Supramolecular assembly composed of different mesogenic compounds possessing a ω-hydroxyalkyl unit exhibits suppressive effects on the A549 human lung cancer cell line†
Yuuka
Takahashi
a,
Masaharu
Hazawa
b,
Kenji
Takahashi
b,
Masanobu
Sagisaka
a,
Ikuo
Kashiwakura
b and
Atsushi
Yoshizawa
*a
aDepartment of Frontier Materials Chemistry, Graduate School of Science and Technology, Hirosaki University, 3, Bunkyo-cho, Hirosaki, 036-8561, Japan. E-mail: ayoshiza@cc.hirosaki-u.ac.jp; Fax: +81-172-39-3558; Tel: +81-172-39-3558
bDepartment of Radiological Life Sciences, Hirosaki University Graduate School of Health Sciences, 3, Bunkyo-cho, Hirosaki, 036-8561, Japan
First published on 3rd December 2010
Abstract
We investigated the anti-tumor activity and mesogenic properties of 4-cyano-4′-(6-hydroxyhexyloxy)biphenyl (I-CN), 4-methyloxy-4′-(6-hydroxyhexyloxy)biphenyl (I-OMe) and their binary mixtures. The anti-tumor properties of the materials were investigated in A549 human lung cancer cells by assessing cell growth, cell cycle distribution and cell signaling pathways using flow cytometry and Western blot analysis. An equimolar mixture of I-CN (2.5 μM) and I-OMe (2.5 μM) exhibited stronger inhibition of cell proliferation in A549 human lung cancer cells than I-CN or I-OMe. The mixture did not affect cell proliferation against WI-38 normal fibroblasts. Both thermotropic and lyotropic mesogenic properties of the mixture indicate that molecular aggregation between I-CN and I-OMe occurs at a concentration of micromolar order. These results reveal that the supramolecular assembly composed of different molecules plays an important role in the biological activity of liquid-crystalline molecules.
Introduction
Drug design for anticancer agents has been an attractive subject for many years. Numerous studies are progressing to develop chemotherapeutic agents either by synthesis or from natural products. Chemotherapy is the administration of drugs that can regulate the uncontrolled proliferation of abnormal cancer cells. Most chemotherapeutic drugs are classifiable as alkylating agents, antimetabolites, anthracyclines, plant alkaloids, topoisomerase inhibitors, monoclonal antibodies, or other anticancer agents.1–7 Although the use of molecular targeting drugs such as the tyrosine kinase inhibitor Imatinib is increasing, few drugs can achieve a complete recovery in cancer patients. Particularly, the development of effective drugs against solid cancer cells, e.g. lung cancer, presents some difficult problems.Generally, a new drug is designed based on the structure–property relations of a single molecule, i.e. introduction of a binding site for a target protein into a molecule. On the other hand, biological structure has links with liquid-crystallinity.8Cell membranes are lamellar bi-layer mesophases of phospholipids, glycolipids, and cholesterol. To reach cancer cells in optimal quantity, a therapeutic agent must pass through an imperfect blood vasculature to the cancer, cross the vessel walls into the interstitium, and penetrate multiple layers of solid cancer cells. Some lyotropic liquid-crystalline materials possessing structural affinity with cell membranes have been applied to develop novel drug delivery systems. Tang, Du et al. developed a novel polyethylene glycol–phosphatidylethanolamine-based nanocarrier of doxorubicin that increased cytotoxicity in vitro and enhanced anticancer activity in vivo with low systematic toxicity.9 The results support the authors' inference that 10 nm to 20 nm nano-assemblies of phospholipids containing doxorubicin would improve the drug's penetration, accumulation and anticancer activity. Both thermotropic and lyotropic liquid-crystalline drugs have attracted considerable interest.10–13 Recently, we reported some liquid-crystalline compounds exhibiting anticancer activity, and described a relation between anticancer activity and liquid-crystallinity.14–16 Conventional cyanobiphenyl derivatives possessing a primary alcohol17–19 showed inhibition activity against A549 human lung cancer cells and did not exhibit suppressive effects on WI-38 normal fibroblasts.15,16 On the other hand, those possessing a secondary alcohol exhibited effects against not only the cancer cells but also against normal cells. These results indicate (1) that liquid-crystallinity has important effects on the inhibition of cell proliferation, and (2) that the liquid-crystalline compounds possessing a primary alcohol can recognize differences between cancer cells and normal cells. Nevertheless, it remains unclear whether a single mesogenic molecule shows anticancer activity or whether an assembly of the mesogenic molecules does so. We have designed supramolecular assemblies by introducing specific interactions among different liquid-crystalline molecules. For this study, we investigated biological activity and mesophase formation of a binary system between different mesogenic compounds, and discuss their molecular organization, which behaves as an effective species for the biological activity of the liquid-crystalline materials.
Results and discussion
Biological activity
We investigated biological activity of 4-cyano-4′-(6-hydroxyhexyloxy)biphenyl (I-CN),15,164-methyloxy-4′-(6-hydroxyhexyloxy)biphenyl (I-OMe), 4-fluoro-4′-(6-hydroxyhexyloxy)biphenyl (I–F),16 and their binary mixtures. The molecular structures are presented in Fig. 1. All compounds have a terminal hydroxy group. Therefore, they have both hydrophilic and hydrophobic properties. Compounds I-CN and I–F have an electron-withdrawing group, whereas compound I-OMe has an electron-donating group. | ||
| Fig. 1 Mesogenic molecules of the mesogenic compounds. | ||
Effects of the mesogenic compounds and their equimolar binary mixtures on the growth of the A549 human lung cancer cell line at a concentration of 5 μM are presented in Fig. 2. The mixtures contain 2.5 μM of each component. No significant difference was found in the inhibition of the cell proliferation among compounds I-CN, I-OMe, I–F, and the control. The compounds showed suppressive effects on cell growth at a concentration of 10 μM. The survival ratios to the control were 0.47 ± 0.02 for I-CN, 0.49 ± 0.03 for I-OMe, and 0.43 ± 0.16 for I–F. With respect to the binary systems, a binary mixture of I-CN possessing an electron-withdrawing group and I-OMe possessing an electron-donating group shows marked inhibition of cell proliferation. On the other hand, the binary mixture of biphenyl derivatives I-CN and I–F, each possessing an electron-withdrawing group, shows no significant difference in the suppressive effect compared to each corresponding biphenyl derivative.
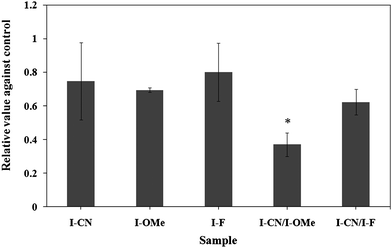 | ||
| Fig. 2 Effects of mesogenic materials on the growth of the A549 human lung cancer cell line. The culture was conducted in liquid media containing 5 μM of each sample for 96 h. The binary mixtures contained 2.5 μM of each component. *p < 0.05 using Student's t-test. | ||
We investigated the dependence of the ratio of I-OMe over I-CN in the binary system on inhibition of the cell proliferation (Fig. 3). It is particularly interest that an equimolar mixture of I-CN and I-OMe was found to exhibit the most suppressive effect against the A549 cell. These results suggest that one-to-one molecular assembly of I-CN and I-OMe is an effective species for the marked inhibition of cancer cell growth.
 | ||
| Fig. 3 Effects of concentration of I-OMe in the I-CN and I-OMe binary system on the growth of the A549 human lung cancer cell line. The culture was conducted in liquid media containing 5 μM of each sample for 96 h. *p < 0.05 using Student's t-test. | ||
We investigated the influence of I-CN, I-OMe, I–F and their binary mixtures on cell cycle progression. The effects of the materials on the cell cycle regulation of A549 were assayed after 24 h of treatment using flow cytometry. Fig. 4 shows that an equimolar mixture of I-CN and I-OMe caused a statistically significant increase of the G1 phase population of A549 cells. However, the mixture showed no induction of either the sub G1 population or apoptosis. Effects of the binary mixture of I-CN and I-OMe on the cell cycle regulation are the same as those of each corresponding component.15,16
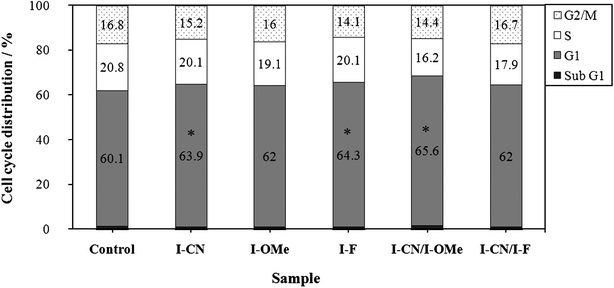 | ||
| Fig. 4 Cell cycle distributions in A549 determined using flow cytometry. The A549 cells were treated with each sample in liquid culture condition (5 μM) for 24 h. *p < 0.05 using Student's t-test. | ||
To elucidate the mechanism of the anticancer activity, the expression and activation of some proteins (ERK, JNK, and CDK2) were evaluated using Western blot analysis in A549 cells treated with each sample. Sample concentrations inducing effective suppressive inhibition of the cell growth were applied. They were 10 μM for I-CN, 10 μM for I-OMe and 5 μM for their binary mixture. Fig. 5 shows that no significant difference exists in the appearance of CDK2 among the control, I-CN, I-OMe, or the mixture. CDK2 is a cell-cycle regulating protein in the transition of the G1 to S phase. The mixture showed marked down-regulation of extracellular signal-regulated kinase ERK. In fact, ERK plays a role in cell survival and proliferation. The mixture decreased the phosphorylation of survival signal protein ERK, which engenders arrest of the cancer cell proliferation. The mixture also showed down-regulation of JNK. p21waf1, which is a CDK inhibitor, has a crucial role in G1 phase arrest in somatic cells harboring the p53 gene.20 Moreover, p21waf1 has another important role as an inhibitor of JNK.21 When G1 phase arrest was induced by DNA damaging stimuli such as X-ray irradiation, p21waf1 was up-regulated through a p53-mediated pathway in A549 cells.22 Therefore, the observed inhibition of JNK activities could demonstrate the negative regulation by p21waf1 induced by p53-mediated G1 phase arrest.
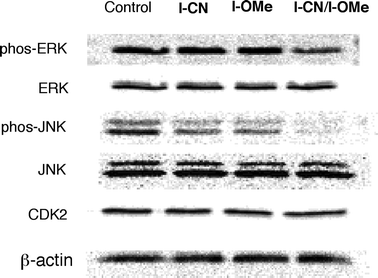 | ||
| Fig. 5 Western blot profile of proliferation-related molecules in A549 cells. A549 cells were treated with each sample for 24 h: I-CN 10 μM, I-OMe 10 μM, the equimolar mixture 5 μM (I-CN at 2.5 μM and I-OMe at 2.5 μM). β-Actin is shown as a loading control. | ||
To determine the influence of the binary mixture of I-CN and I-OMe on normal cells, the mixture and each component were tested against WI-38 fibroblast cells. Fig. 6 shows that neither the mixture nor each separate component showed a significant suppressive effect on the WI-38 fibroblast cell growth. The binary system was found to induce different effects on carcinoma cells and normal fibroblast cells.
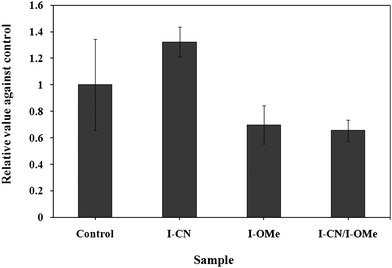 | ||
| Fig. 6 Effects of the mesogenic materials on the growth of WI-38 fibroblast cells. The culture was conducted for 168 h in liquid media containing 5 μM of each sample. | ||
Liquid crystallinity
To confirm the formation of specific intermolecular interaction between the mesogenic molecules, thermotropic behaviour of the binary mixtures between them was investigated. Compound I-CN exhibited a nematic phase; its isotropic-to-nematic transition temperature (TIN) was 109 °C. In contrast, neither of the compounds I-OMe and I–F showed a liquid-crystalline phase; their isotropic-to-crystal temperatures were 144 °C and 93 °C, respectively. Concomitantly with increasing concentration of I-OMe in a binary system of I-CN and I-OMe, the TIN increased. The TIN of the equimolar mixture was 16 K higher than that of I-CN. Stabilization of the nematic phase shows the existence of the intermolecular interaction between I-CN and I-OMe. Actually, I-CN has an electron-withdrawing group, whereas I-OMe has an electron-donating group. Therefore, dipole–dipole interactions are thought to occur between them. On the other hand, TIN of the equimolar mixture of compounds I-CN and I–F was 22 K lower than that of I-CN, suggesting that interaction between them does not exist.We then investigated the lyotropic liquid crystallinity of an equimolar mixture of compounds I-CN and I-OMe. A DMSO solution of the mixture (10 mM) was added to various amounts of water. Fig. 7 shows a photomicrograph of the mixture (500 μM) in DMSO–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19) solution at 37 °C. Although some crystalline phases appeared, many droplet textures were observed, suggesting that lamellar and/or micellar liquid-crystalline phases exist. However, the characteristic droplet texture could not be confirmed by POM at a lower concentration than 500 μM because the texture size might be smaller than 1 μm.
19) solution at 37 °C. Although some crystalline phases appeared, many droplet textures were observed, suggesting that lamellar and/or micellar liquid-crystalline phases exist. However, the characteristic droplet texture could not be confirmed by POM at a lower concentration than 500 μM because the texture size might be smaller than 1 μm.
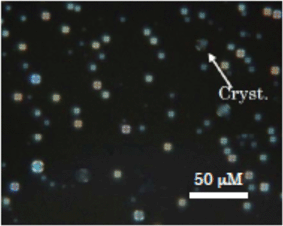 | ||
Fig. 7
Photomicrograph of a sample of the equimolecular mixture of compounds I-CN and I-OMe at 500 μM in DMSO–H2O(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19) solution at 37 °C. 19) solution at 37 °C. | ||
To estimate the ability of the binary mixture to form a molecular assembly, we investigated the turbidity of each sample at various concentrations by observing the absorbance at 500 nm (Table 1). The critical aggregated concentration (CAC) is defined as a concentration at which the absorbance begins to increase, which can be a measure of the molecular aggregation ability of the mesogenic molecules in the solution. Critical aggregated concentrations of some binary systems together with their components are listed in the table. The equimolar mixture of I-CN and I-OMe shows lower CAC than their components, suggesting that the binary system that is stabilized by intermolecular interaction between I-CN and I-OMe has higher ability to form molecular aggregation than its components. However, CAC of the equimolar mixture of I-CN and I–F is higher than I–F and lower than I-CN, indicating that molecular assembly of I-CN and I–F does not form.
| Sample | CAC/μM |
|---|---|
| I-CN | 30 ± 8 |
| I-OMe | 30 ± 11 |
| I–F | 57 ± 38 |
| Equimolar mixture of I-CN and I-OMe | 8 ± 5 |
| Equimolar mixture of I-CN and I–F | 44 ± 10 |
Molecular organization model
An equimolar mixture of I-CN and I-OMe exhibits larger suppressive effects on the growth of the A549 human lung cancer cell line and shows higher ability to form both thermotropic and lyotropic liquid-crystalline phases than I-CN or I-OMe. We discuss molecular aggregation of the mixture. The main driving force of the mesophase formation of amphiphilic compounds is microsegregation between hydrophilic and hydrophobic units. The microsegregation prefers formation of a parallel molecular alignment. On the other hand, cyanobiphenyl derivatives are known to form an antiparallel alignment via dipole–dipole interaction. Actually, I-CN and I-OMe have both a hydroxy unit and a dipole moment. Therefore, competition between parallel alignment via microsegregation and antiparallel alignment via dipole–dipole interaction is thought to occur. With respect to the binary system, both microsegregation and dipole–dipole interaction prefer to form parallel alignment. Fig. 8 shows that the parallel alignment can easily organize bilayered lamellar and/or micellar assemblies. The nano-assemblies are thought to have high ability to penetrate layers of solid cancer cells. | ||
| Fig. 8 Molecular aggregation models for the binary system of I-CN and I-OMe. | ||
Conclusion
An equimolar mixture of mesogenic compounds I-CN and I-OMe was found to exhibit stronger suppressive effects on A549 human lung cancer cells than either of its constituents. The marked inhibition results from cell progression from the G1 into the S phase. The binary system was found to induce down-regulation of survival signal ERK, which engenders the G1 cell arrest. Parallel molecular alignment between I-CN and I-OMe is thought to be organized via both dipole–dipole interaction and a microsegregation effect, which leads to preferential formation of bilayered lamellar and/or micellar assemblies. The supramolecular assembly composed of the different mesogenic compounds plays an important role in exhibiting anticancer activity. Our findings provide novel insight into the creation of new anticancer drugs.Acknowledgements
This study was supported by a Grant for Hirosaki University Institutional Research and by a Grant-in-Aid for Challenging Exploratory Research from the Japan Society for the Promotion of Science (No. 21655045).References
- P. L. Kuo, Y. L. Hsu, T. C. Lin and J. K. Chang, Anti-Cancer Drugs, 2005, 4, 409–415 CrossRef.
- A. Abe, M. Yamane and A. Tomoda, Anti-Cancer Drugs, 2001, 12, 377–382 CrossRef CAS.
- K. Skobridis, M. Kinigopoulou, V. Theodorou, E. Giannousi, A. Russell, R. Chauhan, R. Sala, N. Brownlow, S. Kiriakdis, J. Domin, G. A. Tzakos and N. J. Dibb, ChemMedChem, 2010, 5, 130–139 CrossRef CAS.
- J. H. Chang, C. F. Hung, S. C. Yang, J. P. Wang, S. J. Won and C. N. Lin, Bioorg. Med. Chem., 2008, 16, 7270–7276 CrossRef.
- A. Goel, A. K. Prasad, V. S. Parmar, B. Ghosh and N. Saini, FEBS Lett., 2007, 581, 2447–2454 CrossRef CAS.
- T. C. Chou, X. Zhang, Z. Y. Zhong, Y. Li, L. Feng, S. Eng, D. R. Myles, R. Johnson, N. Wu, Y. I. Yin, R. M. Wilson and S. J. Danishefsky, Proc. Natl. Acad. Sci. U. S. A., 2008, 35, 13157–13162 CrossRef.
- J. H. Zhang, C. D. Fan, B. X. Zhao, D. S. Shin, W. L. Dong, Y. S. Xie and J. Y. Miao, Bioorg. Med. Chem., 2008, 16, 10165–10171 CrossRef CAS.
- J. W. Goodby, V. Görtz, S. J. Cowling, G. Mackenzie, P. Martin, D. Plusquellec, T. Benvegnu, P. Boullanger, D. Lafont, Y. Queneau, S. Chambert and J. Fitremann, Chem. Soc. Rev., 2007, 36, 1971–2032 RSC.
- N. Tang, G. Du, N. Wang, C. Liu, H. Hang and W. Liang, J. Natl. Cancer Inst., 2007, 99, 1004–1015 CrossRef CAS.
- Y. Y. Luk, S. F. Campbell, N. L. Abbott and C. J. Murphy, Liq. Cryst., 2004, 31, 611–621 CrossRef CAS.
- H. Bunjes and T. Rades, J. Pharm. Pharmacol., 2005, 57, 807–816 CrossRef CAS , and references cited therein.
- C. T. Stevenson, D. B. Bennett and D. Lechuga-Ballesteros, J. Pharm. Sci., 2005, 94, 1861–1880 CrossRef CAS , and references cited therein.
- C. Cervin, P. Vandoolaeghe, C. Nistor, F. Tiberg and M. Johnsson, Eur. J. Pharm. Sci., 2009, 36, 377–385 CrossRef CAS.
- A. Yoshizawa, Y. Takahashi, R. Terasawa, S. Chiba, K. Takahashi, M. Hazawa and I. Kashiwakura, Chem. Lett., 2009, 38, 310–311 CrossRef CAS.
- A. Yoshizawa, Y. Takahashi, A. Nishizawa, K. Takeuchi, M. Sagisaka, K. Takahashi, M. Hazawa and I. Kashiwakura, Chem. Lett., 2009, 38, 530–531 CrossRef CAS.
- Y. Takahashi, M. Hazawa, K. Takahashi, A. Nishizawa, A. Yoshizawa and I. Kashiwakura, Invest. New Drugs DOI:10.1007/s10637-010-9411-9.
- A. C. Griffin and S. R. Vidya, Mol. Cryst. Liq. Cryst., 1989, 173, 85–88 CAS.
- V. Percec, M. Lee and C. Ackerman, Polymer, 1992, 33, 703–711 CrossRef CAS.
- V. Percec and M. Lee, Macromolecules, 1991, 24, 1017–1024 CrossRef CAS.
- Y. Xue, N. T. Ramaswamy, X. Hong and J. C. Pelling, Mol. Carcinog., 2003, 36, 38–44 CrossRef CAS.
- J. Shim, H. Lee, H. Kim and E. J. Choi, Nature, 1996, 381, 804–806 CrossRef CAS.
- M. Hazawa, K. Wada, K. Takahashi, T. Mori, N. Kawahara and I. Kashiwakura, Invest. New Drugs, 2008, 27, 111–119.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details. See DOI: 10.1039/c0md00126k |
| This journal is © The Royal Society of Chemistry 2011 |
