vismara, J. Heterocycl. Chem., 1990, 27, 79–96 CrossRef CAS
;
(b) F. Minisci, F. Fontana and E. Vismara, J. Heterocycl. Chem., 1990, 27, 79–96 CrossRef CAS  ;
(c) F. Minisci, E. Vismara and F. Fontana, Heterocycles, 1989, 28, 489–519 CrossRef CAS
;
(c) F. Minisci, E. Vismara and F. Fontana, Heterocycles, 1989, 28, 489–519 CrossRef CAS  .
.
-
(a) D. C. Harrowven and B. J. Sutton, Prog. Heterocycl. Chem., 2004, 16, 27–53 CrossRef CAS
 ;
(b) D. C. Harrowven, B. J. Sutton and S. Coulton, Org. Biomol. Chem., 2003, 1, 4047–4057 RSC
;
(b) D. C. Harrowven, B. J. Sutton and S. Coulton, Org. Biomol. Chem., 2003, 1, 4047–4057 RSC  ;
(c) D. C. Harrowven, B. J. Sutton and S. Coulton, Tetrahedron, 2002, 58, 3387–3400 CrossRef CAS
;
(c) D. C. Harrowven, B. J. Sutton and S. Coulton, Tetrahedron, 2002, 58, 3387–3400 CrossRef CAS  .
.
- R. W. Bowman and J. M. D. Storey, Chem. Soc. Rev., 2007, 36, 1803–1822 RSC
 .
.
- M. Gomberg and W. E. Bachmann, J. Am. Chem. Soc., 1924, 42, 2239–2243 Search PubMed
 .
.
-
(a) R. Möhlau and R. Berger, Ber. Dtsch. Chem. Ges., 1893, 26, 1196 CrossRef
 ;
(b) R. Möhlau and R. Berger, Ber. Dtsch. Chem. Ges., 1893, 26, 1994 CrossRef
;
(b) R. Möhlau and R. Berger, Ber. Dtsch. Chem. Ges., 1893, 26, 1994 CrossRef  .
.
- See ref. 2a, pages 27–30.
- L. K. Dyall and K. H. Pausacker, J. Chem. Soc., 1961, 18–23 RSC
 .
.
- B. M. Lynch and H. S. Chang, Tetrahedron Lett., 1964, 5, 617–620 CrossRef
 .
.
- B. M. Lynch and H. S. Chang, Tetrahedron Lett., 1964, 5, 2965–2968 CrossRef
 .
.
- H. J. M. Dou and B. M. Lynch, Tetrahedron Lett., 1965, 6, 897–901 CrossRef
 .
.
- H. J. M. Dou, Bull. Soc. Chim. Fr., 1966, 1678–1679 CAS
 .
.
- H. J. M. Dou and B. M. Lynch, Bull. Soc. Chim. Fr., 1966, 3815–3820 CAS
 .
.
- H. J. M. Dou and B. M. Lynch, Bull. Soc. Chim. Fr., 1966, 3820–3823 CAS
 .
.
-
(a) R. D. Brown, J. Chem. Soc., 1956, 272–275 RSC
 ;
(b) R. D. Brown and M. L. Heffernan, Aust. J. Chem., 1956, 9, 83–89 CrossRef CAS
;
(b) R. D. Brown and M. L. Heffernan, Aust. J. Chem., 1956, 9, 83–89 CrossRef CAS  .
.
- F. Minisci, R. Galli, M. Cecere, V. Malatesta and T. Caronna, Tetrahedron Lett., 1968, 9, 5609–5612 CrossRef
 .
.
-
(a) P. Ertle, S. Jelfs, J. Mühlbacher, A. Schuffenhauer and P. Selzer, J. Med. Chem., 2006, 49, 4568–4573 CrossRef
 ;
(b) H. B. Broughton and I. A. Watson, J. Mol. Graphics Modell., 2004, 23, 51–58 CrossRef CAS
;
(b) H. B. Broughton and I. A. Watson, J. Mol. Graphics Modell., 2004, 23, 51–58 CrossRef CAS  ;
(c) X. Q. Lewell, A. C. Jones, C. L. Bruce, G. Harper, M. M. Jones, I. M. Mclay and J. Bradshaw, J. Med. Chem., 2003, 46, 3257–3274 CrossRef CAS
;
(c) X. Q. Lewell, A. C. Jones, C. L. Bruce, G. Harper, M. M. Jones, I. M. Mclay and J. Bradshaw, J. Med. Chem., 2003, 46, 3257–3274 CrossRef CAS  ;
(d) S. Gibson, R. McGuire and D. C. Rees, J. Med. Chem., 1996, 39, 4065–4072 CrossRef CAS
;
(d) S. Gibson, R. McGuire and D. C. Rees, J. Med. Chem., 1996, 39, 4065–4072 CrossRef CAS  ;
(e) G. W. Bemis and M. A. Murcko, J. Med. Chem., 1996, 39, 2887–2893 CrossRef CAS
;
(e) G. W. Bemis and M. A. Murcko, J. Med. Chem., 1996, 39, 2887–2893 CrossRef CAS  .
.
- F. Minisci, R. Bernadi, F. Bertini, R. Galli and M. Perchinunno, Tetrahedron, 1971, 27, 3575–3580 CrossRef CAS
 .
.
- M. A. J. Duncton, M. A. Estiarte, M. Cox, D. J. R. O'Mahony, R. J. Johnson, W. T. Edwards and M. G. Kelly, J. Org. Chem., 2009, 74, 6354–6357 CrossRef CAS
 .
.
- For representative reviews see
(a) S. Basili and S. Moro, Expert Opin. Ther. Pat., 2009, 19, 555–574 CrossRef CAS
 ;
(b) D. Kim and N. Lee, Mini-Rev. Med. Chem., 2002, 2, 611–619 CrossRef CAS
;
(b) D. Kim and N. Lee, Mini-Rev. Med. Chem., 2002, 2, 611–619 CrossRef CAS  .
.
- S. Sawada, S. Okijima, R. Aiyama, K. Nokata, T. Furuta, T. Yokokura, E. Sugino, K. Yamaguchi and T. Miyasaka, Chem. Pharm. Bull., 1991, 39, 1446–1454 CAS
 .
.
-
(a) S. Sawada, K. Nokata, H. Nagata, T. Furuta, T. Yokokura and T. Miyasaka, Chem. Pharm. Bull., 1991, 39, 2574–2580 CAS
 ;
(b) S. Sawada, S. Matsuoka, K. Nokata, H. Nagata, T. Furuta, T. Yokokura and T. Miyasaka, Chem. Pharm. Bull., 1991, 39, 3183–3188 CAS
;
(b) S. Sawada, S. Matsuoka, K. Nokata, H. Nagata, T. Furuta, T. Yokokura and T. Miyasaka, Chem. Pharm. Bull., 1991, 39, 3183–3188 CAS  .
.
- S. K. Ahn, N. S. Choi, B. S. Jeong, K. K. Kim, D. J. Journ, J. K. Kim, S. J. Lee, J. W. Kim, C. I. Hong and S. Jew, J. Heterocycl. Chem., 2000, 37, 1141–1144 CrossRef CAS
 .
.
- S. M. Apana, L. W. Anderson and M. S. Berridge, J. Labelled Com. Radiopharm., 2010, 53, 178–182 CAS
 .
.
- Z.-F. Xie, K. Ootsu and H. Akimoto, Bioorg. Med. Chem. Lett., 1995, 5, 2189–2194 CrossRef CAS
 .
.
- M. Li, W. Jin, C. Jiang, W. Tang, T. You and L. Lou, Bioorg. Med. Chem. Lett., 2009, 19, 4107–4109 CrossRef CAS
 .
.
- For a review on the synthesis of leading COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundcamptothecin anti-cancer compounds see: S. T. Liew and L.-X. Yang, Curr. Pharm. Des., 2008, 14, 1078–1097 CrossRef CAS  .
.
-
(a) R. Jain, L. A. Cohen, N. A. El-Kadi and M. M. King, Tetrahedron, 1997, 53, 2365–2370 CrossRef CAS
 ;
(b) For a previous introduction of methyl group in to 1-alkylimidazoles see: C. G. Begg, M. R. Grimmett and L. Yu-Man, Aust. J. Chem., 1973, 26, 415–420 CrossRef CAS
;
(b) For a previous introduction of methyl group in to 1-alkylimidazoles see: C. G. Begg, M. R. Grimmett and L. Yu-Man, Aust. J. Chem., 1973, 26, 415–420 CrossRef CAS  .
.
- S. Narayanan, S. Vangapandu and R. Jain, Bioorg. Med. Chem. Lett., 2001, 11, 1133–1136 CrossRef CAS
 .
.
- N. Kaur, V. Monga and R. Jain, Tetrahedron Lett., 2004, 45, 6883–6885 CrossRef CAS
 .
.
- N. Kaur, X. Lu, M. C. Gershengorn and R. Jain, J. Med. Chem., 2005, 48, 6162–6165 CrossRef CAS
 .
.
- N. Kaur, V. Monga, J. S. Josan, X. Lu, M. C. Gershengorn and R. Jain, Bioorg. Med. Chem., 2006, 14, 5981–5988 CrossRef CAS
 .
.
- N. Kaur, V. Onga, X. Lu, M. C. Gershengorn and R. Jain, Bioorg. Med. Chem., 2007, 15, 433–443 CrossRef CAS
 .
.
- V. Monga, C. L. Meena, N. Kaur, S. Kumar, C. Pawar, S. S. Sharma and R. Jain, J. Heterocycl. Chem., 2008, 45, 1603–1608 CrossRef CAS
 .
.
-
(a) K. B. Hansen, S. A. Springfield, R. Desmond, P. N. Devine, E. J. J. Garbowski and P. J. Reider, Tetrahedron Lett., 2001, 42, 7353–7355 CrossRef CAS
 ;
(b)
K. B. Hansen and S. A. Springfield, Patent WO2002059085 ( 2002)
;
(b)
K. B. Hansen and S. A. Springfield, Patent WO2002059085 ( 2002)  ;
Chem. Abstr., 2002, 137, p. 140528 Search PubMed.
;
Chem. Abstr., 2002, 137, p. 140528 Search PubMed.
- R. Jain, B. Vaitilingam, A. Nayyar and P. B. Palde, Bioorg.
Med. Chem. Lett., 2003, 13, 1051–1054 CrossRef CAS
 .
.
- S. Vangapandu, M. Jain, R. Jain, S. Kaur and P. P. Singh, Bioorg. Med. Chem., 2004, 12, 2501–2508 CrossRef CAS
 .
.
- B. Vaitilingam, A. Nayyar, P. P. Palde, V. Monga, R. Jain, S. Kaur and P. P. Singh, Bioorg. Med. Chem., 2004, 12, 4179–4188 CrossRef CAS
 .
.
- A. Nayyar, V. Monga, A. Malde, E. Coutinho and R. Jain, Bioorg. Med. Chem., 2007, 15, 626–640 CrossRef CAS
 .
.
- For recent reviews see:
(a) A. Koul, E. Arnoult, N. Lounis, J. Guillemont and K. Andries, Nature, 2011, 469, 483–490 CrossRef CAS
 ;
(b) C. Dye and B. G. Williams, Science, 2010, 328, 856–861 CrossRef CAS
;
(b) C. Dye and B. G. Williams, Science, 2010, 328, 856–861 CrossRef CAS  .
.
-
(a) R. W. Carling, A. Madin, A. Guiblin, M. G. N. Russell, K. W. Moore, A. Mitchinson, B. Sohal, A. Pike, S. M. Cook, I. C. Regan, R. M. McKernan, K. Quirk, P. Ferris, G. Marshall, S. A. Thompson, K. A. Wafford, G. R. Dawson, J. R. Atack, T. Harrison, J. L. Castro and L. J. Street, J. Med. Chem., 2005, 48, 7089–7092 CrossRef CAS
 ;
(b)
H. B. Broughton, R. W. Carling, J. L. Castro-Pineiro, A. R. Guiblin, A. Madin, K. W. Moore, M. G. Russell and L. J. Street, Patent WO9804559 ( 1998)
;
(b)
H. B. Broughton, R. W. Carling, J. L. Castro-Pineiro, A. R. Guiblin, A. Madin, K. W. Moore, M. G. Russell and L. J. Street, Patent WO9804559 ( 1998)  ;
Chem. Abstr., 1998, 128, p. 167433 Search PubMed.
;
Chem. Abstr., 1998, 128, p. 167433 Search PubMed.
- C. A. Lipinski, J. L. LaMattina and L. A. Hohnke, J. Med. Chem., 1985, 28, 1628–1636 CrossRef CAS
 .
.
-
(a) P. B. Palde, P. C. Gareiss and B. J. Miller, J. Am. Chem. Soc., 2008, 130, 9566–9573 CrossRef CAS
 ;
(b) P. B. Palde, B. R. McNaughton, N. T. Ross, P. C. Gareiss, C. R. Mace, R. C. Spitale and B. L. Miller, Synthesis, 2007, 2287–2290 CAS
;
(b) P. B. Palde, B. R. McNaughton, N. T. Ross, P. C. Gareiss, C. R. Mace, R. C. Spitale and B. L. Miller, Synthesis, 2007, 2287–2290 CAS  ;
(c)
B. L. Miller, P. B. Palde and P. Gareiss, Patent WO2008048967 ( 2008);
Chem. Abstr., 2008, 148, p. 471607 Search PubMed.
;
(c)
B. L. Miller, P. B. Palde and P. Gareiss, Patent WO2008048967 ( 2008);
Chem. Abstr., 2008, 148, p. 471607 Search PubMed.
- D. H. R. Barton, B. Garcia, H. Togo and S. Z. Zard, Tetrahedron Lett., 1986, 27, 1327–1330 CrossRef CAS
 .
.
- E. Castagnino, S. Corsano, D. H. R. Barton and S. Z. Zard, Tetrahedron Lett., 1986, 27, 6337–6338 CrossRef CAS
 .
.
- C. Fausta, F. Fontana, F. Minisci, G. Pianese, P. Tortoreto and L. Zhao, Tetrahedron Lett., 1992, 33, 687–690 CrossRef
 .
.
- M. Jarman, E. E. Barrie and J. M. Llera, J. Med. Chem., 1998, 41, 5375–5381 CrossRef CAS
 .
.
- For a recent review see S. Roy, B. T. Gregg, G. W. Gribble, V. D. Le and S. Roy, Tetrahedron, 2011, 67, 2161–2195 CrossRef CAS
 .
.
- For reviews see
(a) S. Pursur, P. R. Moore, S. Swallow and V. Gouverneur, Chem. Soc. Rev., 2008, 37, 320–342 RSC
 ;
(b) W. K. Hagmann, J. Med. Chem., 2008, 51, 4359–4369 CrossRef CAS
;
(b) W. K. Hagmann, J. Med. Chem., 2008, 51, 4359–4369 CrossRef CAS  .
.
- D. Uraguchi, K. Yamamoto, Y. Ohtsuka, K. Tokuhisa and T. Yamakawa, Appl. Catal., A, 2008, 342, 137–143 CrossRef CAS
 .
.
-
(a)
A. Yamakawa, K. Yamamoto, D. Uraguchi and K. Tokuhisa, Japanese Patent 2008239572 ( 2008)
 ;
Chem. Abstr., 2008, 149, p. 448374 Search PubMed;
(b)
A. Yamakawa, K. Yamamoto, D. Uraguchi and K. Norihisa, Japanese Patent 2008231039 ( 2008)
;
Chem. Abstr., 2008, 149, p. 448374 Search PubMed;
(b)
A. Yamakawa, K. Yamamoto, D. Uraguchi and K. Norihisa, Japanese Patent 2008231039 ( 2008)  ;
Chem. Abstr., 2008, 149, p. 402215 Search PubMed;
(c)
A. Yamakawa, K. Yamamoto, D. Uraguchi and K. Norihisa, Japanese Patent 2008115122 ( 2008)
;
Chem. Abstr., 2008, 149, p. 402215 Search PubMed;
(c)
A. Yamakawa, K. Yamamoto, D. Uraguchi and K. Norihisa, Japanese Patent 2008115122 ( 2008)  ;
Chem. Abstr., 2008, 148, p. 537971 Search PubMed;
(d)
A. Yamakawa, K. Yamamoto, D. Uraguchi and K. Norihisa, Japanese Patent 2008115105 ( 2008)
;
Chem. Abstr., 2008, 148, p. 537971 Search PubMed;
(d)
A. Yamakawa, K. Yamamoto, D. Uraguchi and K. Norihisa, Japanese Patent 2008115105 ( 2008)  ;
Chem. Abstr., 2008, 148, p. 585739 Search PubMed.
;
Chem. Abstr., 2008, 148, p. 585739 Search PubMed.
- T. Kino, Y. Nagase, Y. Ohtsuka, K. Yamamoto, D. Uraguchi, K. Tokuhisa and T. Yamakawa, J. Fluorine Chem., 2010, 131, 98–105 CrossRef CAS
 .
.
- W. R. Dolbier, Chem. Rev., 1996, 96, 1557–1584 CrossRef CAS
 .
.
- Y. Ohtsuka and T. Yamakawa, Tetrahedron, 2011, 67, 2323–2331 CrossRef CAS
 .
.
-
(a) W. R. Dolbier, S. Ait-Mohand, T. D. Schertz, T. A. Sergeeva, J. A. Cradlebaugh, A. Mitani, G. L. Gard, R. W. Winter and J. A. Thrasher, J. Fluorine Chem., 2006, 127, 1302–1310 CrossRef CAS
 ;
(b) S. A. Mohand and W. R. Dolbier, Org. Lett., 2002, 4, 3013–3015 CrossRef CAS
;
(b) S. A. Mohand and W. R. Dolbier, Org. Lett., 2002, 4, 3013–3015 CrossRef CAS  ;
(c) H. W. Sidebottom, J. M. Tedder and J. C. Walton, Trans. Faraday Soc., 1970, 66, 2038–2044 RSC
;
(c) H. W. Sidebottom, J. M. Tedder and J. C. Walton, Trans. Faraday Soc., 1970, 66, 2038–2044 RSC  ;
(d) H. W. Sidebottom, J. M. Tedder and J. C. Walton, J. Chem. Soc. Chem. Commun., 1970, 253–254 RSC
;
(d) H. W. Sidebottom, J. M. Tedder and J. C. Walton, J. Chem. Soc. Chem. Commun., 1970, 253–254 RSC  ;
(e) H. W. Sidebottom, J. M. Tedder and J. C. Walton, Trans. Faraday Soc., 1969, 65, 2103–2109 RSC
;
(e) H. W. Sidebottom, J. M. Tedder and J. C. Walton, Trans. Faraday Soc., 1969, 65, 2103–2109 RSC  ;
(f) J. R. Case, N. H. Ray and H. L. Roberts, J. Chem. Soc., 1961, 2066–2070 RSC
;
(f) J. R. Case, N. H. Ray and H. L. Roberts, J. Chem. Soc., 1961, 2066–2070 RSC  .
.
- For examples see
(a) T. Mo, X. Mi, E. E. Milner, G. S. Dow and P. Wipf, Tetrahedron Lett., 2010, 51, 5137–5140 CrossRef CAS
 ;
(b) P. Wipf, T. Mo, S. J. Geib, D. Caridha, G. S. Dow, L. Gerena, N. Roncal and E. E. Milner, Org. Biomol. Chem., 2009, 7, 4163–4165 RSC
;
(b) P. Wipf, T. Mo, S. J. Geib, D. Caridha, G. S. Dow, L. Gerena, N. Roncal and E. E. Milner, Org. Biomol. Chem., 2009, 7, 4163–4165 RSC  ;
(c) J. T. Welch and D. S. Lim, Bioorg. Med. Chem., 2007, 15, 6659–6666 CrossRef CAS
;
(c) J. T. Welch and D. S. Lim, Bioorg. Med. Chem., 2007, 15, 6659–6666 CrossRef CAS  .
.
-
(a) E. Baciocchi and E. Muraglia, Tetrahedron Lett., 1993, 34, 3799–3800 CrossRef CAS
 ;
(b) E. Baciocchi and E. Muraglia, Tetrahedron Lett., 1993, 34, 5015–5018 CrossRef CAS
;
(b) E. Baciocchi and E. Muraglia, Tetrahedron Lett., 1993, 34, 5015–5018 CrossRef CAS  ;
(c) F. Minisci, E. Vismara and F. Fontana, J. Org. Chem., 1989, 54, 5224–5227 CrossRef CAS
;
(c) F. Minisci, E. Vismara and F. Fontana, J. Org. Chem., 1989, 54, 5224–5227 CrossRef CAS  .
.
-
(a) F. Antonietti, A. Mele, F. Minisci, C. Punta, F. Recupero and F. Fontana, J. Fluorine Chem., 2004, 125, 205–211 CrossRef CAS
 ;
(b) F. Antonietti, C. Gambarotti, A. Mele, F. Minisci, R. Paganelli, C. Punta and F. Recupero, Eur. J. Org. Chem., 2005, 4434–4440 CrossRef CAS
;
(b) F. Antonietti, C. Gambarotti, A. Mele, F. Minisci, R. Paganelli, C. Punta and F. Recupero, Eur. J. Org. Chem., 2005, 4434–4440 CrossRef CAS  ;
(c) See also X. T. Huang, Z. Y. Long and Q. Y. Chen, J. Fluorine Chem., 2001, 111, 107–113 CrossRef CAS
;
(c) See also X. T. Huang, Z. Y. Long and Q. Y. Chen, J. Fluorine Chem., 2001, 111, 107–113 CrossRef CAS  ;
(d) A. B. Cowell and C. Tamborski, J. Fluorine Chem., 1981, 17, 345–356 CrossRef CAS
;
(d) A. B. Cowell and C. Tamborski, J. Fluorine Chem., 1981, 17, 345–356 CrossRef CAS  .
.
- For a review of fluorous probe molecules for fluorous separation see: H. J. Lehmler, S. Telu, S. M. Vyas, N. S. Shaikh, S. E. Rankin, B. L. Knutson and S. Parkin, Tetrahedron, 2010, 66, 2561–2569 CrossRef CAS
 .
.
-
(a) T. Sugimoto and W. Pfleiderer, Heterocycles, 1997, 45, 765–772 CrossRef CAS
 ;
(b) S. Murata, K. Kiguchi and T. Sugimoto, Heterocycles, 1998, 48, 1255–1274 CrossRef CAS
;
(b) S. Murata, K. Kiguchi and T. Sugimoto, Heterocycles, 1998, 48, 1255–1274 CrossRef CAS  .
.
- S. Yanagisawa, K. Ueda, T. Taniguchi and K. Itami, Org. Lett., 2008, 10, 4673–4676 CrossRef CAS
 .
.
- For reviews see:
(a) C. Ollivier and P. Renaud, Chem. Rev., 2001, 101, 3415–3434 CrossRef CAS
 ;
(b) H. C. Brown and M. M. Midland, Angew. Chem., Int. Ed. Engl., 1972, 11, 692–700 CrossRef CAS
;
(b) H. C. Brown and M. M. Midland, Angew. Chem., Int. Ed. Engl., 1972, 11, 692–700 CrossRef CAS  . See also;
(c) A. Dickschat and A. Studer, Org. Lett., 2010, 12, 3972–3974 CrossRef CAS
. See also;
(c) A. Dickschat and A. Studer, Org. Lett., 2010, 12, 3972–3974 CrossRef CAS  ;
(d) H. Suenaga, K. Nakashima, I. Hamachi and S. Shinkai, Tetrahedron Lett., 1997, 38, 2479–2482 CrossRef CAS
;
(d) H. Suenaga, K. Nakashima, I. Hamachi and S. Shinkai, Tetrahedron Lett., 1997, 38, 2479–2482 CrossRef CAS  ;
(e) G. W. Kabalka, H. C. Brown, A. Suzuki, S. Honma, A. Arase and M. Itoh, J. Am. Chem. Soc., 1970, 92, 710–712 CrossRef CAS
;
(e) G. W. Kabalka, H. C. Brown, A. Suzuki, S. Honma, A. Arase and M. Itoh, J. Am. Chem. Soc., 1970, 92, 710–712 CrossRef CAS  ;
(f) H. C. H. A. van Riel, F. C. Fischer, J. Lugtenburg and E. Havinga, Tetrahedron Lett., 1969, 10, 3085 CrossRef
;
(f) H. C. H. A. van Riel, F. C. Fischer, J. Lugtenburg and E. Havinga, Tetrahedron Lett., 1969, 10, 3085 CrossRef  ;
(g) A. G. Davies and B. P. Roberts, J. Chem. Soc. B, 1967, 17–22 RSC
;
(g) A. G. Davies and B. P. Roberts, J. Chem. Soc. B, 1967, 17–22 RSC  .
.
- H. Miyabe, M. Ueda and T. Naito, J. Org. Chem., 2000, 65, 5043–5047 CrossRef CAS
 .
.
- A. S. Demir, Ö. Reis and M. Emrullahogu, J. Org. Chem., 2003, 68, 578–580 CrossRef CAS
 .
.
- S. K. Guchhait, M. Kashyap and S. Saraf, Synthesis, 2010, 1166–1170 CrossRef CAS
 .
.
- A. S. Demir, H. Findik, N. Saygili and N. T. Subasi, Tetrahedron, 2010, 66, 1308–1312 CrossRef CAS
 .
.
- I. B. Seiple, S. Su, R. A. Rodriguez, R. Gianatassio, Y. Fujiwara, A. L. Sobel and P. S. Baran, J. Am. Chem. Soc., 2010, 132, 13194–13196 CrossRef CAS
 .
.
- For a recent use of alkyl boronic acids and an alkyl trifluoroborate in radical conjugate addition reactions using Minsici-type conditions for radical formation see: Y. Fujiwara, V. Domingo, I. B. Seiple, R. Gianatassio, M. Del Bel and P. S. Baran, J. Am. Chem. Soc., 2011, 133, 3292–3295 CrossRef CAS
 .
.
- For a review on the use of COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundMIDA boronates in cross-coupling reactions see: M. P. Gillis and M. D. Burke, Aldrichchimica Acta, 2009, 42, 17–27 Search PubMed  .
.
- For reviews on COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundpotassium trifluoroborates in organic chemistry see:
(a) S. Darses and J. P. Genêt, Chem. Rev., 2008, 108, 288–325 CrossRef CAS  ;
(b) G. A. Molander and E. Noel, Acc. Chem. Res., 2007, 40, 275–286 CrossRef CAS
;
(b) G. A. Molander and E. Noel, Acc. Chem. Res., 2007, 40, 275–286 CrossRef CAS  .
.
-
(a) G. A. Molander, V. Colombel and V. A. Braz, Org. Lett., 2011, 13, 1852–1855. CrossRef CAS
 For the use of trifluoroborates as radical precursors in conjugate addition reactions see ref. 67 and;
(b) G. Sorin, R. Martinez Mallorquin, Y. Contie, A. Baralle, M. Malacria, J. P. Goddard and L. Fensterbank, Angew. Chem., Int. Ed., 2010, 49, 8721–8723 CrossRef CAS
For the use of trifluoroborates as radical precursors in conjugate addition reactions see ref. 67 and;
(b) G. Sorin, R. Martinez Mallorquin, Y. Contie, A. Baralle, M. Malacria, J. P. Goddard and L. Fensterbank, Angew. Chem., Int. Ed., 2010, 49, 8721–8723 CrossRef CAS  .
.
- For reviews of oxetanes in drug discovery see
(a) J. A. Burkhard, G. Wuitschik, M. Rogers-Evans, K. Müller and E. M. Carreira, Angew. Chem., Int. Ed., 2010, 49, 9052–9067 CrossRef CAS
 ;
(b) G. Wuitschik, E. M. Carreira, B. Wagner, H. Fischer, I. Parrilla, F. Schuler, M. Rogers-Evans and K. Müller, J. Med. Chem., 2010, 53, 3227–3246 CrossRef CAS
;
(b) G. Wuitschik, E. M. Carreira, B. Wagner, H. Fischer, I. Parrilla, F. Schuler, M. Rogers-Evans and K. Müller, J. Med. Chem., 2010, 53, 3227–3246 CrossRef CAS  .
.
- G. Wuitschik, M. Rogers-Evans, K. Müller, H. Fischer, B. Wagner, F. Schuler, L. Polonchuk and E. M. Carreira, Angew. Chem., Int. Ed., 2006, 45, 7736–7739 CrossRef CAS
 .
.
- M. A. J. Duncton, M. A. Estiarte, D. Tan, C. Kaub, D. J. R. O'Mahony, R. J. Johnson, M. Cox, W. T. Edwards, M. Wan, J. Kincaid and M. G. Kelly, Org. Lett., 2008, 10, 3259–3262 CrossRef CAS
 .
.
- For examples see:
(a) P. Melloni, A. Delle Torre, M. Meroni, A. Ambrosini and A. C. Rossi, J. Med. Chem., 1979, 22, 183–191 CrossRef CAS
 ;
(b) E. Testa, L. Fontanella and V. Aresi, Justus Liebigs Ann. Chem., 1965, 688, 165–169 CrossRef CAS
;
(b) E. Testa, L. Fontanella and V. Aresi, Justus Liebigs Ann. Chem., 1965, 688, 165–169 CrossRef CAS  ;
(c) E. Testa and L. Fontanella, Justus Liebigs Ann. Chem., 1964, 671, 106–108 CrossRef CAS
;
(c) E. Testa and L. Fontanella, Justus Liebigs Ann. Chem., 1964, 671, 106–108 CrossRef CAS  ;
(d) E. Testa, L. Fontanella and L. Mariani, Justus Liebigs Ann. Chem., 1962, 660, 135–143 CrossRef CAS
;
(d) E. Testa, L. Fontanella and L. Mariani, Justus Liebigs Ann. Chem., 1962, 660, 135–143 CrossRef CAS  ;
(e) E. Testa, A. Bonati, G. Pagani and E. Gatti, Justus Liebigs Ann. Chem., 1961, 647, 92–100 CrossRef CAS
;
(e) E. Testa, A. Bonati, G. Pagani and E. Gatti, Justus Liebigs Ann. Chem., 1961, 647, 92–100 CrossRef CAS  ;
(f) E. Testa, L. Fontanella, L. Mariani and G. Cristiani, Justus Liebigs Ann. Chem., 1961, 639, 157–165 CrossRef CAS
;
(f) E. Testa, L. Fontanella, L. Mariani and G. Cristiani, Justus Liebigs Ann. Chem., 1961, 639, 157–165 CrossRef CAS  ;
(g) E. Testa, L. Fontanella, G. Cristiani and L. Mariani, Justus Liebigs Ann. Chem., 1960, 635, 119–127 CrossRef CAS
;
(g) E. Testa, L. Fontanella, G. Cristiani and L. Mariani, Justus Liebigs Ann. Chem., 1960, 635, 119–127 CrossRef CAS  ;
(h) E. Testa, L. Fontanella and G. Cristiani, Justus Liebigs Ann. Chem., 1959, 626, 114–120 CrossRef CAS
;
(h) E. Testa, L. Fontanella and G. Cristiani, Justus Liebigs Ann. Chem., 1959, 626, 114–120 CrossRef CAS  .
.
- H. Togo, M. Aoki and M. Yokoyama, Tetrahedron Lett., 1991, 32, 6559–6562 CrossRef CAS
 .
.
- E. Vismara, G. Torri, N. Pastori and M. Marchiandi, Tetrahedron Lett., 1992, 33, 7575–7578 CrossRef CAS
 .
.
- E. Vismara, A. Donna, F. Minisci, A. Naggi, N. Pastori and G. Torri, J. Org. Chem., 1993, 58, 959–963 CrossRef CAS
 .
.
-
(a)
H. Togo, S. Ishigami, M. Fujii and M. Yokoyama, Japanese Patent 05306283 ( 1993)
 ;
Chem. Abstr., 1993, 120, p. 192219 Search PubMed;
(b) U. Hacksell and G. D. Daves, Prog. Med. Chem., 1985, 22, 1–65 CrossRef CAS
;
Chem. Abstr., 1993, 120, p. 192219 Search PubMed;
(b) U. Hacksell and G. D. Daves, Prog. Med. Chem., 1985, 22, 1–65 CrossRef CAS  .
.
-
(a) T. Caronna, G. P. Gardini and F. Minisci, Chem. Commun., 1969, 201 RSC
 ;
(b) T. Caronna, G. Fronza, F. Minisci, O. Porta and G. P. Gardini, J. Chem. Soc., Perkin Trans., 1972, 2, 1477–1481 Search PubMed
;
(b) T. Caronna, G. Fronza, F. Minisci, O. Porta and G. P. Gardini, J. Chem. Soc., Perkin Trans., 1972, 2, 1477–1481 Search PubMed  ;
(c) T. Caronna, G. Fronza, F. Minisci and O. Porta, J. Chem. Soc., Perkin Trans., 1972, 2, 2035–2038 Search PubMed
;
(c) T. Caronna, G. Fronza, F. Minisci and O. Porta, J. Chem. Soc., Perkin Trans., 1972, 2, 2035–2038 Search PubMed  .
.
- L. Désaubry and J. J. Bourguignon, Tetrahedron Lett., 1995, 43, 7875–7876 CrossRef
 .
.
- N. Sato and T. Matsuura, J. Chem. Soc., Perkin Trans. 1, 1996, 1, 2345–2350 RSC
 .
.
-
(a) O. A. Phillips, K. S. K. Murthy, C. Y. Fiakpui and E. E. Knaus, Can. J. Chem., 1999, 77, 216–222 CrossRef CAS
 ;
(b) K. S. K. Murthy and E. E. Knaus, Drug Dev. Res., 1999, 46, 155–162 CrossRef CAS
;
(b) K. S. K. Murthy and E. E. Knaus, Drug Dev. Res., 1999, 46, 155–162 CrossRef CAS  .
.
-
(a) J. M. Pruet, J. D. Robertus and E. V. Anslyn, Tetrahedron Lett., 2010, 51, 2539–2540 CrossRef CAS
 ;
(b) W. Pfleiderer, Tetrahedron Lett., 1984, 25, 1031–1034 CrossRef CAS
;
(b) W. Pfleiderer, Tetrahedron Lett., 1984, 25, 1031–1034 CrossRef CAS  ;
(c) R. Baur, E. Kleiner and W. Pfleiderer, Liebigs Ann. Chem., 1984, 1798–1814 CrossRef CAS
;
(c) R. Baur, E. Kleiner and W. Pfleiderer, Liebigs Ann. Chem., 1984, 1798–1814 CrossRef CAS  ;
(d) R. Baur, T. Sugimoto and W. Pfeiderer, Chem. Lett., 1984, 1025–1028 CrossRef CAS
;
(d) R. Baur, T. Sugimoto and W. Pfeiderer, Chem. Lett., 1984, 1025–1028 CrossRef CAS  ;
(e) R. Baur, T. Sugimoto and W. Pfeiderer, Helv. Chim. Acta, 1988, 71, 531–543 CrossRef CAS
;
(e) R. Baur, T. Sugimoto and W. Pfeiderer, Helv. Chim. Acta, 1988, 71, 531–543 CrossRef CAS  .
.
-
(a) W. Pfleiderer, Advan. Exp. Med. Biol., 1993, 338, 1–16 CAS
 ;
(b) E. C. Taylor, J. Heterocycl. Chem., 1990, 27, 1–12 CrossRef CAS
;
(b) E. C. Taylor, J. Heterocycl. Chem., 1990, 27, 1–12 CrossRef CAS  ;
(c) W. Pfleiderer, Biochem. Clin. Aspects Pteridines, 1982, 1, 3–26 CAS
;
(c) W. Pfleiderer, Biochem. Clin. Aspects Pteridines, 1982, 1, 3–26 CAS  ;
(d) E. C. Taylor, R. N. Henrie and D. J. Dumas, Develop. Biochem., 1979, 4, 71–75 Search PubMed
;
(d) E. C. Taylor, R. N. Henrie and D. J. Dumas, Develop. Biochem., 1979, 4, 71–75 Search PubMed  .
.
- For an example of anti-tumor activity with lumazine hydrazone derivatives see: S. B. Jiménez-Pulido, F. M. Linares-Ordóñez, J. M. Matínez-Martos, M. N. Moreno-Carretero, M. Quirós-Olozábel and M. J. Ramírez-Expósito, J. Inorg. Biochem., 2008, 102, 1677–1683 CrossRef
 .
.
- C. Giordana, F. Minisci, E. Vismara and S. Levi, J. Org. Chem., 1986, 51, 536–537 CrossRef
 .
.
-
(a) F. Minisci and G. P. Gardini, Tetrahedron Lett., 1970, 11, 15–16 CrossRef
 ;
(b) F. Minisci, A. Citterio, E. Vismara and C. Giordano, Tetrahedron, 1985, 41, 4157–4170 CrossRef CAS
;
(b) F. Minisci, A. Citterio, E. Vismara and C. Giordano, Tetrahedron, 1985, 41, 4157–4170 CrossRef CAS  ;
(c) F. Coppa, F. Fontana, E. Lazzarini, F. Minisci, G. Pianese and L. Zhao, Tetrahedron Lett., 1992, 33, 3057–3060 CrossRef CAS
;
(c) F. Coppa, F. Fontana, E. Lazzarini, F. Minisci, G. Pianese and L. Zhao, Tetrahedron Lett., 1992, 33, 3057–3060 CrossRef CAS  .
.
- I. Matin, J. Anvelt, L. Vares, I. Kühn and A. Claesson, Acta Chem. Scand., 1995, 49, 230–232 CrossRef
 .
.
-
(a) R. Iyer, G. Fetterly, A. Lugade and Y. Thanavala, Expert Opin. Pharmacother., 2010, 11, 1943–1955 CrossRef CAS
 ;
(b) S. Wilhelm, C. Carter, M. Lynch, T. Lowinger, J. Dumas, R. A. Smith, B. Schwartz, R. Simantov and S. Kelley, Nat. Rev. Drug Discovery, 2006, 5, 835–844 CrossRef CAS
;
(b) S. Wilhelm, C. Carter, M. Lynch, T. Lowinger, J. Dumas, R. A. Smith, B. Schwartz, R. Simantov and S. Kelley, Nat. Rev. Drug Discovery, 2006, 5, 835–844 CrossRef CAS  ;
(c) S. M. Wilhelm, L. Adnane, P. Newell, A. Villanueva, J. M. Llovet and M. Lynch, Mol. Cancer Ther., 2008, 7, 3129–3140 CrossRef CAS
;
(c) S. M. Wilhelm, L. Adnane, P. Newell, A. Villanueva, J. M. Llovet and M. Lynch, Mol. Cancer Ther., 2008, 7, 3129–3140 CrossRef CAS  .
.
-
(a)
S. Miller, M. Osterhout, J. Dumas, U. Khire, T. B. Lowinger, B. Riedl, W. J. Scott, R. A. Smith, J. E. Wood, D. Gunn, M. Rodriguez and M. Wang, Patent WO9932436 ( 1999)
 ;
Chem. Abstr., 1999, 131, p. 58658 Search PubMed;
(b)
B. Riedl, J. Dumas, U. Khire, T. B. Lowinger, W. J. Scott, R. A. Smith, J. E. Wood, M. K. Monahan, R. Natero, J. Renick and R. N. Sibley, Patent WO200042012 ( 2000);
Chem. Abstr., 2000, 133, p. 120157 Search PubMed.
;
Chem. Abstr., 1999, 131, p. 58658 Search PubMed;
(b)
B. Riedl, J. Dumas, U. Khire, T. B. Lowinger, W. J. Scott, R. A. Smith, J. E. Wood, M. K. Monahan, R. Natero, J. Renick and R. N. Sibley, Patent WO200042012 ( 2000);
Chem. Abstr., 2000, 133, p. 120157 Search PubMed.
-
X. Liu, Chinese Patent 101302193 ( 2008);
Chem. Abstr., 2008, 150, p. 5598 Search PubMed.
-
J. P. Dumas, S. J. Boyer, T. K. Joe, H. Natoum-Mokdad, H. C. E. Kluender, W. Lee, D. Nagarathnam, R. N. Sibley and N. Su, US Patent 6903101 ( 2005)
 ;
Chem. Abstr., 2005, 143, p. 43891 Search PubMed.
;
Chem. Abstr., 2005, 143, p. 43891 Search PubMed.
-
U. Klinkhammer, European Patent 979822 ( 2000)
 ;
Chem. Abstr., 2000, 132, p. 151834 Search PubMed.
;
Chem. Abstr., 2000, 132, p. 151834 Search PubMed.
- H. Bernard, G. Bülow, U. E. W. Lange, H. Mack, T. Pfeiffer, B. Schäfer, W. Seitz and T. Zierke, Synthesis, 2004, 2367–2375 CAS
 .
.
- S. Demirci, S. Göksu, M. Boztaş, F. Tümer and H. Secen, Turk. J. Chem., 2008, 32, 287–295 CAS
 .
.
-
(a) A. Maślankiewicz and E. Michalik, J. Heterocycl. Chem., 1997, 34, 401–405 CrossRef
 ;
(b) A. Maślankiewicz and E. Michalik, J. Heterocycl. Chem., 2005, 42, 1161–1166 CrossRef
;
(b) A. Maślankiewicz and E. Michalik, J. Heterocycl. Chem., 2005, 42, 1161–1166 CrossRef  .
.
-
(a) D. J. Miller, K. Ravikumar, H. Shen, J. K. Suh, S. M. Kerwin and J. D. Robertus, J. Med. Chem., 2002, 45, 90–98 CrossRef CAS
 ;
(b) X. Yan, T. Hollis, M. Svinth, P. Day, A. F. Monzingo, G. W. A. Milne and J. D. Robertus, J. Mol. Biol., 1997, 266, 1043–1049 CrossRef CAS
;
(b) X. Yan, T. Hollis, M. Svinth, P. Day, A. F. Monzingo, G. W. A. Milne and J. D. Robertus, J. Mol. Biol., 1997, 266, 1043–1049 CrossRef CAS  ;
(c) J. D. Robertus, X. Yan, S. Ernst, A. Monzingo, S. Worley, P. Day, T. Hollis and M. Svinth, Toxicon, 1996, 34, 1325–1334 CrossRef CAS
;
(c) J. D. Robertus, X. Yan, S. Ernst, A. Monzingo, S. Worley, P. Day, T. Hollis and M. Svinth, Toxicon, 1996, 34, 1325–1334 CrossRef CAS  .
.
- F. Minisci, W. Buratti, G. P. Gardini, F. Bertini, R. Galli and M. Perchinummo, Tetrahedron, 1971, 27, 3655–3668 CrossRef
 .
.
- Minisci and co-workers have introduced an alternative hydroxmethylation procedure that can eliminate any aldehyde by-product encountered under the original reaction conditions, see: F. Minisci, O. Porta, F. Recupero, C. Punta, C. Gambarotti, B. Pruna, M. Pierini and F. Fontana, Synlett, 2004, 874–876 CrossRef CAS
 . The process uses COMPOUND LINKS
. The process uses COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundethylene glycol as the hydroxymethyl radical precursor, rather than COMPOUND LINKS
Read more about this on ChemSpider
Download mol file of compoundmethanol.
- R. B. Katz, J. Mistry and M. B. Mitchell, Synth. Commun., 1989, 19, 317–325 CrossRef CAS
 .
.
- A. M. Crowe, R. J. Ife, M. B. Mitchell and D. Saunders, J. Labelled Compd. Radiopharm., 1986, 23, 21–33 CrossRef CAS
 .
.
- B. Evans and R. Wolfenden, J. Am. Chem. Soc., 1970, 92, 4751–4752 CrossRef CAS
 .
.
- N. A. Porter, Acc. Chem. Res., 1986, 19, 262–268 CrossRef CAS
 .
.
- T. Nam, C. L. Rector, H. Kim, A. F. P. Sonnen, R. Meyer, W. M. Nau, J. Atkinson, J. Rintoul, D. A. Pratt and N. A. Porter, J. Am. Chem. Soc., 2007, 129, 10211–10219 CrossRef CAS
 .
.
- C. J. Cowden, Org. Lett., 2003, 5, 4497–4499 CrossRef CAS
 .
.
- S. Dallavalle, T. Delsoldato, A. Ferrari, L. Merlini, S. Penco, N. Carenini, P. Perego, M. De Cesare, G. Pratesi and F. Zunino, J. Med. Chem., 2000, 43, 3963–3969 CrossRef CAS
 .
.
- G. Giannini, M. Marzi, W. Cabri, E. Marastoni, G. Battistuzzi, L. Vesci, C. Pisano, G. L. Beretta, M. De Cesare and F. Zunino, Bioorg. Med. Chem. Lett., 2008, 18, 2910–2915 CrossRef CAS
 .
.
- Z. Xie, K. Ootsu and H. Akimoto, Bioorg. Med. Chem. Lett., 1995, 5, 2189–2194 CrossRef CAS
 .
.
- T. Sugimoto, S. Murata, S. Matsuura and W. Pfleiderer, Tetrahedron Lett., 1986, 27, 4179–4180 CrossRef CAS
 .
.
- M. Tada, M. Furuse and H. Kashima, Heterocycles, 1992, 34, 357–362 CrossRef CAS
 .
.
- G. Heinisch, A. Jentzsch and I. Kirchner, Tetrahedron Lett., 1978, 19, 619–622 CrossRef
 .
.
- H. Suemune and T. Miyasaka, Nucleic Acid Symposium Series, 1983, 12, 1–4 CAS
 .
.
- S. Rádl, J. Černý, O. Klecán, J. Stach, L. Plaček and Z. Mandelová, Tetrahedron Lett., 2008, 49, 5316–5318 CrossRef
 .
.
- F. Minisci, E. Vismara and F. Fontana, J. Org. Chem., 1989, 54, 5524–5527 Search PubMed
 .
.
- E. Baciocchi, E. Muraglia and G. Sleiter, J. Org. Chem., 1992, 57, 6817–6820 CrossRef CAS
 .
.
- Y. Mkhynya, Z. Hussain, T. Bauschlicher, P. Schwinte, F. Siebert and W. Gärtner, Eur. J. Org. Chem., 2007, 1287–1293 CrossRef
 .
.
- B. Emmert and A. Asendorf, Ber. Dtsch. Chem. Ges. B, 1939, 72, 1188–1194 CrossRef
 .
.
- C. A. Russell, C. E. Crrawforth and O. Meth-Cohn, J. Chem. Soc., Chem. Commun., 1970, 1406–1407 CAS
 .
.
-
(a) D. J. O'Neill and P. Helquist, Org. Lett., 1999, 1, 1659–1662 CrossRef CAS
 ;
(b) J. A. Weitgenant, J. D. Mortison, D. J. O'Neill, B. Mowery, A. Puranen and P. Helquist, J. Org. Chem., 2004, 69, 2809–2815 CrossRef CAS
;
(b) J. A. Weitgenant, J. D. Mortison, D. J. O'Neill, B. Mowery, A. Puranen and P. Helquist, J. Org. Chem., 2004, 69, 2809–2815 CrossRef CAS  .
.
- C. H. Tilford, R. S. Shelton and M. G. Van Campen, J. Am. Chem. Soc., 1948, 70, 4001–4009 CrossRef CAS
 .
.
- A. K. Tripathi, D. Mukherjee, S. Koul and S. C. Taneja, ARKIVOC, 2009, xiii, 241–251 Search PubMed
 .
.
-
(a) P. Englebienne, A. Van Hoonacker and V. C. Herst, Drug Des. Rev.–Online, 2005, 2, 467–483 CrossRef CAS
 ;
(b) J. S. Mills and G. A. Showell, Expert Opin. Invest. Drugs, 2004, 13, 1149–1157 CrossRef CAS
;
(b) J. S. Mills and G. A. Showell, Expert Opin. Invest. Drugs, 2004, 13, 1149–1157 CrossRef CAS  ;
(c) G. A. Showell and J. S. Mills, Drug Discovery Today, 2003, 8, 551–556 CrossRef CAS
;
(c) G. A. Showell and J. S. Mills, Drug Discovery Today, 2003, 8, 551–556 CrossRef CAS  ;
(d) W. Bains and R. Tacke, Curr. Opin. Drug. Discov. Develop., 2003, 6, 526–543 CAS
;
(d) W. Bains and R. Tacke, Curr. Opin. Drug. Discov. Develop., 2003, 6, 526–543 CAS  .
.
- D. Bom, D. P. Curran, J. Zhang, S. G. Zimmer, R. Bevins, S. Kruszewski, J. N. How, A. Binglang, L. J. Lans and J. G. Burke, J. Controlled Release, 2001, 74, 325–333 CrossRef CAS
 .
.
- See http://www.clinicaltrials.gov/ct2/show/NCT00930540.
- W. Du, B. Kaskar, P. Blumbergs, P. K. Subramanian and D. P. Curran, Bioorg. Med. Chem., 2003, 11, 451–458 CrossRef CAS
 .
.
-
H. Kochat, X. Chen, Q. Huang, S. Peddaiaghari and F. H. Hausheer, Patent WO2000066127 ( 2000);
Chem. Abstr., 2000, 133, p. 350387 Search PubMed.
-
F. H. Hausheer, US Patent 20080261919 ( 2008);
Chem. Abstr., 2008, 149, p. 471692 Search PubMed.
- M. K. H. Doll, J. Org. Chem., 1999, 64, 1372–1374 CrossRef CAS
 .
.
- M. L. Bennasar and T. Roca, J. Org. Chem., 2011, 76, 4213–4218 CrossRef CAS
 .
.
- M. A. González and S. Molina-Navarro, J. Org. Chem., 2007, 72, 7462–7465 CrossRef
 .
.
- N. Houllier, M. C. Lasne, R. Bureau, P. Lestage and J. Rouden, Tetrahedron, 2010, 66, 9231–9241 CAS
 .
.
- F. Gagosz and S. Z. Zard, Org. Lett., 2002, 4, 4345–4348 CrossRef CAS
 .
.
- M. L. Bennasar, T. Roca and F. Ferrando, Org. Lett., 2006, 8, 561–564 CrossRef CAS
 .
.
- M. L. Bennasar, T. Roca and F. Ferrando, J. Org. Chem., 2005, 70, 9077–9080 CrossRef CAS
 .
.
- D. R. Artis, I. S. Cho, S. Jaime-Figueroa and J. M. Muchowski, J. Org. Chem., 1994, 59, 2456–2466 CrossRef CAS
 .
.
- L. D. Miranda, R. Cruz-Almanza and M. Pavón, ARKIVOC, 2002, xii, 15–22 Search PubMed
 .
.
- M. Menes-Arzate, R. Martínez, R. Cruz-Almanza, J. M. Muchowski, Y. M. Osornio and L. D. Miranda, J. Org. Chem., 2004, 69, 4001–4004 CrossRef CAS
 .
.
- Y. M. Osornio, L. D. Mirranda, R. Cruz-Almanza and J. M. Muchowski, Tetrahedron Lett., 2004, 45, 2855–2858 CrossRef CAS
 .
.
- Y. M. Osnorio, L. D. Miranda and J. M. Muchowski, Rev. Soc. Quim. Méx., 2004, 48, 283–287 Search PubMed
 .
.
-
D. R. Adams, J. M. Bentley, J. R. A. Roffey, R. J. Hamlyn, S. Gaur, M. A. J. Duncton, J. E. P. Davidson, M. J. Bickerdike, I. A. Cliffe and H. L. Mansell, Patent WO2000012510 ( 2000); Chem. Abstr., 2000, 123, p. 194290 Search PubMed.
-
(a) C. J. Moody and C. L. Norton, Tetrahedron Lett., 1995, 36, 9051–9052 CrossRef CAS
 ;
(b) C. J. Moody and C. L. Norton, J. Chem. Soc., Perkin Trans. 1, 1997, 1, 2639–2643 RSC
;
(b) C. J. Moody and C. L. Norton, J. Chem. Soc., Perkin Trans. 1, 1997, 1, 2639–2643 RSC  .
.
- S. M. Allin, W. R. S. Barton, W. R. Bowman, E. Bridge, M. R. J. Elsegood, T. McInally and V. McKee, Tetrahedron, 2008, 64, 7745–7758 CrossRef CAS
 .
.
-
(a) C. J. Burrows and J. G. Müller, Chem. Rev., 1998, 98, 1109–1152 CrossRef CAS
 ;
(b) R. A. Manderville, Adv. Phys. Org. Chem., 2009, 43, 177–218 CrossRef CAS
;
(b) R. A. Manderville, Adv. Phys. Org. Chem., 2009, 43, 177–218 CrossRef CAS  ;
(c) K. S. Gates, Chem. Res. Toxicol., 2009, 22, 1747–1760 CrossRef CAS
;
(c) K. S. Gates, Chem. Res. Toxicol., 2009, 22, 1747–1760 CrossRef CAS  .
.
- H. Linschitz and J. S. Connolly, J. Am. Chem. Soc., 1968, 90, 2979–2980 CrossRef
 .
.
- N. C. Yang, L. S. Gorelic and B. Kim, Photochem. Photobiol., 1971, 13, 275–277 CrossRef CAS
 .
.
-
(a) D. Elad, I. Rosenthal and H. Steinmaur, Chem. Commun., 1969, 305–306 RSC
 ;
(b) D. Elad, I. Rosenthal and H. Steinmaus, Chem. Commun., 1969, 905–906 RSC
;
(b) D. Elad, I. Rosenthal and H. Steinmaus, Chem. Commun., 1969, 905–906 RSC  ;
(c) H. Steinmaus, I. Rosenthal and D. Elad, J. Am. Chem. Soc., 1969, 91, 4921–4923 CrossRef CAS
;
(c) H. Steinmaus, I. Rosenthal and D. Elad, J. Am. Chem. Soc., 1969, 91, 4921–4923 CrossRef CAS  ;
(d) H. Steinmaus, I. Rosenthal and D. Elad, J. Org. Chem., 1971, 36, 3594–3598 CrossRef CAS
;
(d) H. Steinmaus, I. Rosenthal and D. Elad, J. Org. Chem., 1971, 36, 3594–3598 CrossRef CAS  ;
(e) D. Elad and J. Salomon, Tetrahedron Lett., 1971, 12, 4783–4784 CrossRef
;
(e) D. Elad and J. Salomon, Tetrahedron Lett., 1971, 12, 4783–4784 CrossRef  ;
(f) R. Ben-Ishai, M. Green, E. Graff, D. Elad, H. Steinmaus and J. Salomon, Photochem. Photobiol., 1973, 17, 155–167 CrossRef CAS
;
(f) R. Ben-Ishai, M. Green, E. Graff, D. Elad, H. Steinmaus and J. Salomon, Photochem. Photobiol., 1973, 17, 155–167 CrossRef CAS  ;
(g) D. Leonov, J. Salomon, S. Sasson and D. Elad, Photochem. Photobiol., 1973, 17, 465–468 CrossRef CAS
;
(g) D. Leonov, J. Salomon, S. Sasson and D. Elad, Photochem. Photobiol., 1973, 17, 465–468 CrossRef CAS  ;
(h) J. Salomon and D. Elad, J. Am. Chem. Soc., 1974, 96, 3295–3299 CrossRef CAS
;
(h) J. Salomon and D. Elad, J. Am. Chem. Soc., 1974, 96, 3295–3299 CrossRef CAS  ;
(i) D. Leonov and D. Elad, J. Am. Chem. Soc., 1974, 96, 5635–5637 CrossRef CAS
;
(i) D. Leonov and D. Elad, J. Am. Chem. Soc., 1974, 96, 5635–5637 CrossRef CAS  ;
(j) D. Leonov and D. Elad, J. Org. Chem., 1974, 39, 1470–1473 CrossRef CAS
;
(j) D. Leonov and D. Elad, J. Org. Chem., 1974, 39, 1470–1473 CrossRef CAS  ;
(k) A. A. Frimer, A. Havron, D. Leonov, J. Sperling and D. Elad, J. Am. Chem. Soc., 1976, 98, 6026–6033 CrossRef CAS
;
(k) A. A. Frimer, A. Havron, D. Leonov, J. Sperling and D. Elad, J. Am. Chem. Soc., 1976, 98, 6026–6033 CrossRef CAS  .
.
- M. Maeda, K. Nushi and Y. Kawazoe, Tetrahedron, 1974, 30, 2677–2682 CrossRef CAS
 .
.
- For a selection of other articles see:
(a) K. Kawai, Y. S. Li, M. F. Song and H. Kasai, Bioorg. Med. Chem. Lett., 2010, 20, 260–265 CrossRef CAS
 ;
(b) C. Crean, N. E. Geacintov and V. Shafirovich, J. Phys. Chem. B, 2009, 113, 12773–12781 CrossRef CAS
;
(b) C. Crean, N. E. Geacintov and V. Shafirovich, J. Phys. Chem. B, 2009, 113, 12773–12781 CrossRef CAS  ;
(c) P. M. Gannett, J. H. Powell, R. Rao, X. Shi, T. Lawson, C. Kolar and B. Toth, Chem. Res. Toxicol., 1999, 12, 297–304 CrossRef CAS
;
(c) P. M. Gannett, J. H. Powell, R. Rao, X. Shi, T. Lawson, C. Kolar and B. Toth, Chem. Res. Toxicol., 1999, 12, 297–304 CrossRef CAS  ;
(d) L. S. Nakao and O. Augusto, Chem. Res. Toxicol., 1998, 11, 888–894 CrossRef CAS
;
(d) L. S. Nakao and O. Augusto, Chem. Res. Toxicol., 1998, 11, 888–894 CrossRef CAS  ;
(e) S. Hix, M.da Silva Morais and O. Augusto, Free Radical Biol. Med., 1995, 19, 293–301 CrossRef CAS
;
(e) S. Hix, M.da Silva Morais and O. Augusto, Free Radical Biol. Med., 1995, 19, 293–301 CrossRef CAS  .
.
- See ref. 143a, page 1141–1142 and ref. 148a.
- See: C. R. Chong and D. J. Sullivan, Nature, 2007, 448, 645–646 CrossRef CAS
 ; for a discussion and relevant quotation from Sir James Black.
; for a discussion and relevant quotation from Sir James Black.
-
(a) S. D. Roughley and A. M. Jordan, J. Med. Chem., 2011, 54, 3451–3479 CrossRef CAS
 ;
(b) T. W. J. Cooper, I. B. Campbell and S. J. F. Macdonald, Angew. Chem., Int. Ed., 2010, 49, 8082–8091 CrossRef CAS
;
(b) T. W. J. Cooper, I. B. Campbell and S. J. F. Macdonald, Angew. Chem., Int. Ed., 2010, 49, 8082–8091 CrossRef CAS  ;
(c) J. S. Carey, D. Laffan, C. Thomson and M. T. Williams, Org. Biomol. Chem., 2006, 4, 2337–2347 RSC
;
(c) J. S. Carey, D. Laffan, C. Thomson and M. T. Williams, Org. Biomol. Chem., 2006, 4, 2337–2347 RSC  ;
(d) R. W. Dugger, J. A. Ragan and D. H. Brown Ripin, Org. Process Res. Dev., 2005, 9, 253–258 CrossRef CAS
;
(d) R. W. Dugger, J. A. Ragan and D. H. Brown Ripin, Org. Process Res. Dev., 2005, 9, 253–258 CrossRef CAS  .
.
Footnote |
| † Current address: Rigel, Inc., 1180 Veteran's Blvd, South San Francisco, CA 94080, United States |
|
| This journal is © The Royal Society of Chemistry 2011 |
Click here to see how this site uses Cookies. View our privacy policy here. 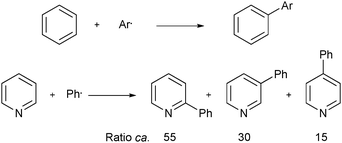
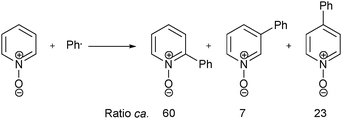






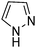


























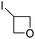
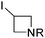
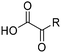
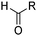


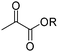

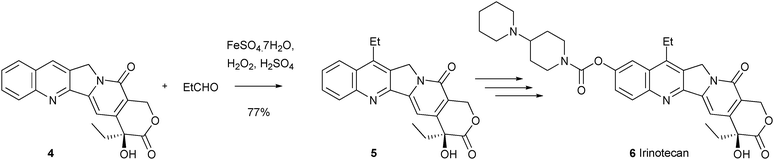
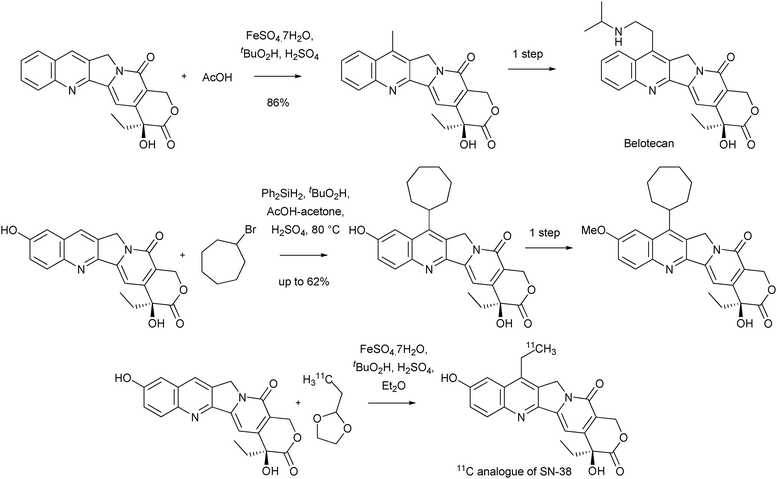
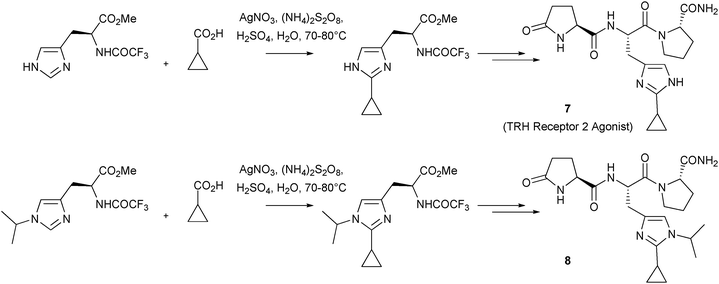
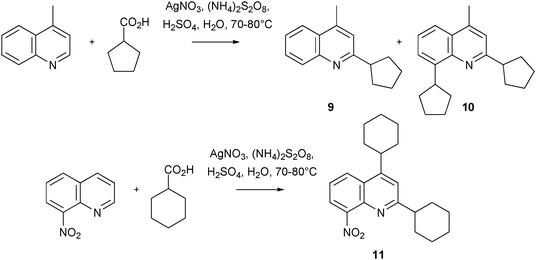
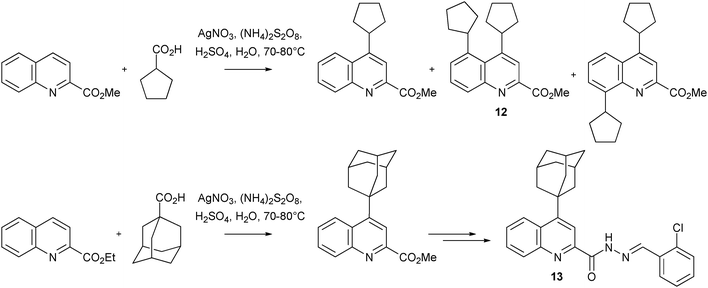
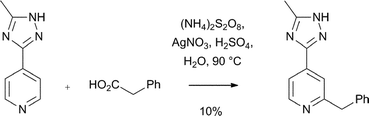
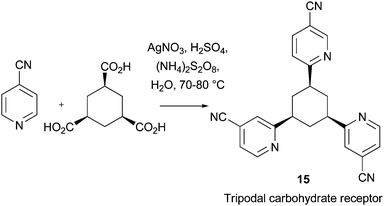
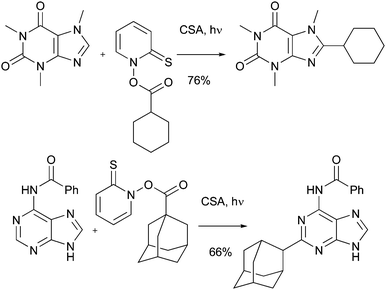
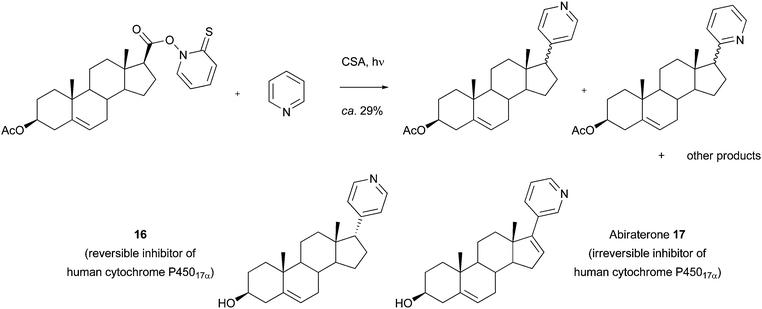
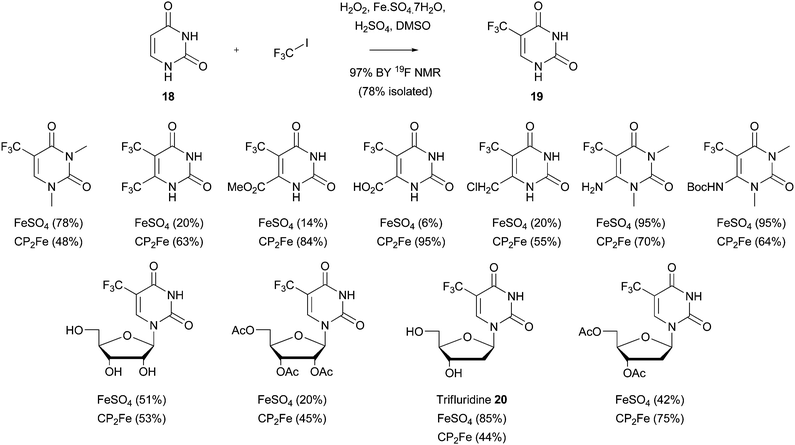
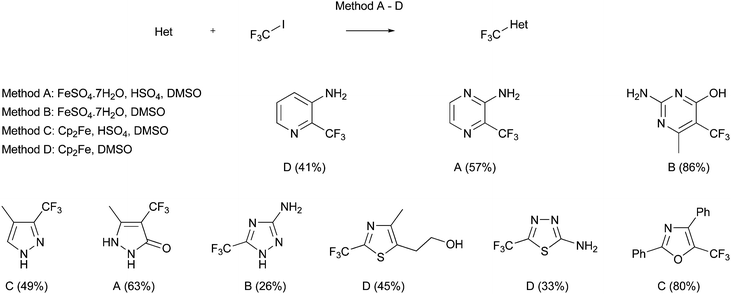
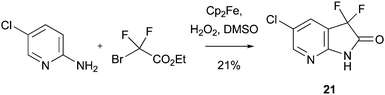

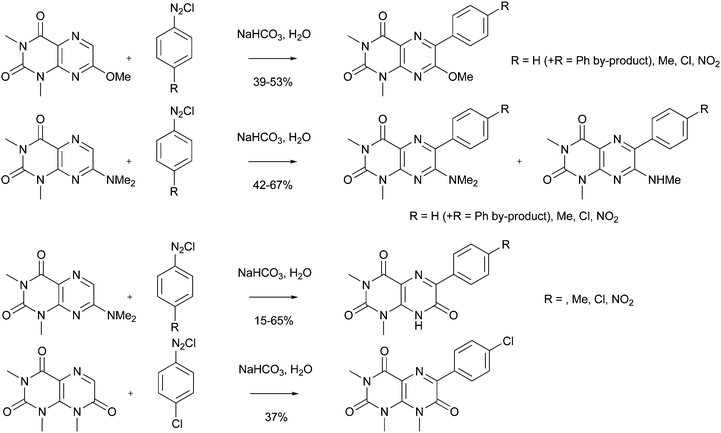
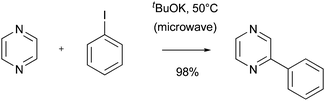
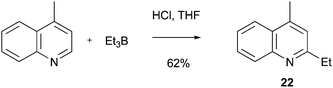
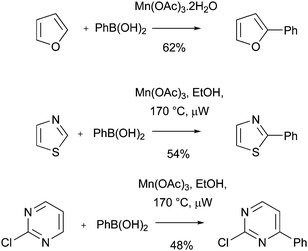
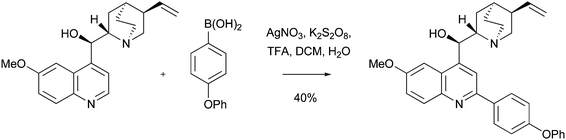
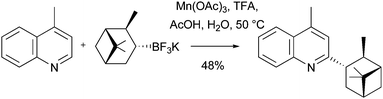



![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 starting material 28 and desired product 29). In this way, a 60% yield (19.3g) of desired amide 29 could be obtained after crystallization. The tactic employed above, of repeating a reaction after workup, may be a useful method for improving yields of other Minisci transformations that proceed in modest conversion.
1 starting material 28 and desired product 29). In this way, a 60% yield (19.3g) of desired amide 29 could be obtained after crystallization. The tactic employed above, of repeating a reaction after workup, may be a useful method for improving yields of other Minisci transformations that proceed in modest conversion.
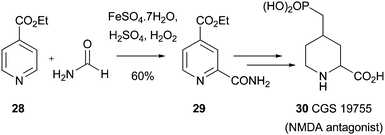
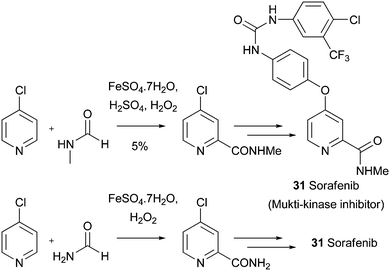
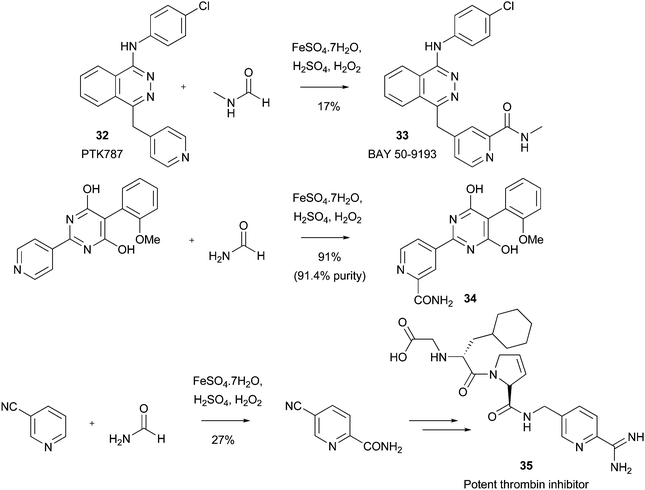
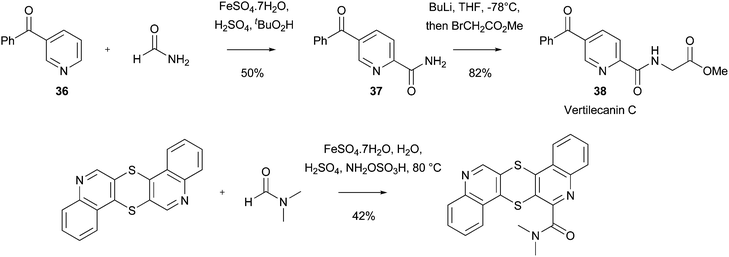
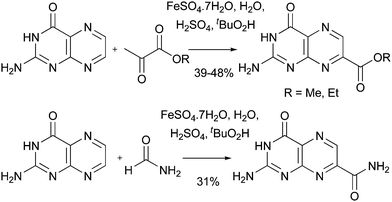
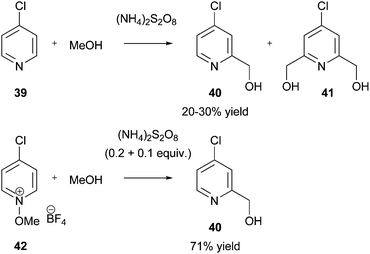
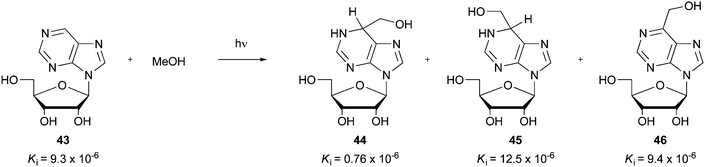
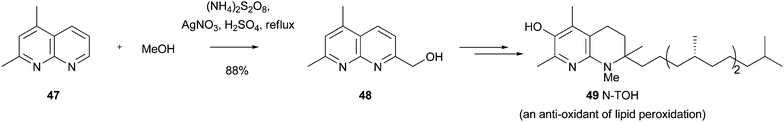
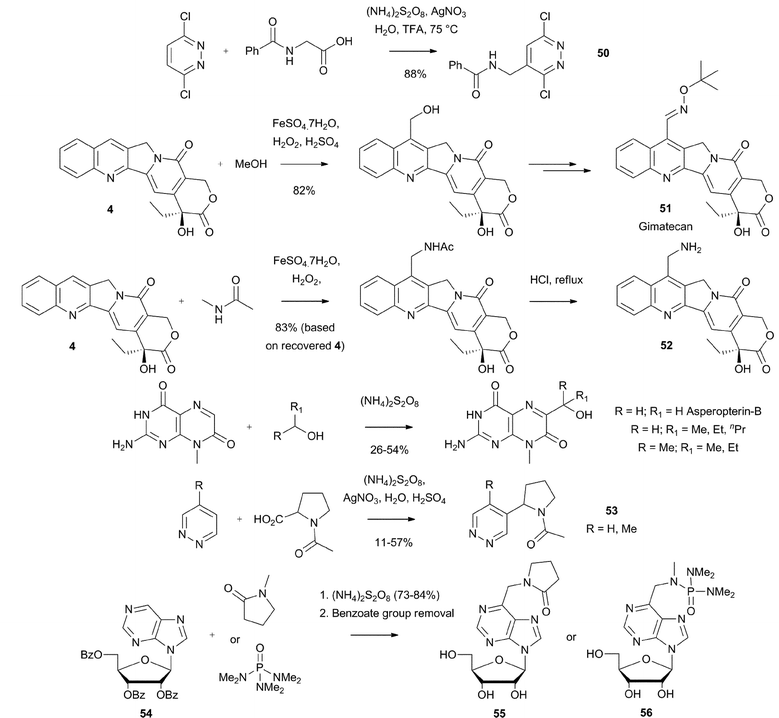
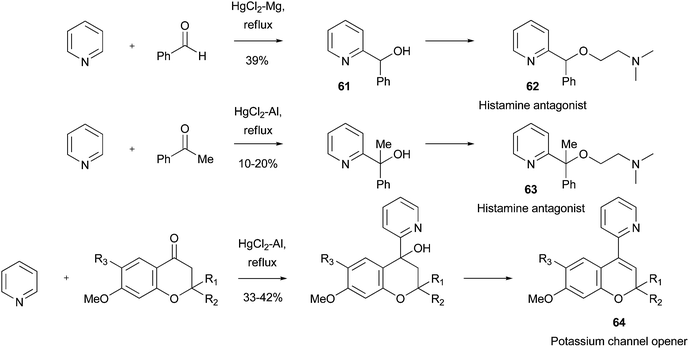
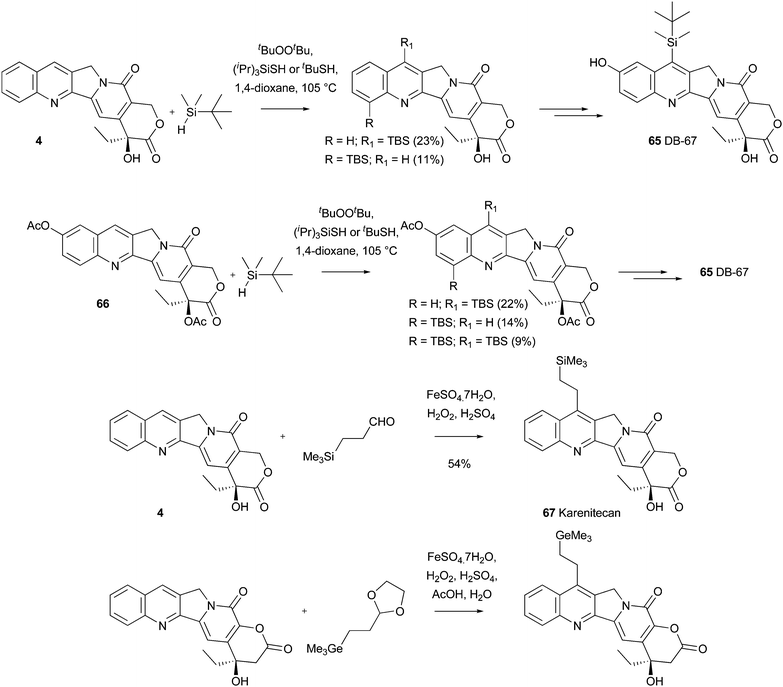
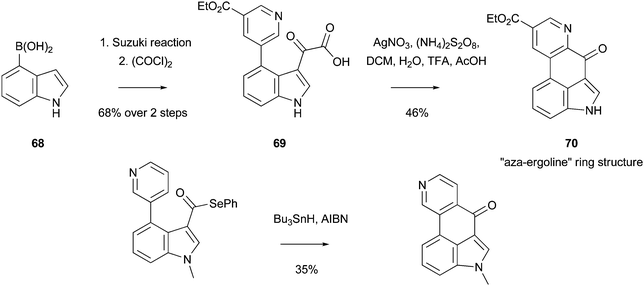


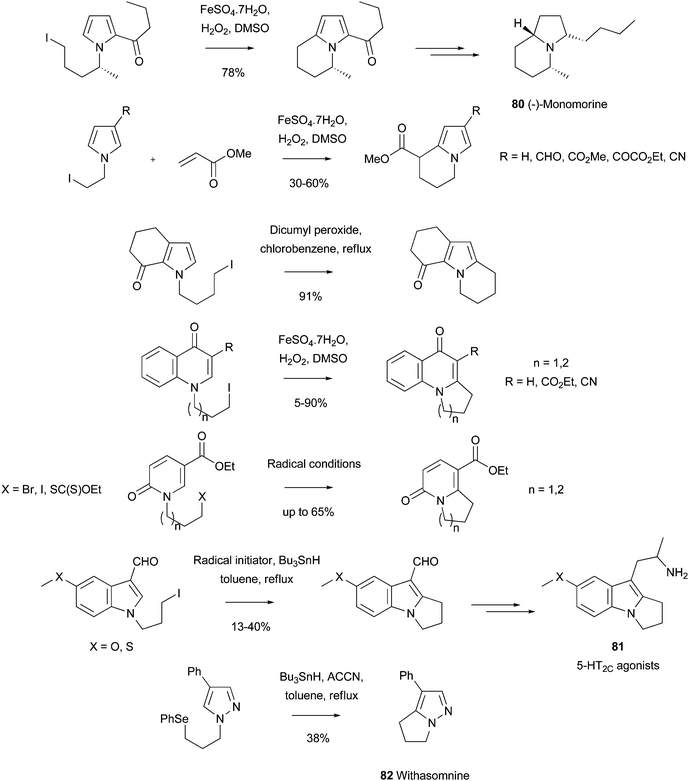


; (b) F. Minisci, F. Fontana and E. Vismara, J. Heterocycl. Chem., 1990, 27, 79–96 CrossRef CAS
; (c) F. Minisci, E. Vismara and F. Fontana, Heterocycles, 1989, 28, 489–519 CrossRef CAS
.
; (b) D. C. Harrowven, B. J. Sutton and S. Coulton, Org. Biomol. Chem., 2003, 1, 4047–4057 RSC
; (c) D. C. Harrowven, B. J. Sutton and S. Coulton, Tetrahedron, 2002, 58, 3387–3400 CrossRef CAS
.
.
.
; (b) R. Möhlau and R. Berger, Ber. Dtsch. Chem. Ges., 1893, 26, 1994 CrossRef
.
.
.
.
.
.
.
.
; (b) R. D. Brown and M. L. Heffernan, Aust. J. Chem., 1956, 9, 83–89 CrossRef CAS
.
.
; (b) H. B. Broughton and I. A. Watson, J. Mol. Graphics Modell., 2004, 23, 51–58 CrossRef CAS
; (c) X. Q. Lewell, A. C. Jones, C. L. Bruce, G. Harper, M. M. Jones, I. M. Mclay and J. Bradshaw, J. Med. Chem., 2003, 46, 3257–3274 CrossRef CAS
; (d) S. Gibson, R. McGuire and D. C. Rees, J. Med. Chem., 1996, 39, 4065–4072 CrossRef CAS
; (e) G. W. Bemis and M. A. Murcko, J. Med. Chem., 1996, 39, 2887–2893 CrossRef CAS
.
.
.
; (b) D. Kim and N. Lee, Mini-Rev. Med. Chem., 2002, 2, 611–619 CrossRef CAS
.
.
; (b) S. Sawada, S. Matsuoka, K. Nokata, H. Nagata, T. Furuta, T. Yokokura and T. Miyasaka, Chem. Pharm. Bull., 1991, 39, 3183–3188 CAS
.
.
.
.
.
.
; (b) For a previous introduction of methyl group in to 1-alkylimidazoles see: C. G. Begg, M. R. Grimmett and L. Yu-Man, Aust. J. Chem., 1973, 26, 415–420 CrossRef CAS
.
.
.
.
.
.
.
; (b) K. B. Hansen and S. A. Springfield, Patent WO2002059085 ( 2002)
; Chem. Abstr., 2002, 137, p. 140528 Search PubMed.
.
.
.
.
; (b) C. Dye and B. G. Williams, Science, 2010, 328, 856–861 CrossRef CAS
.
; (b) H. B. Broughton, R. W. Carling, J. L. Castro-Pineiro, A. R. Guiblin, A. Madin, K. W. Moore, M. G. Russell and L. J. Street, Patent WO9804559 ( 1998)
; Chem. Abstr., 1998, 128, p. 167433 Search PubMed.
.
; (b) P. B. Palde, B. R. McNaughton, N. T. Ross, P. C. Gareiss, C. R. Mace, R. C. Spitale and B. L. Miller, Synthesis, 2007, 2287–2290 CAS
; (c) B. L. Miller, P. B. Palde and P. Gareiss, Patent WO2008048967 ( 2008); Chem. Abstr., 2008, 148, p. 471607 Search PubMed.
.
.
.
.
.
; (b) W. K. Hagmann, J. Med. Chem., 2008, 51, 4359–4369 CrossRef CAS
.
.
; Chem. Abstr., 2008, 149, p. 448374 Search PubMed; (b) A. Yamakawa, K. Yamamoto, D. Uraguchi and K. Norihisa, Japanese Patent 2008231039 ( 2008)
; Chem. Abstr., 2008, 149, p. 402215 Search PubMed; (c) A. Yamakawa, K. Yamamoto, D. Uraguchi and K. Norihisa, Japanese Patent 2008115122 ( 2008)
; Chem. Abstr., 2008, 148, p. 537971 Search PubMed; (d) A. Yamakawa, K. Yamamoto, D. Uraguchi and K. Norihisa, Japanese Patent 2008115105 ( 2008)
; Chem. Abstr., 2008, 148, p. 585739 Search PubMed.
.
.
.
; (b) S. A. Mohand and W. R. Dolbier, Org. Lett., 2002, 4, 3013–3015 CrossRef CAS
; (c) H. W. Sidebottom, J. M. Tedder and J. C. Walton, Trans. Faraday Soc., 1970, 66, 2038–2044 RSC
; (d) H. W. Sidebottom, J. M. Tedder and J. C. Walton, J. Chem. Soc. Chem. Commun., 1970, 253–254 RSC
; (e) H. W. Sidebottom, J. M. Tedder and J. C. Walton, Trans. Faraday Soc., 1969, 65, 2103–2109 RSC
; (f) J. R. Case, N. H. Ray and H. L. Roberts, J. Chem. Soc., 1961, 2066–2070 RSC
.
; (b) P. Wipf, T. Mo, S. J. Geib, D. Caridha, G. S. Dow, L. Gerena, N. Roncal and E. E. Milner, Org. Biomol. Chem., 2009, 7, 4163–4165 RSC
; (c) J. T. Welch and D. S. Lim, Bioorg. Med. Chem., 2007, 15, 6659–6666 CrossRef CAS
.
; (b) E. Baciocchi and E. Muraglia, Tetrahedron Lett., 1993, 34, 5015–5018 CrossRef CAS
; (c) F. Minisci, E. Vismara and F. Fontana, J. Org. Chem., 1989, 54, 5224–5227 CrossRef CAS
.
; (b) F. Antonietti, C. Gambarotti, A. Mele, F. Minisci, R. Paganelli, C. Punta and F. Recupero, Eur. J. Org. Chem., 2005, 4434–4440 CrossRef CAS
; (c) See also X. T. Huang, Z. Y. Long and Q. Y. Chen, J. Fluorine Chem., 2001, 111, 107–113 CrossRef CAS
; (d) A. B. Cowell and C. Tamborski, J. Fluorine Chem., 1981, 17, 345–356 CrossRef CAS
.
.
; (b) S. Murata, K. Kiguchi and T. Sugimoto, Heterocycles, 1998, 48, 1255–1274 CrossRef CAS
.
.
; (b) H. C. Brown and M. M. Midland, Angew. Chem., Int. Ed. Engl., 1972, 11, 692–700 CrossRef CAS
. See also; (c) A. Dickschat and A. Studer, Org. Lett., 2010, 12, 3972–3974 CrossRef CAS
; (d) H. Suenaga, K. Nakashima, I. Hamachi and S. Shinkai, Tetrahedron Lett., 1997, 38, 2479–2482 CrossRef CAS
; (e) G. W. Kabalka, H. C. Brown, A. Suzuki, S. Honma, A. Arase and M. Itoh, J. Am. Chem. Soc., 1970, 92, 710–712 CrossRef CAS
; (f) H. C. H. A. van Riel, F. C. Fischer, J. Lugtenburg and E. Havinga, Tetrahedron Lett., 1969, 10, 3085 CrossRef
; (g) A. G. Davies and B. P. Roberts, J. Chem. Soc. B, 1967, 17–22 RSC
.
.
.
.
.
.
.
.
; (b) G. A. Molander and E. Noel, Acc. Chem. Res., 2007, 40, 275–286 CrossRef CAS
.
For the use of trifluoroborates as radical precursors in conjugate addition reactions see ref. 67 and; (b) G. Sorin, R. Martinez Mallorquin, Y. Contie, A. Baralle, M. Malacria, J. P. Goddard and L. Fensterbank, Angew. Chem., Int. Ed., 2010, 49, 8721–8723 CrossRef CAS
.
; (b) G. Wuitschik, E. M. Carreira, B. Wagner, H. Fischer, I. Parrilla, F. Schuler, M. Rogers-Evans and K. Müller, J. Med. Chem., 2010, 53, 3227–3246 CrossRef CAS
.
.
.
; (b) E. Testa, L. Fontanella and V. Aresi, Justus Liebigs Ann. Chem., 1965, 688, 165–169 CrossRef CAS
; (c) E. Testa and L. Fontanella, Justus Liebigs Ann. Chem., 1964, 671, 106–108 CrossRef CAS
; (d) E. Testa, L. Fontanella and L. Mariani, Justus Liebigs Ann. Chem., 1962, 660, 135–143 CrossRef CAS
; (e) E. Testa, A. Bonati, G. Pagani and E. Gatti, Justus Liebigs Ann. Chem., 1961, 647, 92–100 CrossRef CAS
; (f) E. Testa, L. Fontanella, L. Mariani and G. Cristiani, Justus Liebigs Ann. Chem., 1961, 639, 157–165 CrossRef CAS
; (g) E. Testa, L. Fontanella, G. Cristiani and L. Mariani, Justus Liebigs Ann. Chem., 1960, 635, 119–127 CrossRef CAS
; (h) E. Testa, L. Fontanella and G. Cristiani, Justus Liebigs Ann. Chem., 1959, 626, 114–120 CrossRef CAS
.
.
.
.
; Chem. Abstr., 1993, 120, p. 192219 Search PubMed; (b) U. Hacksell and G. D. Daves, Prog. Med. Chem., 1985, 22, 1–65 CrossRef CAS
.
; (b) T. Caronna, G. Fronza, F. Minisci, O. Porta and G. P. Gardini, J. Chem. Soc., Perkin Trans., 1972, 2, 1477–1481 Search PubMed
; (c) T. Caronna, G. Fronza, F. Minisci and O. Porta, J. Chem. Soc., Perkin Trans., 1972, 2, 2035–2038 Search PubMed
.
.
.
; (b) K. S. K. Murthy and E. E. Knaus, Drug Dev. Res., 1999, 46, 155–162 CrossRef CAS
.
; (b) W. Pfleiderer, Tetrahedron Lett., 1984, 25, 1031–1034 CrossRef CAS
; (c) R. Baur, E. Kleiner and W. Pfleiderer, Liebigs Ann. Chem., 1984, 1798–1814 CrossRef CAS
; (d) R. Baur, T. Sugimoto and W. Pfeiderer, Chem. Lett., 1984, 1025–1028 CrossRef CAS
; (e) R. Baur, T. Sugimoto and W. Pfeiderer, Helv. Chim. Acta, 1988, 71, 531–543 CrossRef CAS
.
; (b) E. C. Taylor, J. Heterocycl. Chem., 1990, 27, 1–12 CrossRef CAS
; (c) W. Pfleiderer, Biochem. Clin. Aspects Pteridines, 1982, 1, 3–26 CAS
; (d) E. C. Taylor, R. N. Henrie and D. J. Dumas, Develop. Biochem., 1979, 4, 71–75 Search PubMed
.
.
.
; (b) F. Minisci, A. Citterio, E. Vismara and C. Giordano, Tetrahedron, 1985, 41, 4157–4170 CrossRef CAS
; (c) F. Coppa, F. Fontana, E. Lazzarini, F. Minisci, G. Pianese and L. Zhao, Tetrahedron Lett., 1992, 33, 3057–3060 CrossRef CAS
.
.
; (b) S. Wilhelm, C. Carter, M. Lynch, T. Lowinger, J. Dumas, R. A. Smith, B. Schwartz, R. Simantov and S. Kelley, Nat. Rev. Drug Discovery, 2006, 5, 835–844 CrossRef CAS
; (c) S. M. Wilhelm, L. Adnane, P. Newell, A. Villanueva, J. M. Llovet and M. Lynch, Mol. Cancer Ther., 2008, 7, 3129–3140 CrossRef CAS
.
; Chem. Abstr., 1999, 131, p. 58658 Search PubMed; (b) B. Riedl, J. Dumas, U. Khire, T. B. Lowinger, W. J. Scott, R. A. Smith, J. E. Wood, M. K. Monahan, R. Natero, J. Renick and R. N. Sibley, Patent WO200042012 ( 2000); Chem. Abstr., 2000, 133, p. 120157 Search PubMed.
; Chem. Abstr., 2005, 143, p. 43891 Search PubMed.
; Chem. Abstr., 2000, 132, p. 151834 Search PubMed.
.
.
; (b) A. Maślankiewicz and E. Michalik, J. Heterocycl. Chem., 2005, 42, 1161–1166 CrossRef
.
; (b) X. Yan, T. Hollis, M. Svinth, P. Day, A. F. Monzingo, G. W. A. Milne and J. D. Robertus, J. Mol. Biol., 1997, 266, 1043–1049 CrossRef CAS
; (c) J. D. Robertus, X. Yan, S. Ernst, A. Monzingo, S. Worley, P. Day, T. Hollis and M. Svinth, Toxicon, 1996, 34, 1325–1334 CrossRef CAS
.
.
. The process uses ethylene glycol as the hydroxymethyl radical precursor, rather than methanol.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
; (b) J. A. Weitgenant, J. D. Mortison, D. J. O'Neill, B. Mowery, A. Puranen and P. Helquist, J. Org. Chem., 2004, 69, 2809–2815 CrossRef CAS
.
.
.
; (b) J. S. Mills and G. A. Showell, Expert Opin. Invest. Drugs, 2004, 13, 1149–1157 CrossRef CAS
; (c) G. A. Showell and J. S. Mills, Drug Discovery Today, 2003, 8, 551–556 CrossRef CAS
; (d) W. Bains and R. Tacke, Curr. Opin. Drug. Discov. Develop., 2003, 6, 526–543 CAS
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
; (b) C. J. Moody and C. L. Norton, J. Chem. Soc., Perkin Trans. 1, 1997, 1, 2639–2643 RSC
.
.
; (b) R. A. Manderville, Adv. Phys. Org. Chem., 2009, 43, 177–218 CrossRef CAS
; (c) K. S. Gates, Chem. Res. Toxicol., 2009, 22, 1747–1760 CrossRef CAS
.
.
.
; (b) D. Elad, I. Rosenthal and H. Steinmaus, Chem. Commun., 1969, 905–906 RSC
; (c) H. Steinmaus, I. Rosenthal and D. Elad, J. Am. Chem. Soc., 1969, 91, 4921–4923 CrossRef CAS
; (d) H. Steinmaus, I. Rosenthal and D. Elad, J. Org. Chem., 1971, 36, 3594–3598 CrossRef CAS
; (e) D. Elad and J. Salomon, Tetrahedron Lett., 1971, 12, 4783–4784 CrossRef
; (f) R. Ben-Ishai, M. Green, E. Graff, D. Elad, H. Steinmaus and J. Salomon, Photochem. Photobiol., 1973, 17, 155–167 CrossRef CAS
; (g) D. Leonov, J. Salomon, S. Sasson and D. Elad, Photochem. Photobiol., 1973, 17, 465–468 CrossRef CAS
; (h) J. Salomon and D. Elad, J. Am. Chem. Soc., 1974, 96, 3295–3299 CrossRef CAS
; (i) D. Leonov and D. Elad, J. Am. Chem. Soc., 1974, 96, 5635–5637 CrossRef CAS
; (j) D. Leonov and D. Elad, J. Org. Chem., 1974, 39, 1470–1473 CrossRef CAS
; (k) A. A. Frimer, A. Havron, D. Leonov, J. Sperling and D. Elad, J. Am. Chem. Soc., 1976, 98, 6026–6033 CrossRef CAS
.
.
; (b) C. Crean, N. E. Geacintov and V. Shafirovich, J. Phys. Chem. B, 2009, 113, 12773–12781 CrossRef CAS
; (c) P. M. Gannett, J. H. Powell, R. Rao, X. Shi, T. Lawson, C. Kolar and B. Toth, Chem. Res. Toxicol., 1999, 12, 297–304 CrossRef CAS
; (d) L. S. Nakao and O. Augusto, Chem. Res. Toxicol., 1998, 11, 888–894 CrossRef CAS
; (e) S. Hix, M.da Silva Morais and O. Augusto, Free Radical Biol. Med., 1995, 19, 293–301 CrossRef CAS
.
; for a discussion and relevant quotation from Sir James Black.
; (b) T. W. J. Cooper, I. B. Campbell and S. J. F. Macdonald, Angew. Chem., Int. Ed., 2010, 49, 8082–8091 CrossRef CAS
; (c) J. S. Carey, D. Laffan, C. Thomson and M. T. Williams, Org. Biomol. Chem., 2006, 4, 2337–2347 RSC
; (d) R. W. Dugger, J. A. Ragan and D. H. Brown Ripin, Org. Process Res. Dev., 2005, 9, 253–258 CrossRef CAS
.