Three-dimensional microwell arrays for cell culture†
Christina L.
Randall
a,
Yevgeniy V.
Kalinin
b,
Mustapha
Jamal
b,
Tanmay
Manohar
a and
David H.
Gracias
*bc
aDepartment of Biomedical Engineering, The Johns Hopkins University, Baltimore, MD 21205, USA
bDepartment of Chemical and Biomolecular Engineering, Johns Hopkins University, Baltimore, MD 21218, USA
cDepartment of Chemistry, Johns Hopkins University, Baltimore, MD 21218, USA. E-mail: dgracias@jhu.edu
First published on 9th November 2010
Abstract
We propose the concept of three-dimensional (3D) microwell arrays for cell culture applications and highlight the importance of oxygen diffusion through pores in all three dimensions to enhance cell viability.
Since cells reside in 3D environments in vivo, it is necessary to develop platforms that enable the culture and study of aggregates of cells while providing adequate diffusion in all three dimensions. For example, cells have been suspended and cultured within gels1–4 or on porous multilayered scaffolds.5–7 Attempts have also been made to integrate channels within these materials to enable vasculature reminiscent of perfusion in 3D.8,9 However, many existing methodologies provide limited control over geometric positioning of cell clusters such as can be achieved in an array as well as a lack of precise tunability of nutrient and waste diffusion in all three dimensions.
Culturing cells within present-day microwell arrays allows precise geometric positioning of cell clusters in culture and is a well-established practice in drug discovery, microbiology, tissue engineering and biotechnology.1,7,10–12 This approach has been widely utilized due to ease of device fabrication, high throughput loading and compatibility with optical microscopy techniques.13,14 Additionally, 2D microwells enable cells to be encapsulated and cultured in liquid media without the need for a gel. However, since conventional microwells are embedded in a flat substrate they do not accurately mimic the natural cellular microenvironment due to a lack of 3D cues from the external media, thus generating physiologically compromised cells.15 For example, due to limited access to the surrounding medium from only one opening (a single 2D interface) in traditional planar microwell arrays, hypoxic conditions resulting in decreased cell or tissue function have been reported.16–18 We propose to extend the concept of a conventional microwell array (Fig. 1a) by creating arrays of microwells with externally exposed or porous sidewalls. As a result, the 3D microwell arrays allow for the encapsulated cells to interact with their surroundings in all three dimensions (Fig. 1b) while retaining several of the attractive features of 2D microwell arrays described above. A more quantitative understanding of the diffusion characteristics of these microwells and of expected differences in cell behavior in 2D and 3D can be observed from numerical simulations. We simulated microwell sizes of 100, 250 and 500 µm while systematically varying the face porosity. Here, we generated a model of O2 consumption by encapsulated pancreatic β-cells. Our choice for simulating this cell line was motivated by its widespread use in diabetes therapy19 and in research related to bioartificial pancreas development.20,21 The simulation parameters (details in the ESI†) were chosen to correspond to experimental conditions while still remaining instructive. The individual 2D and 3D microwells were cylindrical in geometry and were placed in the bottom-center of the medium. Stationary solutions of the spatial variation of O2 concentration were obtained by solving the diffusion equation with a reaction term, 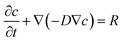 . Here, c is the O2 concentration, D is the diffusion coefficient of O2 in either the medium or through the cellular mass and R is the O2 reaction rate per unit volume. For the boundary conditions, we assumed the O2 concentration at the medium–air interface to be constant and equal to 0.2 mM.21 The O2 cellular consumption rate R for β-TC-6 cells was assumed to vary in accordance with Michaelis–Menten kinetics with a necrosis threshold step-function θ(c > ccr) such that,
. Here, c is the O2 concentration, D is the diffusion coefficient of O2 in either the medium or through the cellular mass and R is the O2 reaction rate per unit volume. For the boundary conditions, we assumed the O2 concentration at the medium–air interface to be constant and equal to 0.2 mM.21 The O2 cellular consumption rate R for β-TC-6 cells was assumed to vary in accordance with Michaelis–Menten kinetics with a necrosis threshold step-function θ(c > ccr) such that,  . Here, cmm is the Michaelis–Menten constant assumed to be 1.0 µM, ccr is the critical necrosis threshold (0.1 µM) and Rmax is the maximal consumption rate. Two values for Rmax often cited in the literature of 16 µM s−1 and 34 µM s−1 were used to generate upper and lower bounds of the viability fraction (f) plots.20,21 We used the step function,
. Here, cmm is the Michaelis–Menten constant assumed to be 1.0 µM, ccr is the critical necrosis threshold (0.1 µM) and Rmax is the maximal consumption rate. Two values for Rmax often cited in the literature of 16 µM s−1 and 34 µM s−1 were used to generate upper and lower bounds of the viability fraction (f) plots.20,21 We used the step function,  so that cells were alive when the O2 concentration was above ccr and consumed O2 or they were dead when the O2 concentration was below ccr and had consumed all of the available O2.
so that cells were alive when the O2 concentration was above ccr and consumed O2 or they were dead when the O2 concentration was below ccr and had consumed all of the available O2.
 | ||
| Fig. 1 Conceptual schematic of (a) a conventional 2D microwell array and (b) our proposed 3D microwell array. (c–f) Numerical simulations and comparison of cell viability in a single 2D versus 3D porous microwell with cylindrical geometry. Spatial variation of viable (green) and necrotic (red) cells within a microwell with (c) one porous face (a conventional 2D microwell) and (d) a microwell with porosity on all faces except the one at the bottom (our proposed 3D microwell). The O2 concentration outside the microwell is color coded as per the legend in the figure and the arrows represent the diffusive flux of O2 in the medium surrounding the microwell. (e) Plots of the fraction f of the volume of the microwell where the O2 concentration is larger than the threshold concentration ccr (0.1 µM) required for viable β-TC-6 cellsversus the porosity of each face. The three panels correspond to cylindrical microwells with heights (or diameters) of 500, 250 and 100 µm. The regions shown in solid (3D microwell) and hatched (2D microwell) are bounded by low and high literature values of consumption rates.20,21 (f) Plot of fversus both the cell density and O2 consumption rate within a microwell with a height (or diameter) of 500 µm and wall porosity φ = 2.3%. The intersection of the dotted line in the plot corresponds to the parameters used in determining the spatial variation of viable cells shown in panels (c) and (d). | ||
Simulations indicated that the fraction of viable cells depends upon the microwell volume, face porosity, encapsulated cell density and cellular O2 consumption rate. Cell viability was observed to be consistently higher in microwells with 3D porosity as compared to 2D porosity (Fig. 1c–e). The spatial variation of viable cells within 2D and 3D microwells indicated that cells are more viable adjacent to porous faces (Fig. 1c and d). This improvement was more pronounced in microwells with larger volumes and lower pore density. A notable simulation result is that cell viability was significantly higher when microwells feature 3D porosity and differences in viability as high as a factor of four were observed.
Since viability also depends on the number of cells encapsulated within the microwell, we simulated varying cell densities or cellular O2 consumption within 500 µm sized microwells (Fig. 1f). The cell density (ρ) variation inside the microwells was modeled by varying the bulk O2 consumption rate according to 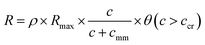 . In the model, we assumed that all cells consume O2 at the same rate and they have a close packed, uniform spatial distribution within the microwell. Simulations revealed that for all cell densities greater than approximately 5%, a significantly higher fraction of viable cells was consistently observed in 3D microwells.
. In the model, we assumed that all cells consume O2 at the same rate and they have a close packed, uniform spatial distribution within the microwell. Simulations revealed that for all cell densities greater than approximately 5%, a significantly higher fraction of viable cells was consistently observed in 3D microwells.
Guided by numerical simulations and by the well-known fact that in vivocells further than approximately 200 µm from the nearest blood vessel become hypoxic,22 we chose 500 µm microwells to conduct a model experimental study. It should be noted that depending on the application (e.g.cell encapsulation therapy), the microwell size could be determined by a balance between encapsulating the largest numbers of cells for an improved therapeutic effect (by using larger wells) while providing adequate diffusion so that cells remain viable (by increasing surface to volume ratio achieved by using smaller wells). Many cell encapsulation devices such as alginate microspheres have diameters ranging from 400 to 800 µm.23
Individual microwells were self-assembled24 from 2D cruciform shaped templates composed of hollow, porous or closed square panels interconnected with solder hinges (Fig. 2a). Self-assembly was driven by the minimization of surface area of the molten hinges and has been described in detail elsewhere.25 A variety of polyhedral shapes with side lengths ranging from 100 nm to 2 mm, pore sizes as small as tens of nanometres, and with metallic or polymeric composition have been synthesized using this approach.26–29 The fabrication process is highly parallel and large numbers of polyhedra can be fabricated in a cost-effective manner. Here, a model geometry was designed in which we systematically varied the number of porous faces of 500 µm sized, cubic microwells between one (Fig. 2b), three and five (Fig. 2c); one face was left open for cell loading. Diffusion in the cubic microwells with one porous face mimics that of conventional 2D microwells, which typically have openings in only one plane; five porous-faced cubic microwells represent our proposed 3D microwells. In all cases, pores were photolithographically structured as 10 × 16 arrays with individual sizes of 8 µm as-fabricated and 6 to 7 µm after gold (Au) coating. Au was electrodeposited onto all surfaces of the microwells to improve biocompatibility, since Au has been demonstrated to be inert to cells30 and can also be readily functionalized using a variety of thiol coatings to further enhance biocompatibility.31
![(a) Layout of the 2D templates for one, three and five porous-faced microwells; in each case, one panel had a large opening for cell loading. (b) Electron microscopy image of a 2D [one porous-faced, which self-assembled from the 2D template at the top of panel (a)] microwell along with a zoomed-in image showing individual pores. (c) Electron microscopy image of a 3D [five porous-faced, which self-assembled from the 2D template at the bottom of panel (a)] microwell. The scale bar for the microwell images and inset indicates lengths of 200 µm and 10 µm respectively. (d) Conceptual schematic diagram of the cell-loading process which involves tumbling the microwells in a concentrated 104cells ml−1 solution. (e) Representative optical microscopy images of stained cells loaded via tumbling illustrating microwells with low (left) and high (right) cell number (scale bar 200 µm). (f) Optical microscopy image of five microwells arrayed and sealed on a polyurethane adhesive (scale bar 500 µm) with an inset showing the seal around the base of an individual microwell (scale bar 200 µm). (g) Optical microscopy image of a microwell array formed on a curved flexible substrate (scale bar 500 µm).](/image/article/2011/LC/c0lc00368a/c0lc00368a-f2.gif) | ||
| Fig. 2 (a) Layout of the 2D templates for one, three and five porous-faced microwells; in each case, one panel had a large opening for cell loading. (b) Electron microscopy image of a 2D [one porous-faced, which self-assembled from the 2D template at the top of panel (a)] microwell along with a zoomed-in image showing individual pores. (c) Electron microscopy image of a 3D [five porous-faced, which self-assembled from the 2D template at the bottom of panel (a)] microwell. The scale bar for the microwell images and inset indicates lengths of 200 µm and 10 µm respectively. (d) Conceptual schematic diagram of the cell-loading process which involves tumbling the microwells in a concentrated 104cells ml−1 solution. (e) Representative optical microscopy images of stained cells loaded via tumbling illustrating microwells with low (left) and high (right) cell number (scale bar 200 µm). (f) Optical microscopy image of five microwells arrayed and sealed on a polyurethane adhesive (scale bar 500 µm) with an inset showing the seal around the base of an individual microwell (scale bar 200 µm). (g) Optical microscopy image of a microwell array formed on a curved flexible substrate (scale bar 500 µm). | ||
As an alternative to the loading of conventional planar arrays using pipettes, microwells were loaded in a parallel manner via tumbling (Fig. 2d). Images of stained cells obtained within microwells indicated that there was variability in the number of loaded cells (Fig. 2e) within each microwell. However, since cells continued to multiply after loading, microwells had relatively homogeneous numbers of cells after incubation for 48 hours prior to first use.28 Haemocytometer counts that were obtained by counting trypsinized cells within microwells suggested cell numbers of 4.1 ± 1.1 (×104) (average over a sample size, n = 30) within each microwell after 48 hours.
Arrays were then formed by first orienting the microwells with their open face upwards using a glass pipette. The desired substrate, typically an adhesive tape or polyurethane adhesive spin-coated on a glass slide, was then brought into contact with the open face of multiple microwells to form the array. Sealing of the open microwell faces was complete when the polymer cured, typically within 30 minutes in cell media. Using this approach, arrays could easily be formed on both rigid (flat, Fig. 2f) and flexible (curved, Fig. 2g) geometries.
It was also possible to create arrays with both precisely positioned and spaced microwells by first positioning the microwells in an SU-8 holder that was patterned with recessed slots (Fig. 3a). In addition to loading microwells with cells prior to positioning, they could also be loaded after positioning in the holder by allowing the cells to settle into the microwells. The desired array substrate was then brought into contact with the spatially positioned microwells thus creating arrays with relatively well defined geometric spacing. The ordered array pattern “3D” on both a flat (Fig. 3b) and curved (Fig. 3c) substrate highlights the utility of this approach.
 | ||
| Fig. 3 (a) Optical images of a 65 µm thick SU-8 holder with recessed slots and a 3 × 3 array of microwells positioned with their open faces oriented upwards. Optical images of ordered 3D microwell arrays on both (b) flat and (c) curved surfaces. The number 3 and letter D are spelt out to highlight versatility in the spacing and positioning offered by this arraying technique. All scale bars are 500 µm. | ||
The relative functionality of β-TC-6 cells encapsulated within 2D versus 3D microwell arrays was assessed by measuring insulin release, as would be typically required in a therapeutically relevant device, over time periods ranging from one day to approximately one month. The insulin concentration was measured using an insulin enzyme-linked immunosorbent assay (ELISA) and was recorded from arrays composed of one, three or five porous faces. We observed a significant difference between the insulin release characteristics from 3D microwell arrays as compared to 2D microwell arrays. For example, the initial insulin release rate in response to glucose stimulation was significantly more rapid from 3D microwell arrays (Fig. 4a).
 | ||
| Fig. 4 (a) Insulin response profiles to a glucose stimulation from one, three and five porous-faced microwell arrays after seven days. Data are plotted as the average ± the standard deviation (sample size n = 5). (b) A graph showing the four hour (steady-state) insulin concentration measured in response to a glucose stimulation for β-TC-6 cells encapsulated within 2D (one porous-faced), three porous-faced and 3D (five porous-faced) microwell arrays. The average and the standard deviation obtained on days 1 (number of samples, n, = 5), 7 (n = 5), 14 (n = 3) and 28 (n = 3) are plotted. The 3D microwell arrays produced significantly greater stimulated insulin at longer times. Representative fluorescence images of 2D (c) and 3D (d) 500 µm sized microwells removed from the array after 7 days. Cells were stained using the Live/Dead (green/red) assay. Microwells with one porous face showed significant numbers of dead cells while those with five porous faces showed high cell viability (scale bar 200 µm). | ||
Additionally, over multiple trials, we found that while insulin release in response to the same glucose stimulation, at steady-state (240 minutes), from the one, three or five porous-faced microwell arrays was similar after encapsulation for one day, the 3D microwell arrays stimulated far greater insulin production after cells were encapsulated for longer durations (Fig. 4b). Specifically, after 28 days, the insulin released from five porous-faced microwell arrays was approximately 2.20 ± 0.14 ng ml−1 as compared to 0.91 ± 0.06 ng ml−1 for three porous-faced microwells. After 28 days, no measurable insulin was produced from 2D microwell arrays, while 3D microwell arrays maintained their insulin concentration levels.
In order to investigate the reason for the significantly enhanced insulin production by 3D microwell arrays as compared to 2D microwell arrays, we removed (peeled-off) individual microwells from the substrates at different time points and performed a Live/Dead cytotoxicity assay on the cells contained within. Starting from our 7 day measurement, we consistently observed significantly higher numbers of live cells within the 3D microwells as compared to the 2D microwells (Fig. 4c and d). This result is in agreement with our simulations and provides an explanation for the insulin measurement results. We note that the numerical simulations presented in Fig. 1e are of microwells containing cells at higher cell densities in order to simulate the formation of a necrotic core. At similar cell densities that were used in the experiments (approx. 20%, corresponding to the dotted line in Fig. 1f and the corresponding image in Fig. 1d), minimal cell death was indeed predicted by simulations (<5%) as is consistent with the Live/Dead assayed 3D microwell arrays (Fig. 4d).
It should be noted that simulations only look at viability based on O2 diffusion, but in reality cell viability is also dependent on other factors. For example, it is known that necrotic cells release chemicals that can impair the viability of proximal live cells, which could also contribute to the differences in observed cell viability over time between 2D and 3D porous-faced microwells. We hypothesize that this factor may also account for the continuously decreasing cell viability (and the corresponding decrease in insulin release) observed at long encapsulation times in 2D microwells (Fig. 4b).
In summary, both our simulations and experiments clearly indicate that 3D microwell arrays provide a significantly improved cell culture platform (in terms of enhanced diffusion and cell viability) as compared to widely utilized conventional 2D microwell arrays. In this study, we have focused on insulin secretion and cell viability based on nutrient diffusion. However, other aspects of individual cell behavior such as genetic expression or cellular morphology may also be altered when cells are cultured in more physiologically relevant 3D systems;16 these studies can be carried out using our model 3D microwell arrays. As compared to other methods for culturing cells in geometries that enable 3D perfusion, our methodology affords high precision in terms of pore definition, size and spacing of cellular clusters. The 3D microwells enable cellular clusters to be precisely positioned on substrates in liquid culture media without the need for a gel matrix. If required, cells cultured in gel matrices can also be encapsulated. Additionally, fabrication of arrays that incorporate microwells with polymeric side walls29 would enable in situ visualization of encapsulated cells for in vitrocell culture applications. Our insulin release results indicate the therapeutic relevance of the 3D microwell array architecture for constructing bioartificial devices and for cell encapsulation therapy. For these in vivo applications, the ability to structure arrays of these microwells even on curved substrates could more accurately mimic anatomically relevant geometries.
Acknowledgements
This research was supported by the NIH Director's New Innovator Award Program, part of the NIH Roadmap for Medical Research, through grant number 1-DP2-OD004346-01 and DP2-OD004346-01S1; and by the Iacocca Family Foundation. We thank Siddharth Singh for his help with mask design and Sukaina Miftah for microwell sorting and positioning.References
- L. Kim, Y. C. Toh, J. Voldman and H. Yu, Lab Chip, 2007, 7, 681–694 RSC.
- S. Rhee, Exp. Mol. Med., 2009, 41, 858–865 Search PubMed.
- D. R. Albrecht, G. H. Underhill, T. B. Wassermann, R. L. Sah and S. N. Bhatia, Nat. Methods, 2006, 3, 369–375 CrossRef CAS.
- K. Bolt, Z. Upton, K. Schrobback, M. Ehrbar, J. A. Hubbell, M. P. Lutolf and S. C. Rizzi, Biomaterials, 2010, 31, 8454–8464 CrossRef.
- K. W. Lee, S. Wang, M. J. Yaszemski and L. Lu, Biomacromolecules, 2010, 11, 682–689 CrossRef CAS.
- D. Gallegro-Perez, N. Higuita-Castro, S. Sharma, R. K. Reen, A. F. Palmer, K. J. Gooch, L. J. Lee, J. J. Lannutti and D. J. Hansford, Lab Chip, 2010, 10, 775–782 RSC.
- A. Khademhosseini, R. Langer, J. Borenstein and J. P. Vacanti, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 2480–2487 CrossRef CAS.
- N. W. Choi, M. Cabodi, B. Held, J. P. Gleghorn, L. J. Bonassar and A. D. Stroock, Nat. Mater., 2007, 8, 908–915 CrossRef.
- L. M. Bellan, S. P. Singh, P. W. Henderson, T. J. Porri, H. G. Craighead and J. A. Spector, Soft Matter, 2009, 5, 1354–1357 RSC.
- M. Charnley, M. Textor, A. Khademhosseini and M. P. Lutolf, Integr. Biol., 2009, 1, 625–634 RSC.
- B. Ma, G. Zhang, J. Qin and B. Lin, Lab Chip, 2009, 9, 232–239 RSC.
- D. Holmes and S. Gawad, Methods Mol. Biol. (Totowa, N. J.), 2010, 583, 55–80 Search PubMed.
- B. H. Weigl, R. L. Bardell and C. R. Cabrera, Adv. Drug Delivery Rev., 2003, 55, 349–377 CrossRef CAS.
- P. J. Hung, P. J. Lee, P. Sabounchi, R. Lin and L. P. Lee, Biotechnol. Bioeng., 2004, 89, 1–8.
- R. C. Dutta and A. K. Dutta, Biotechnol. Adv., 2009, 27, 334–339 CrossRef CAS.
- C. Rappaport, In Vitro Cell. Dev. Biol.: Anim., 2003, 39, 187–192 Search PubMed.
- E. M. Metzen, J. Wolff, J. Fandrey and J. Jelkman, Respir. Physiol., 1995, 100, 101–110 CrossRef CAS.
- J. Malda, T. J. Klein and Z. Upton, Tissue Eng., 2007, 13, 2153–2162 CrossRef CAS.
- E. S. Avgoustiniatos and C. K. Colton, in Bioartificial Organs—Science, Medicine, and Technology, ed. A. Prokop, D. Hunkeler and A. D. Cherrington, New York Acad Sciences, New York, 1997, pp. 145–167 Search PubMed.
- J. L. Dulong and C. Legallais, Biotechnol. Bioeng., 2007, 96, 990–998 CrossRef CAS.
- P. Buchwald, Theor. Biol. Med. Modell., 2009, 6, 5 Search PubMed.
- R. H. Thomlinson and L. H. Gray, Br. J. Cancer, 1955, 9, 539–549 CAS.
- P. Devos, B. DeHaan, J. Pater and R. VanSchilfgaarde, Transplantation, 1996, 62, 893–899 CrossRef CAS.
- B. Gimi, T. G. Leong, Z. Gu, M. Yang, D. Artemov, Z. M. Bhujwalla and D. H. Gracias, Biomed. Microdevices, 2005, 7, 341–345 CrossRef.
- T. G. Leong, P. Lester, T. Koh, E. Call and D. H. Gracias, Langmuir, 2007, 23, 8747–8751 CrossRef CAS.
- T. G. Leong, A. Zarafshar and D. H. Gracias, Small, 2010, 6, 792–806 CrossRef CAS.
- C. L. Randall, T. G. Leong, N. Bassik and D. H. Gracias, Adv. Drug Delivery Rev., 2007, 59, 1547–1561 CrossRef CAS.
- C. L. Randall, A. Gillespie, S. Singh, T. G. Leong and D. H. Gracias, Anal. Bioanal. Chem., 2009, 393, 1217–1224 CrossRef CAS.
- A. Azam, K. Laflin, M. Jamal, R. Fernandes and D. H. Gracias, Biomed. Microdevices, 2010 DOI:10.1007/s10544-010-9470-x.
- B. Merchant, Biologicals, 1998, 26, 49–59 CrossRef CAS.
- J. C. Love, L. A. Estroff, J. K. Kriebel, R. G. Nuzzo and G. M. Whitesides, Chem. Rev., 2005, 105, 1103–1170 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Methods for array fabrication, cell culture procedures and a detailed description of numerical simulations. See DOI: 10.1039/c0lc00368a |
| This journal is © The Royal Society of Chemistry 2011 |
