A simple and smart telemedicine device for developing regions: a pocket-sized colorimetric reader†‡
Dae-Sik
Lee
*a,
Byoung Goo
Jeon
b,
Chunhwa
Ihm
c,
Je-Kyun
Park
d and
Mun Yeon
Jung
a
aBT Convergence Division, Electronics and Telecommunications Research Institute (ETRI), Korea. E-mail: dslee@etri.re.kr
bDepartment of Physics, KAIST, Daejeon, Korea
cEulji University Hospital, Daejeon, Korea
dDepartment of Bio and Brain Engineering, KAIST, Daejeon, Korea
First published on 26th November 2010
Abstract
We have proposed a novel mobile healthcare platform, combining a pocket-sized colorimetric reader (13.5 × 6.5 × 2.5 cm3) and commercially available 10-parameter urinalysis paper strips (glucose, protein, glucose, bilirubin, urobilinogen, ketones, nitrite, pH, specific gravity, erythrocytes, and leukocytes), capable of sending data with a smart phone. The reader includes a novel colorimetric multi-detection module, which consists of three-chromatic light-emitting diodes, silicon photodiodes and a novel poly(methylmethacrylate) (PMMA) optical splitter. We employed data reading methods using conversions of the signal data (red, blue, and green) to the hue (H) color map or the Y model data, and used a curve-fitting method for the quantification. The reader is battery-powered, inexpensive, light-weight, and very speedy in analysis. And, it was applied to detection of a thousand of human urine samples and demonstrated reliable quantification of urinary glucose and protein. The features can be used by unskilled people on-site to transfer the analyzed data to experts off-site.
Introduction
Lab-on-a-chip (LOC) technologies have a tremendous but unproven potential to contribute to the improvement of people's health, especially in developing countries. Such devices have to be inexpensive, portable, accurate, reliable, robust, battery-powered and well suited to the medical and social cultures of the developing world.1–3Portable LOC devices are beginning to be used in remote settings, as a result of developments in integrating the process of fluid actuation, sample preparation, and signal detection. As they stand, these devices are not yet appropriate for use in the resource-poor settings; however, their advances put LOC research in a prime position to tackle important issues of global health.2 A handheld reader combined with a disposable sensor seems to be the most promising approach for implementing a powerful and versatile format that can meet the demands.1 Thus there is strong need for the development of portable and cost-effective readers.2,3–5
In order to implement a handheld optical detector for the colorimetric bioassay, new types of detectors using webcams or charge-coupled device (CCD) cameras have been reported.6–8 For these systems, the contact image sensor (CIS) or CCD are typically used as image detectors.8,9 These devices are relatively expensive, have low throughput due to long response time. On the other hand, a semiconductor diode has the great merits of simple structure, very low cost, fast response, and low power consumption, and does not require image processing; rather, it has excellent performance along with the information technology advances.9,10 As for optical light-guiding devices, plastic optical components are promising because they are inexpensive and reliable.11
Paper strip tests have been improved to accommodate a wide range of samples, like saliva for the Orasure HIV test; in some cases they allow quantitative readings of analytes like urine glucose and urine albumin.4,12,13 With respect to applications in disease staging, there have been many unmet needs for point-of-care (POC) capabilities in quantitative assays until now. In each application, a pocket-sized reader could bring great value to test strips.
In this technical note, we describe a new pocked-sized system, called the Healthy-100 (wishing everyone to be Healthy with a longevity of 100), that analyzes assays in test strips with not only the ability of semi-quantification but also the ability to quantify concentrations of urinary glucose and protein. The system can analyze and exchange its assay results with off-site experts for clinical evaluation (Fig. 1). We demonstrate this integrated concept by combining a simple handheld device capable of reading the colorimetric bioassays results, and transmitting the readout. For demonstrating the clinical reliability of the handheld system, a performance comparison between the system and hospital equipment was carried out.
 | ||
| Fig. 1 A health care strategy for performing inexpensive bioassays with a handheld reader and a cellphone in remote areas and for exchanging the results of the tests with off-site experts. | ||
Experimental
The pocket-sized colorimetric reader (13.5 × 6.5 × 2.5 cm3) is designed to be inexpensive, operable with very low power consumption, battery powered, simple to use, amenable to read muli-analytes tests simultaneouly, and quantitative in some degree. The overall description for instrumental fabrication is shown in the ESI.† Here a novel detection method and a fabrication protocol of PMMA optical splitter (POS) are mainly described.Detection method
The main idea for the optical configurations is that it uses inexpensive and simple diodes, that is, a white high intensity light-emitting diode (LED) module (Seoul Semiconductor Co., Korea) as a light source and twelve silicon photodiodes (SPDs) (Hamamatsu Co., Japan) as a light detector. The LED module has a tri-chromatic source (ESI Fig. S-2†). Thus, by switching the tri-chromatic LEDs in the white light module sequentially with a time interval, we can easily implement a three wavelength-swept light source, which can be very powerful in extracting the color changes. Via the newly-designed POS, light transports evenly to the testing parts and the reflected light is measured through twelve SPDs, eleven for the paper strips and one for the initial light corrections. The reflected light from the test parts at the three sequential center wavelengths 625 nm, 530 nm, and 460 nm, is named R, G, and B, respectively (Fig. 2). The values are changed into the H color space. | (1) |
 | ||
| Fig. 2 Schematics for the detection mode of the pocket reader. (a) A drawing and picture of the reader. (b) The optical components and operation mode. (c) The procedure gethering digitized signals. While LEDs generate R, G, and B sequentially with a second time interval, twelve SPDs gether the signals. And, t1, t2, and t3 are 2 s equally, and as for t4, it takes several ms. | ||
We can confirm that the H value model is well suited for paper strips to be used as the analytes of RBCs, protein, pH, and specific gravity. When color changes on a paper strip are mainly based on red colors, we designed a new color model, named Y, in which the ratio of G values and R values can be important. It is defined in the below equation.
 | (2) |
Design and fabrication of POS
A POS to transport and divide light from an LED module to eleven test parts on dipsticks has been designed and fabricated. A program called Light Tools™ 7.0, a typical illumination analysis software, was utilized to determine the optimized structure for even light distribution and transportation efficiency. The basic concept is shown in Fig. 3(a). It is assumed that the reflector is in a condition of total reflection. The working principal is very simple: the light emitted from the LED located at the entrance is reflected totally to the arc-typed reflector and divided into each test part. In order to obtain an even distribution of the divided light over each test part (dimensions of 5 mm × 5 mm), an LED with a divergence angle of 120°, corresponding to full width at half maximum in Gaussian distribution is adopted to cover each test part. The simulated results are shown in Fig. 3(b). The radius of the arc reflectors is designed so that the beam can focus on the SPDs well. Thus, the substantial light intensity to be measured can be improved. Merits of the POS are that it is inexpensive and very effective in using light from the LED with a wide emitting area. The optical output efficiency is defined as the sum of POS channel output over LED output. A schematic diagram is shown in the ESI Fig. S-3.† Total output efficiency of the POS is estimated to be about 26.5%. Since the widely-diverging light is apt to be transmitted out of the POS, light confined within the angles of ±30° can be utilized.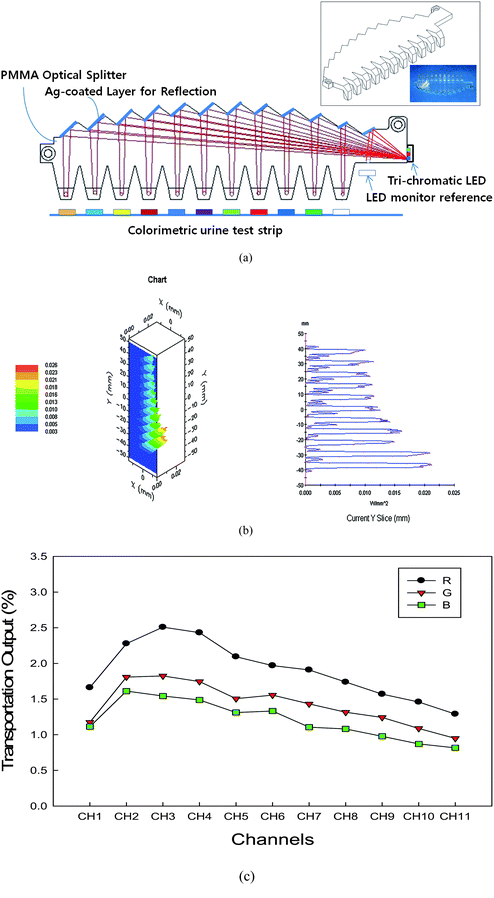 | ||
| Fig. 3 Schematics for describing the POS. (a) Four key elements; a light inlet, twelve reflecting surfaces, 11 light splitting channels, and 11 light outlets with inset showing schematic drawing and picture of the POS. (b) Simulation results. (c) The real optical efficiency test output. Upper left inset shows 3D intensity profile at detection plane which is 4 mm away from POS outputs. Upper right inset shows an intensity profile emitted along the 11 channel outputs. It shows a typical intensity profile along the x-direction. We can confirm that the distributed intensity at each test part area is even and well-confined to the paper test parts. | ||
The schematic design and a picture of the fabricated POS (10 × 0.3 × 2.6 cm3) are shown at inset in Fig. 3(a). The POS was fabricated by using the computer numerical control processing and the injection-molding process. For forming reflecting surfaces, we used a spray-coating process with a silver suspension. A primer material was used to enhance adhesion between POS and the silver-coating layer. The measured reflectance of the silver surfaces is 83%, 74%, and 62%, at wavelengths of 635 nm, 530 nm, and 460 nm, respectively. The reflectance can be enhanced by the optimization of the silver spray-coating processes or by the selection of a new primer material. When the light is injected into the POS, the real distribution of emitted light intensity at each test part is even as shown in Fig. 3(c). These can be well explained with simulation results. The differences in intensity are caused by the discoloration in the process of silver-coating.
Choice of biological fluid, self calibration method and characterization are described in the ESI† in detail.
Results and discussion
Quantitative assays with artificial urine
The color signals for the assays correlate with the concentration of the analytes as shown in Fig. 4. The data and error bars in this figure are the mean and relative standard deviation, respectively. Using H values as a function of the concentration, a modified hyperbola curve-fitting of the protein data and an exponential decay curve-fitting of the glucose data gave coefficients of determination (R2) of 0.9996 and 0.9987, respectively. The responses are linear between 0 and 250 mg/dL glucose, with a limit of detection of 10 mg/dL glucose. The concentration range covers the entire range of concentrations of urine glucose detected clinically (0–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg/dL).14 Levels of glucose in urine above 25 mg/dL are indicative of disease. We tested protein concentrations in ranges of 0–1000 mg/dL, which corresponds to the clinical range.14
000 mg/dL).14 Levels of glucose in urine above 25 mg/dL are indicative of disease. We tested protein concentrations in ranges of 0–1000 mg/dL, which corresponds to the clinical range.14
 | ||
| Fig. 4 Analytical calibration plots for different concentrations of (a) protein, (b) glucose, (c) RBCs, and (d) WBCs in control artificial urine. | ||
Using H and Y values as a function of red blood cells (RBCs) and white blood cells (WBCs) concentrations, respectively, a hyperbola curve-fitting of the RBCs data and an exponential decay curve-fitting of the WBCs data gave coefficients of determination (R2) of 0.994 and 1.0. As for leukocytes, the detection range is appropriate for clinical use and is accurate down to 25 WBCs/μL. As for erythrocytes, the detection range is appropriate for clinical use and is accurate down to 10 RBCs/μL.
Quantitative assays with human urine samples
We performed a precision and comparison study to evaluate the analytical and clinical performance of the pocket reader. First, the precision analysis was assessed with three level urinary control materials, Uritol I, II and III (YD Diagnostics, Korea). Uritrol I is a normal control, Uritrol II has abnormal data for nitrite, ketone, glucose, RBCs and WBCs. Uritrol III is also an abnormal control for bilirubin and protein. For day-to-day precision, all control materials were analyzed once a day for fourteen days. We first analyzed the samples on the Uriscan pro II, then on the Healthy-100. The correlations between them were evaluated using % agreement within the ± one color block.When urinary glucose or proteins were more than trace grade, the corresponding quantitative analysis was performed since there are differences in grading methods between them.15 Day-to day precision was evaluated by assessing each of the three urine controls in one day for fourteen days. Control precisions were satisfied for both instruments using Uritrol I and II. As for the precision using Uritrol III, precision for protein was satisfied and for bilirubin the results were acceptable, but the data of the Healthy-100 was graded one grade lower than that of the Uriscan pro II. The % agreement with the ± one color block was more than 94% in all items (data are shown in the ESI†).
Eighty one urine samples for glucose and 133 samples for protein were evaluated to determine the quantification ability of the reader. The microscopic findings for RBCs and WBCs compared with the Healthy-100 were graded to evaluate the sensitivity and specificity of the microscopic hematuria (more than 5 cells/HPF, high power field ×400), pyuria (more than 5 cells/HPF) and clinically important pyuria (more than 10 cells/HPF). The correlation coefficients between both instruments were 0.997 for glucose and 0.9856 for protein, as shown in Fig. 5. The sensitivity of RBCs for microscopic hematuria was 95.0% and the specificity was 76.9%. The sensitivity and specificity of WBCs for pyuria were 54.8% and 97.5%, and for clinically important pyuria the values were 67.9% and 96. 4% (Table 1). The grade Negative, +1, +2, and +3 in Healthy-100 mean under 4, 5–9, 10–29, and more than 30 cells/HPF, respectively. So, if the sample has grade +1 in the reader and 5–9 cells/HPF in microscopic findings, we marked in italics. Italics mean the zone grades in Healthy-100 are consistent with the microscopic findings.
| (a) Erythrocytes (n = 994) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Microscopic, erythrocytes/HPF | ||||||||
| Less than 1 | 1–4 | 5–9 | 10–29 | More than 30 | > 1/2 in sight | Total | ||
| Grade | Negative | 623 | 104 | 14 | 4 | 1 | 0 | 746 |
| 1+ | 32 | 65 | 34 | 18 | 1 | 0 | 150 | |
| 2+ | 4 | 8 | 9 | 13 | 18 | 0 | 52 | |
| 3+ | 0 | 0 | 2 | 7 | 24 | 13 | 46 | |
| Total | 659 | 177 | 59 | 42 | 44 | 13 | 994 | |
| (b) Leukocytes (n = 996) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Microscopic, leukocytes/HPF | ||||||||
| Less than 1 | 1–4 | 5–9 | 10–29 | More than 30 | > 1/2 in sight | Total | ||
| Grade | Negative | 712 | 57 | 17 | 14 | 3 | 0 | 803 |
| 1+ | 34 | 29 | 22 | 12 | 6 | 4 | 107 | |
| 2+ | 6 | 8 | 4 | 19 | 14 | 4 | 55 | |
| 3+ | 0 | 1 | 1 | 4 | 17 | 8 | 31 | |
| Total | 752 | 95 | 44 | 49 | 40 | 16 | 996 | |
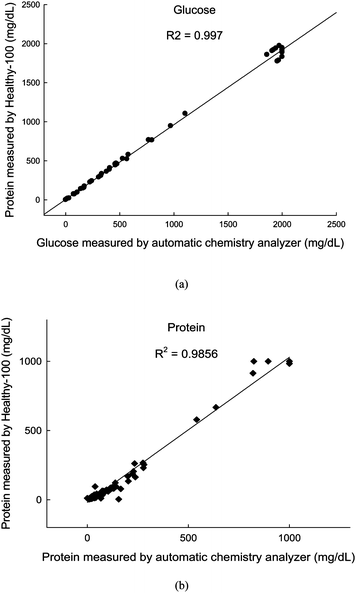 | ||
| Fig. 5 Comparison between the values measured by the automatic chemistry analyzer and those measured by the Healthy-100 for quantification of (a) glucose, and (b) protein. | ||
As for urine protein, the comparison results of the quantitative method and those of the pocket urinalysis system were well correlated. For rather large amounts of protein, there was a slight deviation in reading between them. This discrepancy stemmed from the fact that the test strips are based mainly on albumin, but at higher protein levels, the quantitative equipment can detect other proteins like immunoglobulins, which increased together with albumin.15 As for urine glucose, the % agreement was an exact match and the ± one color block values for both instruments were 96.0%, and 99.4% respectively (data are shown in the ESI†). The comparison of results obtained by the quantitative method and those with the reader were found to have as high a coefficient of determination as 0.997. This value is higher than that for protein, which well agrees with the results reported before.16
Penders et al. reported that the quantification of urine glucose, protein, RBCs, and WBCs, using reflectance reading of urinalysis test strips is complementary with flow cytometeric results, compared with the conventional semi-quantitative grading.12 Since we also found an excellent agreement of the pocket system results with those of the quantitative method, as well as with those of the microscopic method, we strongly believe that the pocket system will be clinically reliable.
Working properties
We estimated that all parts and components would cost about $15.56, when expecting the product volumes as 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 units, as shown in Table 2. Considering these results, we think a final selling price of about 25 dollars is feasible. The device is very light at about 150 g, similar to the weight of a cell phone. The system operates with very low electricity requirements. By using a power-controlling circuit design, the electric power consumption was dramatically decreased. Thus, standby current is about 0.095 mA, and the consumption current is about 10 mA and 30 mA, when power is on and when the optical reading process is engaged, respectively. The consumption power is so low that, with a battery of only 4.2 V and 2000 mAh capacity, over 40
000 units, as shown in Table 2. Considering these results, we think a final selling price of about 25 dollars is feasible. The device is very light at about 150 g, similar to the weight of a cell phone. The system operates with very low electricity requirements. By using a power-controlling circuit design, the electric power consumption was dramatically decreased. Thus, standby current is about 0.095 mA, and the consumption current is about 10 mA and 30 mA, when power is on and when the optical reading process is engaged, respectively. The consumption power is so low that, with a battery of only 4.2 V and 2000 mAh capacity, over 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 iterative measurements are possible.
000 iterative measurements are possible.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 units
000 units
| No. | Part type | Quantity | Price ($) | Amount ($) |
|---|---|---|---|---|
| 1 | MCU | 1 | 2.5 | 2.5 |
| 2 | LED Driver | 1 | 0.8 | 0.8 |
| 3 | Regulator | 2 | 0.1 | 0.2 |
| 4 | Diode | 1 | 0.1 | 0.1 |
| 5 | USB Drive | 1 | 1.0 | 1.0 |
| 6 | Real time clock | 1 | 0.7 | 0.7 |
| 7 | Crystal | 2 | 0.1 | 0.2 |
| 8 | Tri-chromatic LED | 1 | 0.2 | 0.2 |
| 9 | MOSFET | 2 | 0.02 | 0.04 |
| 10 | Passive | 40 | 0.01 | 0.4 |
| 11 | USB connector | 1 | 0.07 | 0.07 |
| 12 | Switch | 3 | 0.05 | 0.15 |
| 13 | Graphics LCD | 1 | 3.0 | 3.0 |
| 14 | Photo diode | 12 | 0.05 | 0.6 |
| 15 | Bluetooth | 1 | 5.0 | 5.0 |
| 16 | Bluetooth Antenna | 1 | 0.3 | 0.3 |
| 17 | PCB | 1 | 0.3 | 0.3 |
| Total | 15.56 |
Conclusion
By combining a pocket-sized colorimetric reader with dipsticks in a device that is able to transmit digital information over a smart phone, we have proposed an integrated solution for detecting disease in areas that are difficult to access by trained experts. As a practical implementation, a novel pocket-sized urinalysis system combined with urinalysis dipsticks, capable of sending data to a smart phone, has been described. We believe it will be a cost-effective platform for telemedicine applications for global healthcare. The reader includes an inexpensive colorimetric multi-analyte detection module. We employed a data reading method using conversion of the signal data to the H or the Y space. It also has functions of self-calibration and urine color compensation. As a result, the reader is battery-powered, inexpensive (about $ 15.56 for all components), very low in power consumption (10 mA when working), capable of multi-analysis (10-items in urine), light-weight (about 150 g), and very speedy in producing readings (within six seconds). The urinalysis strip tests using this pocket system have shown results that are in excellent agreement with those of the quantitative equipment used in a hospital, as well as with the microscopic method for urine glucose, protein, RBCs, and WBCs.References
- P. Yager, T. Edwards, E. Fu, K. Helton, K. Nelson, M. R. Tam and B. H. Weigl, Microfluidic diagnostic technologies for global public health, Nature, 2006, 442, 412–418 CrossRef CAS.
- C. D. Chin, V. Linder and S. K. Sia, Lab-on-a-chip devices for global health: Past studies and future opportunities, Lab Chip, 2007, 7, 41–57 RSC.
- S. K. Sia, V. Linder, B. A. Parviz, A. Siegel and G. M. Whitesides, An Integrated Approach to a Portable and Low-Cost Immunoassay for Resource-Poor Settings, Angew. Chem., Int. Ed., 2004, 43, 498–502 CrossRef CAS.
- A. W. Martinez, S. T. Philips, E. Carrilho, S. W. ThomasIII, Hayat Sindi and G. M. Whitesides, Simple telemedicine for Developing Regions: Camera Phones and Paper-Based Microfluidic Devices for Real-Time, Off-Site Diagnosis, Anal. Chem., 2008, 80, 3699–3707 CrossRef CAS.
- D. Tseng, O. Mudanyali, C. Oztoprak, S. O. Isikman, I. Sencan, O. Yaglidere and A. Ozcan, Lensfree microscopy on a cellphone, Lab Chip, 2010, 10, 1787–1792 RSC.
- B. Kuswandi, Nuriman, J. Huskens and W. Verboom, Optical sensing systems for microfluidic devices:a review, Anal. Chim. Acta, 2007, 601, 141–155 CrossRef CAS.
- W. Wongwilai, S. Lapnantnoppakhun, S. Grupan and K. Grudpan, Webcam camera as a detector for a simple lab-on-a-chip time based approach, Talanta, 2010, 81, 1137–1141 CrossRef CAS.
- J. Delanghe, New screening diagnostic techniques in urinalysis, Acta, Clin. Belg., 2007, 62, 155–161 Search PubMed.
- R. A. Yotter and D. M. Wilson, A review of photodetectors for sensing light-emitting reporters in biological systems, IEEE Sens. J., 2003, 3, 288–303 CrossRef CAS.
- E. F. Schubert and J. K. Kim, Solid-state light sources getting smart, Science, 2005, 308, 1274–1278 CrossRef CAS.
- J. Zubia and J. Arrue, Plastic optical fibers: An introduction to their technological processes and applications, Opt. Fiber Technol., 2001, 7, 101–140 CrossRef.
- J. Penders, T. Fiers and J. R. Delanghe, Quantitative evaluation of urinalysis test strips, Clin. Chem., 2002, 48, 2236–2241 CAS.
- E. Carrilho, S. T. Phillips, S. J. Vella, A. W. Martinez and G. M. Whitesides, Paper microzone plates, Anal. Chem., 2009, 81, 5990–5998 CrossRef CAS.
- W. J. Marshall, and S. K. Bangert, Clinical biochemistry: Metabolic and clinical aspects, 1st ed.; Churchill Livingstone: Edinburgh, NY, US, 1995 Search PubMed.
- J. L. Orsonneau, P. Douet, C. Massoubre, P. Lustenberger and S. Bernard, An improved pyrogallol red-molybdate method for determining total urinary protein, Clin Chem., 1989, 35, 2233–2236 CAS.
- K. A. Yun, T. J. Han, S. C. Chun and W. K. Min, The performance evaluation of yeongdong URiSCAN GEN 10SGL Urine dipstick using other quantitative, microscopic, and culture methods, Korean J. Lab. Med., 2001, 21, 471–479.
Footnotes |
| † Electronic supplementary information (ESI) available: Further experimental details including Fig. S1 to S3 and Movie S1 (working demonstration of the pocket urinalysis system). See DOI: 10.1039/c0lc00209g |
| ‡ Published as part of a LOC themed issue dedicated to Korean Research: Guest Editors: Professor Je-Kyun Park and Kahp-Yang Suh |
| This journal is © The Royal Society of Chemistry 2011 |
