Milk, revealed “silent” chemistry: new mode of cycloretinal synthesis†
Bennie J.
Bench‡
,
Jennifer
Foulke-Abel‡
and
Coran M. H.
Watanabe
*
Department of Chemistry, Texas A&M University, College Station, TX 77843, USA
First published on 5th November 2010
Abstract
Bovine milk is by far the most commonly consumed milk in the western world. The protein composition in milk consists of casein and whey proteins, of which β-lactoglobulin (BLG) is the principal constituent of the latter. Here we provide biochemical evidence that this milk protein, in purified form and in pasteurized store-bought milk, promotes the formation of cycloretinal (all-transretinal dimer), and a variety of other cycloterpenals of biological relevance [Fishkin et al., Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 7091–7096; Fishkin et al., Chirality, 2004, 16, 637–641; Kim et al., Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 19273–19278]. Cycloretinal is an eye metabolite and among several toxic byproducts of the visual cycle firmly established to cause age-related macular degeneration. Experiments in rabbits further demonstrate that BLG/milk can survive the digestive system and promote this reaction in vivo [Caillard et al., Am. J. Physiol., 1994, 266(6), G1053–G1059]. Proteomic studies on age-related macular degeneration patients have detected BLG in the eye of these patients further suggesting that this milk protein could contribute to disease progression [Crabb et al., Proc. Natl. Acad. Sci. U. S. A., 2002, 99(23), 14682–14687].
Introduction
The cycloterpenals are a family of natural products whose name we coined to reflect their terpenoid biosynthetic origin and cyclohexadienal structural motif.1,2Cyclocitral and cycloretinal (Scheme 1) are representative members of this natural product class. While cyclocitral was isolated from a North Sea bryzoan, Flustra foliacea, and exhibits antibiotic activity,3–5cycloretinal has been isolated from the human eye and is one of several compounds associated with age-related macular degeneration,6–8 the most common cause of blindness affecting adults over the age of 50.9,10 This medical condition results in a loss of central vision that begins with the accumulation of characteristic yellow and white deposits in the macula or central part of the retina of the eye that contain by-products of the visual cycle such as cycloretinal and A2E (Scheme 1).6–8,11–14 Photochemical activation of A2E has been shown to give rise to oxidation of the conjugated double bonds (retinoid side arms) of the molecule. The resulting epoxides are highly reactive and damaging to retinal pigment epithelial cells leading to the formation of DNA lesions and protein crosslinking, as well as cellular apoptosis (Scheme 1).15,16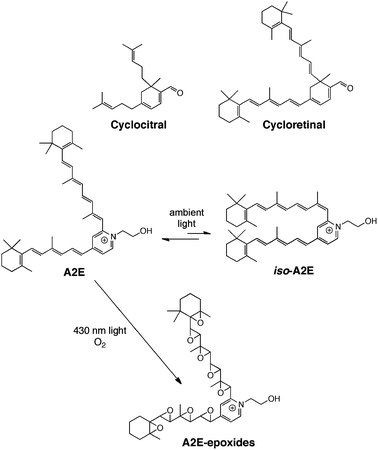 | ||
| Scheme 1 | ||
The physiological activity of the cycloterpenals is further supported by the screening of a 100 member synthetic library of these compounds, consisting of both homodimers and heterodimers.1,2Cell-based assays revealed several with notable activities in a PC12 assay (a model system for neuronal differentiation) and against a cancerous T-cell line.2
During the course of a photochemical investigation examining the role of host proteins in affecting substrate polyene isomerization, β-ionylideneacetaldehyde was shown to undergo a partial transformation to give an unexpected C-30 ring-fused dimer (cyclo-β-ional) mediated by the milk protein β-lactoglobulin (BLG).17 No further research has been reported.
Unprocessed cow's milk contains on average, 3.4% protein, 3.6% fat, 4.6% lactose, and 0.7% minerals. Bovine β-lactoglobulin (BLG) constitutes between 12–15% of the protein content of milk (∼4 g L−1). The protein has been studied extensively for its physical and biochemical properties for over 70 years because of its size (18.4 kDa), stability, and availability.18 Despite these investigations, to date BLG remains a protein of unassigned function. While BLG may serve as a nutritional supplement, its ability to bind a variety of hydrophobic ligands with high affinity is suggestive of a transport role for ligands such as retinoids and various fatty acids.19,20BLG might also play a role in digestion of milk fats as ruminant BLG has been isolated with fatty acids bound.
With the reported role of cycloretinal and/or its derivatives in the onset of age-related macular degeneration,6–8,21 we made the connection that BLG could promote cycloretinal formation and have carried out investigations, both in vitro and in vivo, with pasteurized milk and purified protein establishing a link between BLG chemistry and age-related macular degeneration. The results of these investigations are detailed herein.
Results
β-Lactoglobulin was evaluated for its ability to promote cycloterpenal formation of a variety of ligands. These self-condensation reactions employed conditions that had been optimized for substrate ratio, pH, and duration of the reaction (highlighted in Fig. 1 and Fig. S1, ESI†). | ||
Fig. 1 Optimization of BLG reaction conditions. Yield as a function of protein to substrate ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1 1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1 2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1 3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 1 5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10; reaction duration (days 1–5), and buffer pH (3, 5, 7, 9, 11). See also Fig. S1, ESI.† 10; reaction duration (days 1–5), and buffer pH (3, 5, 7, 9, 11). See also Fig. S1, ESI.† | ||
Varying the protein to substrate ratio from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 revealed that a protein to substrate concentration of 1
10 revealed that a protein to substrate concentration of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 was optimal. Finalized conditions thus consisted of incubating substrates with a 1% BLG solution at pH 7, 37 °C employing a protein to substrate concentration of 1
3 was optimal. Finalized conditions thus consisted of incubating substrates with a 1% BLG solution at pH 7, 37 °C employing a protein to substrate concentration of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. Product formation was monitored over the course of 4 days and was confirmed by 1H-NMR (Fig. S2, ESI†) and mass spectrometry. Substrates ranging from long chain alkyl aldehydes, small aromatics, and bulky aromatics were evaluated (Table 1). Isolated product yields ranged from 24% to 60%. Reaction yields did not reflect any specific trends related to substrate classification. Cycloretinal 2 gave a 44% yield, where conversion was abolished by treatment of the protein with sodium dodecyl sulfate (Fig. S2, ESI†) and represents the first protein mediated synthesis reported on this lipofuscin (a yellow autofluorescent pigment of the eye). An HPLC profile of the reaction mixture is provided as ESI† (Fig. S3).
3. Product formation was monitored over the course of 4 days and was confirmed by 1H-NMR (Fig. S2, ESI†) and mass spectrometry. Substrates ranging from long chain alkyl aldehydes, small aromatics, and bulky aromatics were evaluated (Table 1). Isolated product yields ranged from 24% to 60%. Reaction yields did not reflect any specific trends related to substrate classification. Cycloretinal 2 gave a 44% yield, where conversion was abolished by treatment of the protein with sodium dodecyl sulfate (Fig. S2, ESI†) and represents the first protein mediated synthesis reported on this lipofuscin (a yellow autofluorescent pigment of the eye). An HPLC profile of the reaction mixture is provided as ESI† (Fig. S3).
| Compound | % Yield | Compound | % Yield |
|---|---|---|---|
| 1 | 43 | 6 | 58 |
| 2 | 44 | 7 | 24 |
| 3 | 55 | 8 | 60 |
| 4 | 38 | 9 | 32 |
| 5 | 25 | 10 | 58 |
β-Lactoglobulin was also able to promote the formation of heterodimers. For example, incubation of cinnamaldehyde and citral with BLG resulted in the formation of the four expected racemic products (Fig. 2).2Cinnamaldehyde is unable to dimerize. Biomimetic organic syntheses with proline have revealed that cycloterpenal formation proceeds through a Schiff base.1 As a control reaction, we examined the ability of bovine serum albumin (a protein containing 60 lysine residues) to effect the formation of cyclocitral. Imine formation with aldehydes is ubiquitous in enzymology. For example, lysine in opsin forms a Schiff base intermediate with retinal to give the visual pigment rhodopsin in the eye.22 Formation of cyclocitral was only observed with BLG and not BSA as detected by both 1H-NMR and mass spectrometry (Fig. 3A and B). Likewise, when citral was tested against the E. coli proteome (Fig. 3C, consisting of >1000 proteins, ∼4500 genes), or retinal pigment epithelial cell extract (Fig. S4, ESI†) conversion to cyclocitral was not observed. Therefore, BLG-catalyzed cycloterpenal formation, while quite promiscuous with substrates, is quite specific and not a general reaction exhibited by proteins.
 | ||
| Fig. 2 BLG-mediated cross condensation products formed between citral and cinnamaldehyde. | ||
 | ||
| Fig. 3 NMR analysis of the protein mediated dimerization of citral. Alignments of the aldehydic region of the 1H-NMR spectrum of organics extracted from an aqueous solution of citral incubated with (A) BSA and (B) BLG. Panel (C) represents the 1H-NMR spectrum of organics extracted before and after citral incubation with an E. colicell-free extract. See also Fig. S2–S4, ESI.† | ||
Having demonstrated that BLG could promote the formation of cycloretinal and other cycloterpenals, we evaluated the ability of store-bought, pasteurized milk to promote the reaction. The reaction was carried out with whole milk, 2% milk, skim milk, and buttermilk (Fig. 4). Each was incubated with excess citral for 4 days at 37 °C. Skim milk resulted in the highest yield (14%). The 2% milk exhibited a yield of 7.3%, while whole milk and buttermilk gave a 5% and 1% yield, respectively. As BLG is known to bind a variety of lipophilic molecules with high affinity, the lower gross catalytic ability with higher fat content may be intuitively rationalized as a form of inhibition.18,19
 | ||
| Fig. 4 NMR analysis of citral incubated with milk: shows alignments of the aldehydic region of the 1H-NMR spectrum of organics extracted from an aqueous solution of citral incubated with: (A) whole milk, (B) 2% milk, (C) skim milk and (D) buttermilk. See also Fig. S5, ESI.† | ||
As a control reaction, to ensure that small metabolites were not responsible for invoking the dimerization process, skim milk and 2% milk were passed through a Sephadex G-25 column. The flow-through was then subjected to the same reaction conditions as detailed above (Fig. S5, ESI†). Reaction yields were unaffected lending further support for the involvement of BLG in promoting cycloterpenal synthesis.
In human blood BLG is present at concentrations of 1–4 μg L−1.23 As humans do not possess a BLG homolog, the BLG present in blood is derived exclusively from milk or milk products, e.g. cheese, yogurt. The protein is acid stable and is transported intact into the blood stream by intestinal transepithelial cells. Moreover, proteomic studies performed on eye tissue of macular degeneration patients have revealed the presence of BLG within drusen (retinal pigment epithelium deposits containing assorted lipids, polysaccharides, glycosaminoglycans and proteins).24 To further evaluate the physiological relevance of BLG in promoting cycloterpenal formation in vivo, specifically that of cycloretinal, we examined the ability of BLG (in pure form and in milk) to support cycloretinal formation in a rabbit. Eight New Zealand White Rabbits (2.5 kg) were allocated to the control (basic diet) (n = 1), BLG/water control (n = 1), skim milk control (n = 1), BLG/water + retinal (n = 2), and skim milk + retinal (n = 2). All of the rabbits had free access to a standard rabbit diet and water. They were each administered their respective solutions by gavage twice daily for 7 days. Following the feeding regimen, the rabbits were anesthesized and exsanguinated for subsequent analysis. The blood was extracted with ethyl acetate, the organics were dried over anhydrous magnesium sulfate, and concentrated in vacuo. The extracts were analyzed by 1H-NMR spectroscopy and mass spectrometry (Fig. 5A). Cycloretinal was only detected in rabbits that were supplied a solution of BLG + retinal or skim milk + retinal. To further demonstrate the presence of “active” BLG in blood, we analyzed rabbit blood serum for the presence of BLG. The serum was diluted (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) with buffer and analyzed by SDS-PAGE. The gel (Fig. 5B) revealed that BLG was readily absorbed into the blood stream of rabbits with the exception of the control rabbit (which only received a basic diet and showed no trace of the protein.) These results are consistent with studies, which have shown that absorption of intact BLG into the blood stream proceeds by intracellular transfer through the intestinal transepithelial cells.25,26 Blood isolated from a rabbit provided a BLG-supplemented diet promoted the formation of cycloretinal from retinal, while the control (basic diet) rabbit showed no signs of product formation (Fig. S8, ESI†). Therefore, BLG remains active in the bloodstream.
10) with buffer and analyzed by SDS-PAGE. The gel (Fig. 5B) revealed that BLG was readily absorbed into the blood stream of rabbits with the exception of the control rabbit (which only received a basic diet and showed no trace of the protein.) These results are consistent with studies, which have shown that absorption of intact BLG into the blood stream proceeds by intracellular transfer through the intestinal transepithelial cells.25,26 Blood isolated from a rabbit provided a BLG-supplemented diet promoted the formation of cycloretinal from retinal, while the control (basic diet) rabbit showed no signs of product formation (Fig. S8, ESI†). Therefore, BLG remains active in the bloodstream.
![NMR analysis of cycloretinal formation in rabbit blood: [A] shows alignments of the aldehydic region of the 1H-NMR spectrum of organics extracted from rabbit blood samples: (1) control (basic diet), (2) BLG control, (3) skim milk control, (4) retinal control, (5) BLG + retinal and (6) skim + retinal; detection of BLG in rabbit blood [B] SDS-PAGE analysis of blood serum: (M) molecular weight marker, kD, (1) BLG standard, (2) BLG control, (3) BLG + retinal, (4) skim milk, (5) skim milk + retinal and (6) control (basic diet); ∼Note: cycloretinal prior to feeding did not occur.](/image/article/2011/MB/c0mb00186d/c0mb00186d-f5.gif) | ||
| Fig. 5 NMR analysis of cycloretinal formation in rabbit blood: [A] shows alignments of the aldehydic region of the 1H-NMR spectrum of organics extracted from rabbit blood samples: (1) control (basic diet), (2) BLG control, (3) skim milk control, (4) retinal control, (5) BLG + retinal and (6) skim + retinal; detection of BLG in rabbit blood [B] SDS-PAGE analysis of blood serum: (M) molecular weight marker, kD, (1) BLG standard, (2) BLG control, (3) BLG + retinal, (4) skim milk, (5) skim milk + retinal and (6) control (basic diet); ∼Note: cycloretinal prior to feeding did not occur. | ||
Discussion
Approximately 6 to 10 million Americans suffer from vision loss due to age-related macular degeneration with hundreds of thousands of new cases being documented each year.9,10,24 Here we have demonstrated that the milk protein β-lactoglobulin can promote the synthesis of the eye lipofuscin cycloretinalin vitro and in vivo as well as other cycloterpenals. Two convergent mechanisms for the reaction are provided in Fig. 6. These two “double-bound” mechanisms parallel our previously proposed mechanism for proline-mediated reactions in organic solvents, which was supported by time-course analyses and the direct observation of intermediates by NMR and mass spec.1,2 The validity of these reaction mechanisms will be fully examined in the future and reported elsewhere. The reaction occurs with pasteurized, store-bought milk and with BLG within whole blood. While humans do not possess a BLG homolog, the protein is present within the bloodstream23 and must be diet derived.24 Liposfuscin granules begin to form in the retina at the age of 20 and can constitute as much as 20% of the cell volume by the age of 80.27Cycloretinal and a cycloretinal-phosphotidylethanolamine conjugate have both been successfully isolated from rod outer segments.6 It is postulated that these lipofuscins form in the rod outer segment and become deposited in retinal pigment epithelial cells by photo-receptor outer segment disk phagocytosis.6 The ability of the milk protein BLG to promote cycloretinal formation suggests that this protein might also play a role in the accumulation of lipofuscins. Although cycloterpenal synthesis is unlikely to represent the primary function of BLG (given the slowness of the reaction), this moonlighting activity28 could play an important role in the progression of this debilitating eye disease. While model reactions using a combination of proline and triethylamine for catalysis can yield cycloretinal,17 we have found that no reaction occurs under physiological conditions (Fig. S6, ESI†). This supports the involvement of a proteinin vivo. Indeed, some individuals exhibiting early to intermediate signs of macular degeneration are more likely to progress to the advanced stages of the disease (characterized by blood vessel formation below the retina leading to blood and fluid leak) if consuming a diet rich in high fat dairy products (whole milk, ice cream, hard cheese, butter).29 Nonetheless, BLG cannot be the only route to the formation of cycloretinal as mice do not produce BLG and a Abcr-knockout mouse model of age-related macular degeneration has been shown to accumulate this lipofuscin in the eye.6,8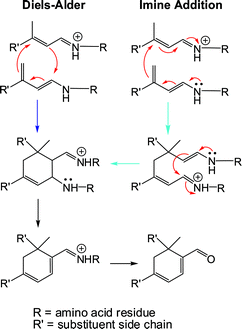 | ||
| Fig. 6 Proposed mechanisms for the BLG-mediated formation of cycloretinal and other biologically relevant cycloterpenals. The reaction can be envisioned to proceed through a Diels–Alder or imine addition, where substrate molecules are bound to the protein through amino acid residues (e.g.lysine). | ||
In the long term, investigations on BLG and its ability to biosynthesize cycloretinal might lead to the development and design of a new class of age-related macular degeneration therapeutics or lead to the synthesis of proteomic agents in the identification of other proteins within the human genome that might also perform this reaction. These investigations will be pursued in due course.
Methods
Reagents and instrumentation
Fresh THF was dried by passage through an MBRAUN solvent purification system and stored over molecular sieves. All other solvents and reagents were purchased and used without further purification unless noted. 1H (300 MHz) and 13C (75 MHz) spectra were recorded on a Varian Inova or Mercury300 spectrometer. Chemical shifts for 1H and 13C NMR spectra are reported in ppm referenced to solvent. Mass data were recorded on an API QSTAR PULSAR (ES) instrument. Chromatographic separations were achieved by flash silica chromatography (silica gel 60 mesh, EMD Biosciences). HPLC separations were performed on a Varian ProStar system. Experiments were carried out using BLG from Sigma (L3908, St. Louis, MO, 90% BLG) or Davisco Foods International, Inc. (JE-003-6-922, La Sueur, MN, 93.6% BLG).General procedure for BLG promoted biosynthesis of cycloterpenal homodimers and heterodimers
In a 1000 mL Erlenmeyer flask, 500 mL of a 1% BLG solution in phosphate buffered saline (PBS, 0.01 M phosphate, 0.0027 M KCl, 0.137 M NaCl, pH 7.0) and 3 equiv. of substrate (0.5 M in absolute ethanol, 1.630 mL) were combined. This mixture was placed in a shaker (37 °C, 250 rpm) for 4 days. The reaction was quenched by the addition of 500 mL of ethyl acetate to the BLG suspension, which was stirred for 30 min at room temperature to extract the product. To avoid emulsions, the mixture was centrifuged at 9000 rpm at 4 °C for 30 min. The organic layer was collected, dried over anhydrous magnesium sulfate, concentrated in vacuo, and the products purified and characterized (Fig. S2, ESI†).1,2 For the synthesis of heterodimers, 0.5 M of each aldehyde substrate (in absolute ethanol, 0.543 mL each) was used following the procedure outlined above.Milk promoted biosynthesis of cycloterpenals
To demonstrate the dimerization with processed milk, skim milk, 2% milk, whole milk and buttermilk were purchased. The milk contained approximately 8–9 grams of protein according to their labels. Initial experiments were performed with 200 mL of milk, which was diluted with 300 mL of PBS (pH 7.0) and incubated with aldehyde substrate (2.20 mL of 0.5 M solution in absolute ethanol) at 37 °C with shaking (250 rpm) for 4 days. Following incubation, the solutions were extracted with 300 mL of ethyl acetate, concentrated in vacuo and the metabolites analyzed using the methods described above.To ensure that there were no small metabolites responsible for the dimerization process, each milk was passed through a Sephadex G-25 column (GE Healthcare, Uppsala, Sweden, Product # 17-0033-01). The G-25 resin (60 g) was swollen to 300 mL with PBS (pH 7.0) and packed into a glass column (5 × 30.5 cm). Milk (90 mL) was passed through the column followed by the addition of PBS until a total volume of 250 mL was obtained. The filtered milk was incubated with aldehyde substrate (1.10 mL of 0.5 M solution in absolute ethanol) and retested utilizing the same conditions as detailed above (Fig. S5, ESI†).
In vivo study utilizing New Zealand white rabbits
The following protocol (AUP#2008-70) was approved by the Texas A&M University Institutional Animal Care and Use Committee (IACUC). Eight adult New Zealand White rabbits (2.5 kg, Myrtle's Rabbitry, Thompsons Station, TN) were randomly allocated to the control (normal diet, n = 1), BLG/water control (n = 1), skim milk control (n = 1), retinal control (n = 1), BLG/water + retinal (n = 2), and skim milk + retinal (n = 2). All rabbits had free access to a standard rabbit diet and water. The rabbits were housed separately in standard cages in the LARR facility at Texas A&M University and maintained under standard conditions. Each day, the rabbits were provided the solutions by gavage twice daily for a week. Each solution was administered in 50 mL volumes with the exception of the retinal control, which was supplied as a 1 mL solution (40 mg kg−1; retinal was solubilized in 150 mL of ethanol). The BLG/water solution was supplied at 1.3 g per kg in 50 mL of water.After seven days, the rabbits were anesthesized with a solution containing 10 mg per kg of ketamine with 3 mg per kg xylezine by intravenous injection allowing 5 min for the cocktail to take effect. Depth of anesthesia was monitored prior to blood removal by squeezing the foot of the rabbit. Cardiac blood was removed by opening the thorax to visualize the heart before carrying out cardiac puncture, which also results in exsanguination. This process allowed us to obtain approximately 40–50 mL of blood from each rabbit. The blood (40 mL) was processed by extraction. Ethyl acetate (300 mL) was added and mixed with a magnetic stirrer for 1 h. The suspension was centrifuged for 30 min. at 9000 rpm, 4 °C. The organic layer was removed, dried with anhydrous magnesium sulfate, and concentrated in vacuo.
To assess BLG content in the samples, blood from the control (normal diet), BLG/water control, BLG/water + retinal, skim milk control, and skim milk + retinal were centrifuged at 4000 rpm for 30 min at 4 °C to separate the red blood cells from serum. After centrifugation, 1 μL of serum was diluted with 9 μL of PBS, pH 8.0 and 10 μL of SDS-PAGE Buffer (125 mM Tris, pH 6.8, 4% SDS, 20% glycerol, 0.2 mg mL−1bromophenol blue, 0.2 mM DTT). Samples were heated to 90 °C for 10 min and analyzed by SDS-PAGE (15%, 200 V for 35 min).
Synthesis of α,β-unsaturated aldehyde substrates
The α,β-unsaturated aldehyde substrates required to test the cycloterpenal biosynthetic reactions were synthesized from their corresponding α,β-unsaturated nitriles following literature protocols.1,2Farnesal was synthesized from farnesol.1All-trans-retinal was generated from β-ionone in the following manner (Fig. S7, ESI†). Initially, β-ionylideneacetaldehyde (C-15 aldehyde) was synthesized in two steps via a Horner–Wadsworth–Emmons reaction with β-ionone, followed by DIBAL reduction.30 The C-15 aldehyde was subsequently converted to the all-trans C-18 ketonei by performing an aldol condensation (with subsequent dehydration) between acetone and β-ionylideneacetaldehyde in the presence of 1.0 M sodium hydroxide, resulting in a 92% yield.31 A Horner–Wadsworth–Emmons reaction on the methyl ketone gave the all-transC-20 β-methyl nitrileii in 88% yield.32,33 Reducing the C-20 nitrile with diisobutylaluminium hydride results in the formation of all-transretinaliii with an 86% yield.30,32,34,35Acknowledgements
The authors gratefully acknowledge the Welch Foundation (A-1587) for financial support of this work.References
- B. J. Bench, C. Liu, C. R. Evett and C. M. Watanabe, Proline Promoted Synthesis of Ring-Fused Homodimers: Self-condensation of Alpha, Beta-unsaturated Aldehydes, J. Org. Chem., 2006, 71(25), 9458–9463 CrossRef CAS.
- B. J. Bench, S. E. Tichy, L. M. Perez, J. Benson and C. M. H. Watanabe, Synthesis and Cellular Effects of Cycloterpenals: Cyclohexadienal-Based Activators of Neurite Outgrowth, Bioorg. Med. Chem., 2008, 16(16), 7573–7581 CrossRef CAS.
- L. Peters, G. M. Konig, A. D. Wright, R. Pukall, E. Stackebrandt, L. Eberl and K. Riedel, Secondary Metabolites of Flustra Foliacea and Their Influence on Bacteria, Appl. Environ. Microbiol., 2003, 69, 3469–3475 CrossRef CAS.
- L. Peters, A. D. Wright, S. Kehraus, D. Gundisch, M. C. Tilotta and G. M. Konig, Prenylated Indole Alkaloids from Flustra Foliacea with Subtype Specific Binding on NAChRs, Planta Med., 2004, 70, 883–886 CrossRef CAS.
- L. Peters, A. D. Wright, A. Krick and G. M. Konig, Variation of Brominated Indoles and Terpenoids within Single and Different Colonies of the Marine Bryozoan Flustra Foliacea, J. Chem. Ecol., 2004, 30, 1165–1181 CrossRef CAS.
- E. N. Fishkin, J. R. Sparrow, R. A. Allikmets and K. Nakanishi, Isolation and Characterization of a Retinal Pigment Epithelial Cell Fluorophore: An All-trans-Retinal Dimer Conjugate, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 7091–7096 CrossRef CAS.
- N. Fishkin, G. Pescitelli, J. R. Sparrow, K. Nakanishi and N. Berova, Absolute Configurational Determination of an All-trans-Retinal Dimer Isolated from Photoreceptor Outer Segments, Chirality, 2004, 16, 637–641 CrossRef CAS.
- S. R. Kim, Y. P. Jang, S. Jockusch, N. E. Fishkin, N. J. Turro and J. R. Sparrow, The All-trans-Retinal Dimer Series of Lipofuscin Pigments in Retinal Pigment Epithelial Cells in a Recessive Stargardt Disease Model, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 19273–19278 CrossRef CAS.
- E. M. Stone, Macular Degeneration, Annu. Rev. Med., 2007, 58, 477–490 CrossRef CAS.
- J. R. Evans, Risk Factors for Age-related Macular Degeneration, Prog. Retinal Eye Res., 2001, 20, 227–253 Search PubMed.
- L. B. Avalle, Z. Wang, J. P. Dillon and E. R. Gaillard, Observation of A2E Oxidation Products in Human Retinal Lipofuscin, Exp. Eye Res., 2004, 78(4), 895–898 CrossRef CAS.
- N. Fishkin, Y. P. Jang, Y. Itagaki, J. R. Sparrow and K. Nakanishi, A2-Rhodopsin: a New Fluorophore Isolated From Photoreceptor Outer Segments, Org. Biomol. Chem., 2003, 1, 1101–1105 RSC.
- Y. P. Jang, H. Matsuda, Y. Itagaki, K. Nakanishi and J. R. Sparrow, Characterization of Peroxy-A2E and Furan-A2E Photooxidation Products and Detection in Human and Mouse Retinal Pigment Epithelial Cell Lipofuscin, J. Biol. Chem., 2005, 280, 39732–39739 CrossRef CAS.
- S. R. Kim, K. Nakanishi, Y. Itagaki and J. R. Sparrow, Photooxidation of A2-PE, a Photoreceptor Outer Segment Fluorophore, and Protection by Lutein and Zeaxanthin, Exp. Eye Res., 2006, 82, 828–839 CrossRef CAS.
- J. R. Sparrow, H. R. Vollmer-Snarr, J. Zhou, Y. P. Jang, S. Jockusch, Y. Itagaki and K. Nakanishi, A2E-Epoxides Damage DNA in Retinal Pigment Epithelial Cells Vitamin E and Other Antioxidants Inhibit A2E-Epoxide Formation, J. Biol. Chem., 2003, 278, 18207–18213 CrossRef CAS.
- J. R. Sparrow, N. Fishkin, J. Zhou, B. Cai, Y. P. Jang, S. Krane, Y. Itagaki and K. Nakanishi, A2E, a Byproduct of the Visual Cycle, Vision Res., 2003, 43, 2983–2990 CrossRef CAS.
- A. E. Asato, C. Watanabe, X. Y. Li and R. S. H. Liu, The Proline and Beta-Lactoglobulin Mediated Asymmetric Self-Condensation of Beta-Ionylideneacetaldehyde, Retinal and Related-Compounds, Tetrahedron Lett., 1992, 33(22), 3105–3108 CrossRef CAS.
- L. Sawyer and G. Kontopidis, The Core Lipocalin, Bovine Beta-Lactoglobulin, Biochim. Biophys. Acta, Protein Struct. Mol. Enzymol., 2000, 1482, 136–148 Search PubMed.
- D. R. Flower, Beyond the Superfamily: the Lipocalin Receptors, Biochim. Biophys. Acta, 2000, 1482, 327–336 CAS.
- Y. Cho, C. A. Batt and L. Sawyer, Probing the Retinol-Binding Site of Bovine Beta-Lactoglobulin, J. Biol. Chem., 1994, 269(15), 11102–11107 CAS.
- T. V. Bui, Y. Han, R. A. Radu, G. H. Travis and N. L. Mata, Characterization of Native Retinal Fluorophores Involved in Biosynthesis of A2E and Lipofuscin-Associated Retinopathies, J. Biol. Chem., 2006, 281(26), 18112–18119 CrossRef CAS.
- T. P. Sakmar, R. R. Franke and H. G. Khorana, The Role of the Retinylidene Schiff Base Counterion in Rhodopsin in Determining Wavelength Absorbance and Schiff Base pKa, Proc. Natl. Acad. Sci. U. S. A., 1991, 88, 3079–3083 CrossRef CAS.
- J. A. Lovegrove, D. L. Osman, J. B. Morgan and S. M. Hampton, Transfer of Cow's Milk Beta-Lactoglobulin to Human Serum After a Milk Load: a Pilot Study, Gut, 1993, 34, 203–207 CrossRef CAS.
- J. W. Crabb, M. Miyagi, X. Gu, K. Shadrach, K. A. West, H. Sakaguchi, M. Kamei, A. Hasan, L. Yan, M. E. Rayborn, R. G. Salomon and J. G. Hollyfield, Drusen Proteome Analysis: an Approach to the Etiology of Age-Related Macular Degeneration, Proc. Natl. Acad. Sci. U. S. A., 2002, 99(23), 14682–14687 CrossRef CAS.
- I. Caillard and D. Tome, Modulation of Beta-Lactoglobulin Transport in Rabbit Ileum, Am. J. Physiol., 1994, 266(6), G1053–G1059 CAS.
- D. Marcon-Genty, D. Tome, O. Kheroua, A. M. Dumontier, M. Heyman and J. F. Desjeux, Transport of Beta-Lactoglobulin Across Rabbit Ileum In vitro, Am. J. Physiol., 1989, 256, G943–G948 CAS.
- S. Warburton, K. Southwick, R. M. Hardman, A. M. Secrest, R. K. Grow, H. Xin, A. T. Woolley, G. F. Burton and C. D. Thulin, Examining the Proteins of Functional Retinal Lipofuscin Using Proteomic Analysis as a Guide for Understanding its Origin, Mol. Vision, 2005, 11, 1122–1134 Search PubMed.
- S. D. Copley, Enzymes with Extra Talents: Moonlighting Functions and Catalytic Promiscuity, Curr. Opin. Chem. Biol., 2003, 7(2), 265–272 CrossRef CAS.
- J. M. Seddon, B. Rosner, R. D. Sperduto, L. Yannuzzi, J. A. Haller, N. P. Blair and W. Willett, Dietary Fat and Risk for Advanced Age-Related Macular Degeneration, Arch. Ophthalmol., 2001, 119, 1191–1199 Search PubMed.
- D. F. Taber, K. Raman and M. D. Gaul, Enantioselective ring construction: synthesis of (+)-estrone methyl ether, J. Org. Chem., 1987, 52(1), 28–34 CrossRef CAS.
- S. A. Tanumihardjo, Synthesis of 10,11,14,15-13C4 and 14,15-13C2-retinyl acetate, J. Labelled Compd. Radiopharm., 2001, 44(5), 365–372 CrossRef CAS.
- A. F. L. Creemers and J. Lugtenburg, The Preparation of All-trans Uniformly 13C-Labeled Retinal via a Modular Total Organic Synthetic Strategy. Emerging Central Contribution of Organic Synthesis toward the Structure and Function Study with Atomic Resolution in Protein Research, J. Am. Chem. Soc., 2002, 124, 6324–6334 CrossRef CAS.
- O. Uchikawa, K. Fukatsu, R. Tokunoh, M. Kawada, K. Matsumoto, Y. Imai, S. Hinuma, K. Kato, H. Nishikawa, K. Hirai, M. Miyamoto and S. Ohkawa, Synthesis of a Novel Series of Tricyclic Indan Derivatives as Melatonin Receptor Agonists, J. Med. Chem., 2002, 45(19), 4222–4239 CrossRef CAS.
- J. Lugtenburg, The Synthesis of 13C-labelled Retinals, Pure Appl. Chem., 1985, 57(5), 753–762 CrossRef CAS.
- A. Valla, B. Valla, R. Le Guillou, D. Cartier, L. Dufosse and R. Labia, New Syntheses of Retinal and Its Acyclic Analog γ-Retinal by an Extended Aldol Reaction with a C6 Building Block that Incorporates a C5 Unit after Decarboxylation. A Formal Route to Lycopene and β-Carotene, Helv. Chim. Acta, 2007, 90, 512–520 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, NMR spectra, and HPLC profiles. See DOI:10.1039/c0mb00186d |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2011 |

