Thioredoxin reductase, an emerging target for anticancer metallodrugs. Enzyme inhibition by cytotoxic gold(III) compounds studied with combined mass spectrometry and biochemical assays†
Chiara
Gabbiani
a,
Guido
Mastrobuoni
b,
Francesca
Sorrentino
c,
Barbara
Dani
c,
Maria Pia
Rigobello
c,
Alberto
Bindoli
d,
Maria Agostina
Cinellu
e,
Giuseppe
Pieraccini
f,
Luigi
Messori
a and
Angela
Casini
*g
aDepartment of Chemistry, University of Florence, Via della Lastruccia 3, 50019, Sesto Fiorentino, Italy
bBerlin Institute for Medical System Biology at the Max-Delbrück-Center for Molecular Medicine, Robert-Rössle-Straße 10, 13125, Berlin, Germany
cDepartment of Chemical Biology, University of Padua, Viale G. Colombo 3, 35121, Padova, Italy
dInstitute of Neuroscience (CNR), c/o Department of Chemical Biology, University of Padua, Viale G. Colombo 3, 35121, Padova, Italy
eDepartment of Chemistry, University of Sassari, Via Vienna 2, 07100, Sassari, Italy
fMass Spectrometry Centre (CISM), University of Florence, Via U. Schiff 6, 50019, Sesto Fiorentino, Italy
gInstitut des Sciences et Ingénierie Chimiques, Ecole Polytechnique Fédérale de Lausanne (EPFL), CH–1015, Lausanne, Switzerland. E-mail: angela.casini@epfl.ch; Fax: +41 21 6939865; Tel: +41 21 6939860
First published on 1st December 2010
Abstract
The seleno-enzyme thioredoxin reductase (TrxR) is a putative target for cytotoxic gold complexes. We investigated the mechanism of TrxR inhibition by a group of structurally diverse gold(III) compounds; the antiarthritic gold(I) drugs auranofin and aurothiomalate were also studied for comparison purposes. The tested compounds – either gold(III) or gold(I) – were found to produce potent enzyme inhibition only after pre-reduction of the enzyme with NADPH, indicating that TrxR inhibition is the result of protein structure modifications occurring upon cofactor binding. MALDI-ToF MS experiments on the intact enzyme provided evidence for extensive enzyme metallation, while experiments on trypsinized gold(III)-protein adducts identified a specific protein fragment – namely 236IGEHMEEHGIK246 – bearing an attached gold(I) ion. Independent mechanistic information on the system was derived from BIAM assays capable of monitoring selective metal binding to cysteine and/or selenocysteine residues. While the effects produced by auranofin could be essentially ascribed to gold(I) coordination to the active site selenol, the effects caused by the various gold(III) compounds were better interpreted in terms of oxidative protein damage.
Following the clinical success of cisplatin, many platinum and non-platinum metallodrugs are currently investigated as experimental antitumor agents.1–5 Much effort is specifically directed to the search of their actual mode of action and of their preferential “protein targets” as it is increasingly evident that not always DNA is the “primary” and/or “only” target for this variegate ensemble of compounds;6–8 target identification might hopefully lead to the discovery of more effective, “mechanism-oriented”, anticancer metallodrugs.
Putative targets for anticancer metallodrugs are mammalian thioredoxin reductases (TrxRs) (E.C. 1.8.1.9), large homodimeric proteins playing a crucial role in the intracellular redox balance.9 Two major isoforms are known, a cytosolic (TrxR1)10 and a mitochondrial one (TrxR2);11 their main function is the reduction of 12 kDa disulfide protein thioredoxin (Trx) to its dithiolic form.12,13 The view that TrxRs are effective “druggable” targets for inorganic compounds is supported by a recent mechanistic investigation of arsenic trioxide, a potent TrxR inhibitor now approved for promyelocytic leukaemia treatment.14 Very recently, another detailed study has appeared describing enzyme inhibition by a Pt(II) terpyridine complex.15
Notably, TrxR contains a cysteine-selenocysteine redox pair constituting the C-terminal active sites.16 The solvent-accessible selenolate group, arising from enzyme reduction, very likely constitutes a high affinity binding site for “soft” metal ions. Tightly blocking the active site selenocysteine (Sec) through metal coordination should result into potent TrxR inhibition, as earlier found for Au(I), Hg(II) and Pt(II) compounds.17–19 Though conclusive structural evidence of direct metal binding to the active-site selenolate has not achieved yet, detailed mechanistic investigations on the reactions of selected Pt(II) and Au(I) compounds with a TrxR1 mutant lacking Sec (namely the Sec497 → Cys mutant) strongly supported this hypothesis.20,21
Nowadays, gold compounds hold great promise as potential anticancer drugs. A conspicuous number of gold(III) and gold(I) compounds, with highly different chemical structures, were indeed reported to manifest outstanding antiproliferative activities against several human cancer cell lines.12,22–26 Inhibition of TrxR seems to be a common mechanistic trait to explain (at least partially) their cytotoxic actions as strong inhibition of thioredoxin reductase may eventually lead to apoptosis through a mitochondrial pathway.27
Within this frame we have explored the interactions of TrxR with a few representative gold(III) compounds. The present investigation takes advantage of the availability of high-performance mass spectrometry (MS) instruments capable of dealing with proteins as large as TrxR (∼55 kDa per monomer). At the same time, independent information was derived from application of specific biochemical assays.
The group of tested gold compounds (Chart 1) comprises [Au(dien)Cl]Cl2 (Audien),28,29 [Au(bipy)(OH)2][PF6] (bipy = bipyridine) (Aubipy),30 [(Au(pydmb-H)(AcO)2] (pydmb = 2-(1,1-dimethylbenzyl)-pyridine), AcO = CH3COO−) (Aupy), [Au(bipydmb-H)(2,6-xylidine-H)][PF6] (bipydmb = 6-(1,1-dimethylbenzyl)-2,2′-bipyridine) (Auxyl)31 and [(Au(cyclam)) (ClO4)2Cl] (Aucyclam),28 most of which were previously reported to be highly cytotoxic. The antiarthritic gold(I) drugs (2,3,4,6-tetra-O-acetyl-1-(thio-kS)-β-D-glucopyranosato) (triethylphosphine), auranofin, and aurothiomalate, that are cytotoxic in vitro (but not in vivo),32 were also included in the test panel for comparison purposes. The potent inhibitory effects induced by these compounds on both cytosolic TrxR1 and mitochondrial TrxR2 (either micromolar or even nanomolar) were previously documented (see Table S1 in the ESI†). Aucyclam is the only compound of the panel causing modest enzyme inhibition.
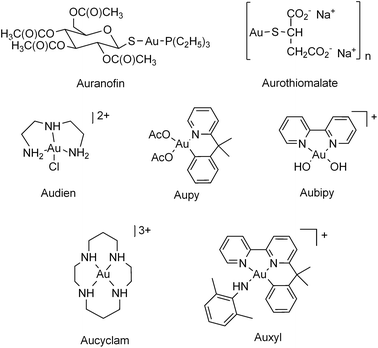 | ||
| Chart 1 Structures of the gold complexes selected for this study. | ||
The mechanism of enzyme inhibition has been analyzed in depth. We have observed that in order to afford effective TrxR inhibition by gold compounds, enzyme pre-reduction by NADPH is strictly required in such a way to convert the essential Cys/Sec residues to their S(Se)H, “gold-reactive”, forms (Fig. 1). On the contrary, in the absence of NADPH, or in the presence of the dithiol oxidizing agent diamide, the enzyme turns to its oxidized, unreactive form (–S–Se–). Moreover, structural analysis of mammalian TrxR1 showed that at the C-terminal of the enzyme at least three different conformations (namely CI, CII and CIII) for the last six amino acid residues are possible.16 These structural rearrangements occur during redox cycling and cofactor binding (e.g. NADPH binding) and are fundamental to orient the C-terminus to the surface of TrxR where the substrate is expected.
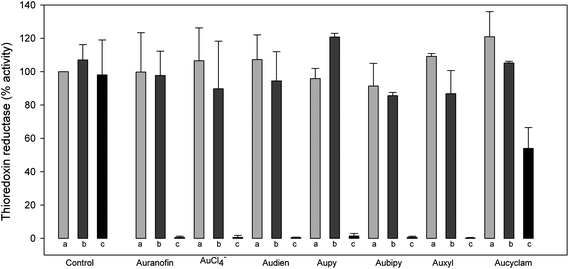 | ||
| Fig. 1 Effect of different redox conditions on the inhibition of TrxR1 by gold(I/III) compounds. TrxR1 (0.120 mg ml−1) was pre-incubated in 0.2 M Na, K-phosphate buffer (pH 7.4) containing 2 mM EDTA in different redox conditions: (a) without NADPH, (b) with 1 mM diamide, (c) with 0.2 mM NADPH. Afterwards, the gold compounds (2 μM) were added and the activity of thioredoxin reductase was estimated as described in the ESI section.† | ||
The reaction of TrxR1 with selected gold compounds was further analysed through MALDI (Matrix-Assisted Laser Desorption Ionization) ToF (Time of Flight) MS. MALDI spectra were recorded on TrxR1 samples, pre-reduced with NADPH, either untreated or incubated with an excess of each gold complex, for 1 h at 37 °C; a similar approach had been previously adopted by Daponte et al., when studying the reaction of gold phospholes with human glutathione reductase.33Fig. 2 reports representative MALDI spectra of Aubipy-TrxR1 samples prepared at different Au:protein ratios. A peak in the spectrum of untreated TrxR1 (Fig. 2a) can be observed at ca. 55600 that is assigned as TrxR1 species (z = +1). Upon reaction with the complex, the mass increase of this main peak with respect to the untreated TrxR1 sample, clearly depends on the applied gold-to-protein molar ratios. It appears that at 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (Fig. 2b) several gold atoms or gold-containing molecular fragments can bind TrxR1 causing a mass shift of ca. 1200 Da, and that the shift can reach ca. 3000 Da at 10
1 ratio (Fig. 2b) several gold atoms or gold-containing molecular fragments can bind TrxR1 causing a mass shift of ca. 1200 Da, and that the shift can reach ca. 3000 Da at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (Fig. 2a). However, due to the fact that the gold(III) compound can undergo reduction processes and loss of the ligands upon protein binding34 it has not been possible to accurately assign the number and molecular structures of the gold adducts. Similar considerations apply to the other Au complexes. Inductively coupled plasma atomic emission spectroscopy (ICP-AES) analysis on Aubipy/TrxR1 samples (3
1 ratio (Fig. 2a). However, due to the fact that the gold(III) compound can undergo reduction processes and loss of the ligands upon protein binding34 it has not been possible to accurately assign the number and molecular structures of the gold adducts. Similar considerations apply to the other Au complexes. Inductively coupled plasma atomic emission spectroscopy (ICP-AES) analysis on Aubipy/TrxR1 samples (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio) confirms that the compound binds TrxR1 almost quantitatively (see ESI† for details on sample preparation).
1 ratio) confirms that the compound binds TrxR1 almost quantitatively (see ESI† for details on sample preparation).
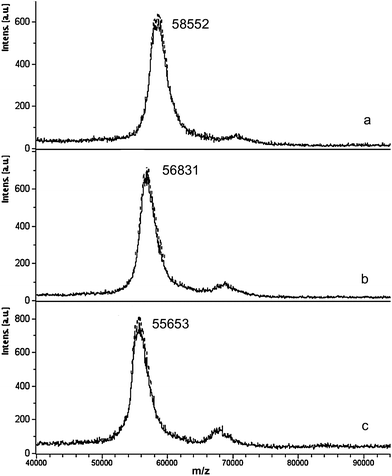 | ||
Fig. 2 MALDI MS spectra of pre-reduced rat TrxR1 incubated with Aubipy at metal:protein ratio 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (a) or 3 1 (a) or 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (b) for 1 h at 37 °C compared to the untreated pre-reduced protein (c). 1 (b) for 1 h at 37 °C compared to the untreated pre-reduced protein (c). | ||
Fig. 3 shows the MALDI spectra obtained for a variety of gold-TrxR1 adducts in comparison to the untreated TrxR1. In general, for all tested gold adducts, mass increments ranging from 1000 to 3000 Da, were measured for TrxR1 (Fig. 3) that are suggestive of protein binding of several gold-containing molecular fragments (up to 5–10 equivalents). These observations provide evidence for extensive protein metallation and point out that a certain number of high-affinity binding sites are available on the protein surface for gold coordination. Similar results were reported for the reaction of human serum albumin with antiarthritic gold(I) compounds.35 At variance, no evident mass shift was observed in the case of Aucyclam in accordance with its known poor reactivity with proteins.36
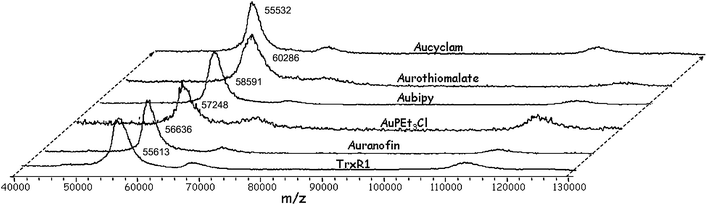 | ||
Fig. 3 MALDI MS spectra of pre-reduced rat TrxR1 after incubation with different Au compounds (metal:protein ratio: 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for Au(I) and 10 1 for Au(I) and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for Au(III) compounds) for 1 h at 37 °C. The spectrum of pre-reduced rat TrxR1 is also shown for comparison (labelled TrxR1). The most intense peaks in the spectra correspond to TrxR1 species in the charge state z = +1, while the peaks around 1100000 m/z correspond to TrxR1 dimers at z = +1. 1 for Au(III) compounds) for 1 h at 37 °C. The spectrum of pre-reduced rat TrxR1 is also shown for comparison (labelled TrxR1). The most intense peaks in the spectra correspond to TrxR1 species in the charge state z = +1, while the peaks around 1100000 m/z correspond to TrxR1 dimers at z = +1. | ||
To obtain further insight on the occurring metal-protein interactions and identify those residues that undergo metallation, a MS strategy similar to that described by Holmgren et al. for the TrxR1/trisenox system was applied relying on MS determinations of trypsinized samples.14
Thus, MALDI ToF MS analysis was carried out on trypsinized pre-reduced TrxR1 treated with increasing amounts of gold(III) complexes (ranging from a 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 gold/protein ratios), for 1 h at 37 °C, as reported in the ESI.† The spectrum of the TrxR1 trypsin-digest well corresponds to the reported one.14 However, it was not possible to attribute the peptide that was assigned as corresponding to the C-terminal active site (487SGGDILQSGCUG498 fragment at 1144.8 Th) due to the impossibility to observe the typical isotopic pattern of Selenium. Representative MALDI spectra for TrxR1 samples treated with Aubipy, Audien or AuCl4− are shown in Fig. 4B, C and D, respectively. In all cases a new peak appeared at 1475.5 Th, tentatively assigned to the tryptic peptide 236IGEHMEEHGIK246 loaded with a single Au atom. This interpretation was subsequently confirmed by performing MS/MS analysis as described in the ESI, Fig. S1.† These latter experiments also show that the bound gold ion is in the oxidation state +1 being probably coordinated to at least one His residue. Gold(III) reduction has been recently reported to occur also upon binding to a synthetic tetrapeptide modelling the Sec containing active site of TrxR.37 Finally, no significant spectral modifications were found in TrxR1 samples treated with Aucyclam (data not shown) in accordance with its poor reactivity.
1 gold/protein ratios), for 1 h at 37 °C, as reported in the ESI.† The spectrum of the TrxR1 trypsin-digest well corresponds to the reported one.14 However, it was not possible to attribute the peptide that was assigned as corresponding to the C-terminal active site (487SGGDILQSGCUG498 fragment at 1144.8 Th) due to the impossibility to observe the typical isotopic pattern of Selenium. Representative MALDI spectra for TrxR1 samples treated with Aubipy, Audien or AuCl4− are shown in Fig. 4B, C and D, respectively. In all cases a new peak appeared at 1475.5 Th, tentatively assigned to the tryptic peptide 236IGEHMEEHGIK246 loaded with a single Au atom. This interpretation was subsequently confirmed by performing MS/MS analysis as described in the ESI, Fig. S1.† These latter experiments also show that the bound gold ion is in the oxidation state +1 being probably coordinated to at least one His residue. Gold(III) reduction has been recently reported to occur also upon binding to a synthetic tetrapeptide modelling the Sec containing active site of TrxR.37 Finally, no significant spectral modifications were found in TrxR1 samples treated with Aucyclam (data not shown) in accordance with its poor reactivity.
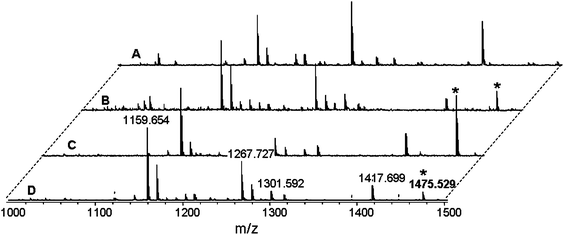 | ||
Fig. 4 Representative MALDI spectra of tryptic digests of NADPH-reduced rat TrxR1 untreated (A) or pre-incubated with excess (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) of Aubipy (B), AuCl4− (C) or Audien (D) respectively. In the gold(III) treated samples, a new peak appears at 1475.5 Th (*) that was attributed to addition of 1 Au ion to the 236IGEHMEEHGIK246 peptide. 1) of Aubipy (B), AuCl4− (C) or Audien (D) respectively. In the gold(III) treated samples, a new peak appears at 1475.5 Th (*) that was attributed to addition of 1 Au ion to the 236IGEHMEEHGIK246 peptide. | ||
Complementary information on the protein metallation processes was obtained through application of a biochemical assay relying on the thiol-tagging reagent, BIAM (biotin-conjugate iodoacetamide).38,39 BIAM alkylates selectively TrxR in dependence of pH: at pH 6.0 only selenocysteines (and low pKa cysteines) are alkylated, while at pH 8.5 both cysteines and selenocysteines are modified. In our experiments, TrxR1 was first treated with each metal complex; afterwards, sample aliquots were transferred to test tubes containing BIAM, either at pH 6.0 or 8.5, and the mixtures subjected to SDS PAGE. Proteins labelled with BIAM were detected with horseradish peroxidase as described in the supporting information. The obtained results are depicted in Fig. 5.
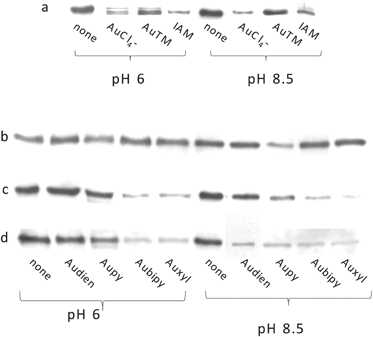 | ||
| Fig. 5 Alkylation with BIAM of rat TrxR1 after treatment with Au(I/III) complexes at increasing concentrations with respect to a control. Iodoacetamide (IAM) was used as a reference Cys-blocking agent. AuCl4−, AuTM were 100 μM (a). Audien, Aupy, Aubipy, Auxyl were 1 μM (b), 5 μM (c) and 10 μM (d). | ||
Notably, with gold(I) compounds, a pattern similar to that already observed for curcumin modification of TrxR138 was apparent (Fig. 5a), with blotting band intensity of treated samples, at pH 6.0, far weaker than the control. Again, this result is highly suggestive of selective gold(I) binding to active site Sec. At variance, in the case of gold(III) complexes, the results of the BIAM experiment are indicative of a lower selectivity. Indeed, the majority of the applied gold(III) compounds were able to alter most Cys residues and the Sec of TrxR1 though at different extents (Fig. 5 b–d). Gold(III) compounds, endowed with marked oxidizing properties, cause extensive oxidation of thiol groups as clearly apparent from the experiments reported in Fig. 5. In fact, alkylation with BIAM at pH 8.5 indicates that, at relatively high concentrations of the various compounds (Fig. 5d), almost all thiols are in the oxidized state, thus blocking BIAM binding. In other words, for gold(III) complexes, oxidation of thiol/selenol groups seems to prevail over metal coordination. Again, Aucyclam turned out to be nearly ineffective in contrasting BIAM modification given its low oxidising character and its poor binding properties. Remarkably, no significant differences in the reactivity of TrxR toward BIAM were observed following treatment with a few known ruthenium(III) inhibitors39 implying that their mechanism of inhibition is markedly different (ESI†, Fig. S2 and S3).
In conclusion, enzymatic assays have clearly revealed that enzyme inhibition by gold compounds takes place only after enzyme pre-reduction by NADPH implying that inhibition is the consequence of protein structural modifications occurring at the C-terminal site (most likely induced by the cofactor binding). MALDI ToF MS results have allowed a direct monitoring of the whole enzyme and provided clear evidence for its extensive metallation (“auration”) at a number of distinct binding sites. MS studies of trypsinized adducts of TrxR1 with various gold(III) compounds revealed that the 236IGEHMEEHGIK246 peptide is metallated and bears a gold(I) ion. On turn, BIAM assays suggested that the active site Sec is the primary anchoring sites for gold(I) compounds, whilst progressive oxidative damage of Cys and Sec residues was evidenced in the case of gold(III) complexes.
Acknowledgements
A.C the Swiss National Science Foundation (SNSF) (in particular AMBIZIONE project n° PZ00P2-121933/1), the Swiss Confederation (Action COST D39 - Accord de recherche - SER project n° C09.0027) and COST D39 for financial support. L.M. and C.G. gratefully acknowledge Beneficentia Stiftung (Vaduz, Liechtenstein), Ente Cassa di Risparmio di Firenze and Regione Toscana (NANO-TREAT project) for financial support.References
- J. Reedijk, Eur. J. Inorg. Chem., 2009, 1303 CrossRef CAS.
- M. Coluccia and G. Natile, Anti-Cancer Agents Med. Chem., 2007, 7, 111 CrossRef.
- X. Y. Wang and Z. J. Guo, Dalton Trans., 2008, 1521 RSC.
- C. G. Hartinger and P. J. Dyson, Chem. Soc. Rev., 2009, 38, 391 RSC.
- M. D. Hall, R. C. Dolman and T. W. Hambley, Metal Ions in Biolgical Systems, Vol. 42: Metal Complexes in Tumor Diagnosis and as Anticancer Agents, 2004, 42, 297 Search PubMed.
- P. J. Dyson and G. Sava, Dalton Trans., 2006, 1929 RSC.
- A. R. Timerbaev, C. G. Hartinger, S. S. Aleksenko and B. K. Keppler, Chem. Rev., 2006, 106, 2224 CrossRef CAS.
- S. P. Fricker, Metallomics, 2010, 2, 366 RSC.
- E. S. J. Arner and A. Holmgren, Eur. J. Biochem., 2000, 267, 6102 CrossRef CAS.
- P. Y. Gasdaska, J. R. Gasdaska, S. Cochran and G. Powis, FEBS Lett., 1995, 373, 5 CrossRef CAS.
- A. Miranda-Vizuete, A. E. Damdimopoulos, J. R. Pedrajas, J. A. Gustafsson and G. Spyrou, Eur. J. Biochem., 1999, 261, 405 CrossRef CAS.
- A. Bindoli, M. P. Rigobello, G. Scutari, C. Gabbiani, A. Casini and L. Messori, Coord. Chem. Rev., 2009, 253, 1692 CrossRef CAS.
- K. Becker, S. Gromer, R. H. Schirmer and S. Muller, Eur. J. Biochem., 2000, 267, 6118 CrossRef CAS.
- J. Lu, E. H. Chew and A. Holmgren, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 12288 CrossRef CAS.
- Y. C. Lo, T. P. Ko, W. C. Su, T. L. Su and A. H. J. Wang, J. Inorg. Biochem., 2009, 103, 1082 CrossRef CAS.
- K. Fritz-Wolf, S. Urig and K. Becker, J. Mol. Biol., 2007, 370, 116 CrossRef.
- A. B. Witte, K. Anestal, E. Jerremalm, H. Ehrsson and E. S. J. Arner, Free Radical Biol. Med., 2005, 39, 696 CrossRef CAS.
- Y. Omata, M. Folan, M. Shaw, R. L. Messer, P. E. Lockwood, D. Hobbs, S. Bouillaguet, H. Sano, J. B. Lewis and J. C. Wataha, Toxicol. in Vitro, 2006, 20, 882 CrossRef CAS.
- C. M. L. Carvalho, E. H. Chew, S. I. Hashemy, J. Lu and A. Holmgren, J. Biol. Chem., 2008, 283, 11913 CrossRef CAS.
- R. Millet, S. Urig, J. Jacob, E. Amtmann, J. P. Moulinoux, S. Gromer, K. Becker and E. Davioud-Charvet, J. Med. Chem., 2005, 48, 7024 CrossRef CAS.
- S. Urig, K. Fritz-Wolf, R. Reau, C. Herold-Mende, K. Toth, E. Davioud-Charvet and K. Becker, Angew. Chem., Int. Ed., 2006, 45, 1881 CrossRef CAS.
- L. Ronconi and D. Fregona, Dalton Trans., 2009, 10670 RSC.
- S. Nobili, E. Mini, I. Landini, C. Gabbiani, A. Casini and L. Messori, Medicinal Research Reviews, 2009 Search PubMed.
- E. R. Tiekink, Inflammopharmacology, 2008, 16, 138 CrossRef CAS.
- R. W. Y. Sun and C. M. Che, Coord. Chem. Rev., 2009, 253, 1682 CrossRef CAS.
- E. Vergara, A. Casini, F. Sorrentino, O. Zava, E. Cerrada, M. P. Rigobello, A. Bindoli, M. Laguna and P. J. Dyson, ChemMedChem, 2010, 5, 96 CrossRef CAS.
- P. J. Barnard and S. J. Berners-Price, Coord. Chem. Rev., 2007, 251, 1889 CrossRef CAS.
- L. Messori, F. Abbate, G. Marcon, P. Orioli, M. Fontani, E. Mini, T. Mazzei, S. Carotti, T. O'Connell and P. Zanello, J. Med. Chem., 2000, 43, 3541 CrossRef CAS.
- M. Coronnello, G. Marcon, S. Carotti, B. Caciagli, E. Mini, T. Mazzei, P. Orioli and L. Messori, Oncology Research, 2001, 12, 361.
- G. Marcon, S. Carotti, M. Coronnello, L. Messori, E. Mini, P. Orioli, T. Mazzei, M. A. Cinellu and G. Minghetti, J. Med. Chem., 2002, 45, 1672 CrossRef CAS.
- L. Messori, G. Marcon, M. A. Cinellu, M. Coronnello, E. Mini, C. Gabbiani and P. Orioli, Bioorg. Med. Chem., 2004, 12, 6039 CrossRef CAS.
- C. K. Mirabelli, R. K. Johnson, C. M. Sung, L. Faucette, K. Muirhead and S. T. Crooke, Cancer Research, 1985, 45, 32 CAS.
- M. Deponte, S. Urig, L. D. Arscott, K. F. Wolf, R. Reau, C. Herold-Mende, S. Koncarevic, M. Meyer, E. Davioud-Charvet, D. P. Ballou, C. H. Williams and K. Becker, J. Biol. Chem., 2005, 280, 20628 CrossRef CAS.
- A. Casini, C. Hartinger, C. Gabbiani, E. Mini, P. J. Dyson, B. K. Keppler and L. Messori, J. Inorg. Biochem., 2008, 102, 564 CrossRef CAS.
- J. Talib, J. L. Beck and S. F. Ralph, JBIC, J. Biol. Inorg. Chem., 2006, 11, 559 CrossRef.
- G. Marcon, L. Messori, P. Orioli, M. A. Cinellu and G. Minghetti, Eur. J. Biochem., 2003, 270, 4655 CrossRef CAS.
- A. Pratesi, C. Gabbiani, M. Ginanneschi and L. Messori, Chem. Commun., 2010, 46, 7001–7003 RSC.
- J. G. Fang, J. Lu and A. Holmgren, J. Biol. Chem., 2005, 280, 25284 CrossRef CAS.
- P. Mura, M. Camalli, A. Bindoli, F. Sorrentino, A. Casini, C. Gabbiani, M. Corsini, P. Zanello, M. P. Rigobello and L. Messori, J. Med. Chem., 2007, 50, 5871 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, Table S1, Figure S1, S2 and S3. See DOI: 10.1039/c0md00181c |
| This journal is © The Royal Society of Chemistry 2011 |
