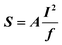Aptamer sandwich-based carbon nanotube sensors for single-carbon-atomic-resolution detection ofnon-polar small molecular species†‡
Joohyung
Lee
a,
Minjoung
Jo
b,
Tae Hyun
Kim
c,
Ji-Young
Ahn
b,
Dong-ki
Lee
d,
Soyoun
Kim
*b and
Seunghun
Hong
*ae
aDepartment of Physics and Astronomy, Seoul National University, Seoul, 151-742, Korea
bDepartment of Biomedical Technology, Dongguk University, Seoul, 100-175, Korea. E-mail: skim@dongguk.edu
cDepartment of Chemistry, Soonchunhyang University, Asan, Chungnam, 336-745, Korea
dDepartment of Chemistry, Sungkyunkwan University, Suwon, Korea. E-mail: dklee@skku.edu
eDepartment of Biophysics and Chemical Biology, Seoul National University, Seoul, 151-742, Korea. E-mail: seunghun@snu.ac.kr
First published on 22nd October 2010
Abstract
A portable sensor platform for the detection of small molecular species is crucial for the on-site monitoring of environmental pollutants, food toxicants, and disease-related metabolites. However, it is still extremely difficult to find highly selective and sensitive sensor platforms for general small molecular detection. Herein, we report aptamer sandwich-based carbon nanotube sensor strategy for small molecular detection, where aptamers were utilized to capture target molecules as well as to enhance the sensor signals. We successfully demonstrated the detection of non-polar bisphenol A molecules with a 1 pM sensitivity. Significantly, our sensors were able to distinguish between similar small molecular species with single-carbon-atomic resolution. Furthermore, using the additional biotin modification on labeling aptamer, we enhanced the detection limit of our sensors down to 10 fM. This strategy allowed us to detect non-polar small molecular species using carbon nanotube transistors, thus overcoming the fundamental limitation of field effect transistor-based sensors. Considering the extensive applications of sandwich assay for the detection of rather large biomolecules, our results should open up completely new dimension in small molecular detection technology and should enable a broad range of applications such as environmental protection and food safety.
Introduction
A portable sensor platform for the detection of small molecules in solution is crucial for the on-site monitoring of environmental pollutants, food toxicants, and disease-related metabolites. Bisphenol A (BPA), in particular, which is generally known as an endocrine disruptor, is among the most serious environmental contaminants. BPA is used as a monomer compound in the products of polycarbonate plastics, and it is found from water source such as river or ocean. Various techniques have been demonstrated for the detection of BPA, for instance, high performance liquid chromatography (HPLC),1gas chromatography coupled with mass spectrometry2 and electrochemical methods.3 However, those techniques often suffer from its bulky system size, complicated instrumentation, and the limitation of sensitivity and selectivity. Carbon nanotubes (CNTs) hold salient advantages as sensing elements because their one dimensional quantum confinement properties should make charge transport extremely sensitive to scattering by adsorbates. Especially, the semiconducting single walled CNTs (swCNTs) are very sensitive to their chemical environment due to the high sensitivity of their band gap energies to the local dielectric or redox environment, which can be exploited for chemical sensing.4–9 However, previous CNT field effect transistor (FET) based sensors have a fundamental limitation that it is difficult to detect non-polar small molecules.Herein, we report a method to build aptamer sandwich-based CNT sensors for the highly selective and sensitive detection of small pollutant molecules including non-polar species. This is the first successful demonstration of aptamer sandwich based CNT sensors for small molecules. Furthermore, it allowed us to overcome the fundamental limitation of FET-based sensors and to detect non-polar molecular species. Considering the broad range of applications of sandwich-based assay for large-biomolecular detection, this aptamer sandwich based CNT sensor for small molecular species should have a significant impact on a variety of applications such as medical diagnostics, environmental control, and food safety.
Experimental
Fabrication of carbon nanotube-based transducers
Fig. 1 shows the schematic diagram depicting the preparation process of our aptamer sandwich based sensors and experimental setup. The method we used for fabrication of the swCNT-FET sensing device was similar to that reported previously.10,11 First, a methyl-terminated octadecyltrichlorosilane (OTS) self-assembled monolayer (SAM) was patterned on a SiO2 substrate via conventional photolithography to create non-polar, passivating molecular patterns (Fig. 1A). To form a OTS SAM, the AZ5214 photoresist patterned SiO2 substrate was dipped in OTS solution (OTS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane = 1
hexane = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 v/v) for 10 min at room temperature and 80% humidity. The SiO2 substrate then was immersed in swCNT suspensions (0.05 mg ml−1 in o-dichlorobenzene) for ∼10 s and then rinsed thoroughly with clean o-dichlorobenzene. In the swCNT suspensions, swCNTs were assembled and aligned directly on the bare SiO2 regions while the OTS SAM prevented the swCNT adsorption (Fig. 1B). Finally, the electrodes were patterned by photolithography and thermal deposition of Pd and Au (30 nm Au on 10 nm Pd) followed by a lift-off process. In each swCNT-FET, two electrodes with a 4 µm gap were connected by swCNT network channel (Fig. 1C).
500 v/v) for 10 min at room temperature and 80% humidity. The SiO2 substrate then was immersed in swCNT suspensions (0.05 mg ml−1 in o-dichlorobenzene) for ∼10 s and then rinsed thoroughly with clean o-dichlorobenzene. In the swCNT suspensions, swCNTs were assembled and aligned directly on the bare SiO2 regions while the OTS SAM prevented the swCNT adsorption (Fig. 1B). Finally, the electrodes were patterned by photolithography and thermal deposition of Pd and Au (30 nm Au on 10 nm Pd) followed by a lift-off process. In each swCNT-FET, two electrodes with a 4 µm gap were connected by swCNT network channel (Fig. 1C).
 | ||
| Fig. 1 Schematic diagram showing sensor fabrication and experimental setup. (A) Patterning hydrophobic OTS molecular layer on SiO2 substrate viaphotolithography. (B) CNT assembly on the bare SiO2 regions by self-assembly process. (C) Fabrication of electrodesviaphotolithography. (D) Immobilization of aptamer on Au electrodes. (E) Sensing experiment of BPAviaaptamer sandwich-based assay using our sensors. | ||
Functionalization of electrodes with aptamer
The Au electrodes in the swCNT-FET devices were functionalized with an anti-BPA aptamer to fabricate a BPA sensor based on swCNTs (Fig. 1D). First, the device was pretreated with mercaptohexanol (MCH) by dipping it in MCH solution (10 mM in deionized water) overnight. The device was then immersed in an aptamer solution (1 µM in 10 mM Tris–HCl) for 10 h so that the Au electrodes were coated with a ssDNA BPA-binding aptamer with a thiol group at its 5′ end.12 To prevent disulfide bonding between aptamers, we mixed TCEP in aptamer solution (10 mM in 10 mM Tris–HCl).Sensing measurements
The electrical detection of BPA was carried out by monitoring the source–drain current change caused by the introduction of BPA or other molecules (source–drain bias ∼0.1 V) using Keithley 4200 semiconductor analyzer (Fig. 1E). First, we added various concentrations (1 pM–100 nM) of a BPA solution in binding buffer (100 mM Tris–HCl, 200 mM NaCl, 25 mM KCl, 10 mM MgCl2, 2.5 ppm DMSO) to our sensor while monitoring the change in source–drain current. We also performed the same experiment using aptamer-functionalized BPA. The functionalized BPA was prepared as follows. We first mixed equal concentrations of BPA and aptamer, and then we heated the solution at 95 °C for 5 min and cooled it to room temperature over a 1 to 2 hour time period. To verify the selectivity of our BPA sensor, we tested other molecules that have a structure similar to BPA (Bisphenol B (BPB), Bisphenol (BP) and 6F Bisphenol (6F), all at a concentration of 1 nM).Result and discussion
Electrical characterization of carbon nanotube-based sensors
Fig. 2A is an atomic force microscope (AFM) topography image of a swCNT-FET showing a swCNT network pattern between the source and drain electrodes. The length of swCNT network channel is 4 µm and the width is 2 µm. Note that the swCNT patterns had well-defined boundaries without any crossing of swCNTs. | ||
| Fig. 2 (A) Optical (left) and AFM topography (right) image of a swCNT channel. Note that the swCNT channel is well-defined in the bare SiO2 region. Scale bar in AFM image is 2 µm. (B) Secondary structure of anti-BPA aptamer. (C) Liquid gate characteristics of a swCNT-FET before (black line) and after (red line) aptamer immobilization. Here, the source–drain bias Vds was maintained at 0.1 V, and liquid gate voltage Vlg swept between −0.5 V and 0.5 V. Note that the conductance of the swCNT-FET decreased after aptamer immobilization due to the Schottky barrier modulation. (D) Noise characteristics of a swCNT-FET before (black square) and after (red square) aptamer immobilization on the Au electrode surfaces. Both devices exhibited 1/f noise characteristics. After aptamer immobilization, the noise amplitude increased due to the increase of the resistance of the swCNT-FET. | ||
As a receptor molecule, we chose aptamers which are synthetic oligonucleotides that can be generated to recognize specific targets with high sensitivity and selectivity. Aptamer sequence was obtained from well-known Systematic Evolution of Ligands by Exponential enrichment (SELEX) process (ESI‡). Secondary structure models of anti-BPA aptamers were predicted with the Mfold program (Fig. 2B, ESI‡).
Fig. 2C shows a liquid gating effect of a swCNT-FET using a Pt reference electrode as a liquid gate in binding buffer solution before and after aptamer immobilization. Note that the swCNT-FET exhibited a typical p-type characteristic. The conductance of swCNT junction was decreased after aptamer immobilization. It is consistent with previous works where negatively charged oligonucleotide adsorbed onto electrode surface increased Schottky barriers between the swCNTs and the electrodes and, as a result, reduced the conductance of the swCNT-FETs.12,13
The noise characteristics of the device were measured via Fast Fourier-transform analyzer (Fig. 2D). Usually, the Hooge's model is used as the empirical description of noise phenomena by the following formula.
Molecular detection via sandwich assay
We applied the sandwiched aptamer assay strategy to our swCNT-FET sensor with anti-BPA aptamer on its electrode surface (Fig. 3A). For sensing experiments, the sensor was placed in a binding buffer (100 mM Tris-HCl, 200 mM NaCl, 25 mM KCl, 10 mM MgCl2, 2.5 ppm DMSO), and current changes in it were monitored after the addition of various analytes. Under these circumstances, any charged analytes that are adsorbed onto the Au electrode can cause a change in the Schottky barrier height between the swCNTs and the electrodes, resulting in electrical sensor responses.12,13,19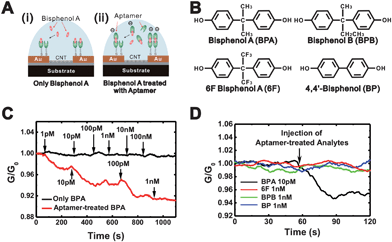 | ||
| Fig. 3 Detection of non-polar BPA molecules viaaptamer sandwich-based swCNT-FET sensors. (A) Schematic diagram showing the sensing experiment set-up using swCNT-FETs functionalized with BPA aptamer. (i) Sensing experiment of bare BPA. (ii) Sensing experiment of BPA molecules treated with aptamer. (B) Members of the bisphenol family with similar structures. (C) Real-time conductance measurement data obtained from the aptamer-functionalized swCNT-FET sensor after the injection of bare BPA molecules (black line) or aptamer-treated BPA molecules (red line). The aptamer-functionalized swCNT-FET sensors exhibited the conductance change only when 1 pM aptamer-treated BPA molecules were injected. (D) Response of aptamer-functionalized swCNT-FET sensors to the injection of BPA, BPB, 6F, and BP molecules that had been treated with the BPA aptamer. It exhibited response only to the aptamer-treated BPA molecules. | ||
First, we performed a control experiment to probe the response of the swCNT-FET sensors to pure BPA molecules (Fig. 3A, (i)). Under these conditions, we did not observe any conductance change with BPA solutions up to the 100 nM level (black line data in Fig. 3C). Presumably, it is because BPA molecules are neutral and could not affect the conductance of swCNT-FETs.
We reasoned that the solution for such a problem could be the sandwiched-aptamer assay strategy (Fig. 3A, (ii)). To test this hypothesis, we first functionalized BPA molecules by mixing them with the negatively charged ssDNA aptamer (labeling aptamer). When the functionalized BPA solution was applied to our aptamer-functionalized swCNT-FET sensors, they exhibited large signals even at very low BPA concentrations (red line data in Fig. 3C). Using this strategy, we could detect BPA concentrations as low as 1 pM. The conductance change of swCNT-FETs in response to functionalized BPA can be explained by Schottky barrier modulation caused by the charge in the aptamer bound to BPA. When the functionalized BPA molecules were selectively adsorbed onto the capturing aptamer residing on the Au electrode of the swCNT device, the charge in the labeling aptamer bound to BPA changed the work function of the Au electrodes. Such a work function change induced an increase in the Schottky barrier between the swCNTs and the Au electrodes, resulting in reduction of the source–drain current.
The selectivity of our aptamer sandwich based CNT sensors was tested by applying other small molecules (BPB, BP, and 6F) that have structures similar to that of BPA (Fig. 3B). It should be noted that the difference between BPA and BPB is just a single alkane chain that includes one carbon and two hydrogen atoms. Our sensors responded only to BPA, which clearly illustrates the high-level selectivity inherent in our sensing system. Furthermore, as a negative control, we introduced only aptamer without BPA into the system, and we detected no significant signals (Fig. S1 in the ESI‡).
The high sensitivity and selectivity of our sandwiched aptamer-based assay can be attributed to two important features of our sensing system. First, the negative charges associated with the aptamers bound to BPA enhanced the signal to the swCNT-FET device. Second, the sandwiched aptamer strategy requires the selective binding of two aptamers to a BPA molecule, which can dramatically improve sensor selectivity.
Signal enhancement viabiotin labels
To enhance the sensitivity of our sensor, we tried to add more charges to the pre-treated aptamer. We used the anti-BPA aptamer which was modified with biotin at its 5′ end (Fig. 4A). | ||
| Fig. 4 Signal enhancement using biotin-labeled aptamer. (A) Schematic diagram showing signal enhancement using biotin-labeled aptamer. (B) Real-time conductance measurement data obtained from the aptamer functionalized swCNT-FET sensor after the injection of biotin-labeled aptamer-treated BPA molecules. A significant change of source–drain current was observed at the 10 fM of analytes. (C) Log concentration vs. sensitivity of electrode functionalized CNT sensor for injection of biotin labeled aptamer treated BPA (black square), bare aptamer treated BPA (red circle) and BPA (blue triangle). The sensitivity of sensor was enhanced about ∼100 times by the biotin labeled aptamer compared to the bare aptamer modification. | ||
Fig. 4B shows the real time response of swCNT sensor for BPA which was treated with biotin labeled anti-BPA aptamer. A significant change (ΔG/G0 ≈ 4.5%) in the source–drain current was detected from 10 fM of BPA. In the case of using bare anti-BPA aptamers, sensors exhibited detectable changes (ΔG/G0 ≈ 2.6%) only from 1 pM concentrations. Fig. 4C compares the sensor sensitivity (ΔG/G0) of swCNT network sensors for differently pretreated BPA molecules. Significantly, in the case of biotin labeled aptamer treatment, the sensors channels exhibited a larger sensitivity to the exposure of the same concentration of analytes.
Since the pKa of biotin is 4.65, at pH 7.4 this molecule is present partially in the neutralized and partially in the negatively charged form. Presumably, this negative charge increases the change of electrode work function, so sensitivity of sensors is enhanced. This signal enhancement process could be applied to other FET based sensor using sandwich assay.
Conclusions
In summary, we report the development of aptamer sandwich-based CNT sensors for the detection of small non-polar pollutant molecules with a single-carbon-atomic resolution. Here, we first developed BPA aptamer suitable for the sandwich assay and utilized it as a recognition element for sandwich-based CNT sensors. Furthermore, using the additional biotin modification on labeling aptamer, we enhanced the sensitivity of our sensors for one hundred times. This is, at least, hundred times lower in detection limit than that of the existing methods.20–23 It should be mentioned that one of the key factors determining the usefulness of our strategy for future applications might be the availability of high-quality aptamers suitable for sandwich assay. In any case, our work should be a significant progress for sensor applications because it is the first demonstration of sandwich-based CNT sensors. Significantly, these results overcome the fundamental limitation of general FET-based sensors and should make a major breakthrough in various applications such as environmental protection and food safety.Acknowledgements
This project was supported by the Seoul R&BD Program (GR070045) and the NRF grant (No. 2010-0000799). SH acknowledges financial support from the WCU program and the Converging Research Center Program (No. 2010-k001138). SK acknowledges the support form KIEST (No. 2010-10002-0065-0/ 2010-09001-0076-0), NRF (No. 2010-0008018) and MKE (No. 10017190-2009-33). DKL was supported by Global Research Laboratory grant from MEST and MKE (No. 10032113-2010-13). THK thanks the support from the NRF grant (No. 2010-0005574). THK thanks the support from the NRF grant (No. 2010-0005574).References
- Y. Wen, B. S. Zhou, Y. Xu, S. W. Jin and Y. Q. Feng, J. Chromatogr., A, 2006, 1133, 21–28 CrossRef CAS.
- H. Ohkuma, K. Abe, M. Ito, A. Kokado, A. Kambegawa and M. Maeda, Analyst, 2002, 127, 93–97 RSC.
- H. Yin, Y. Zhou, L. Cui, X. Liu, S. Ai and L. Zhu, J. Solid State Electrochem., 2010 DOI:10.1007/s10008-010-1089-6.
- T. H. Kim, S. H. Lee, J. Lee, H. S. Song, E. H. Oh, T. H. Park and S. Hong, Adv. Mater., 2009, 21, 91–94 CrossRef CAS.
- J. Kong, N. R. Franklin, C. W. Zhou, M. G. Chapline, S. Peng, K. J. Cho and H. J. Dai, Science, 2000, 287, 622–625 CrossRef CAS.
- N. L. Rosi and C. A. Mirkin, Chem. Rev., 2005, 105, 1547–1562 CrossRef CAS.
- E. S. Snow, F. K. Perkins, E. J. Houser, S. C. Badescu and T. L. Reinecke, Science, 2005, 307, 1942–1945 CrossRef CAS.
- H. M. So, K. Won, Y. H. Kim, B. K. Kim, B. H. Ryu, P. S. Na, H. Kim and J. O. Lee, J. Am. Chem. Soc., 2005, 127, 11906–11907 CrossRef CAS.
- H. M. So, D. W. Park, E. K. Jeon, Y. H. Kim, B. S. Kim, C. K. Lee, S. Y. Choi, S. C. Kim, H. Chang and J. O. Lee, Small, 2008, 4, 197–201 CrossRef CAS.
- M. Lee, J. Im, B. Y. Lee, S. Myung, J. Kang, L. Huang, Y. K. Kwon and S. Hong, Nat. Nanotechnol., 2006, 1, 66–71 CrossRef CAS.
- J. Im, L. Huang, J. Kang, M. Lee, D. J. Lee, S. G. Rao, N. K. Lee and S. Hong, J. Chem. Phys., 2006, 124, 224707 CrossRef.
- X. W. Tang, S. Bansaruntip, N. Nakayama, E. Yenilmez, Y. L. Chang and Q. Wang, Nano Lett., 2006, 6, 1632–1636 CrossRef CAS.
- J. Kang, J. Lee, T. H. Kim, J. Park, M. J. Seong and S. Hong, Nanotechnology, 2008, 19, 135305 CrossRef.
- S. Soliveres, J. Gyani, C. Delseny, A. Hoffmann and F. Pascal, Appl. Phys. Lett., 2007, 90, 082107 CrossRef.
- M. Briman, K. Bradley and G. Gruner, J. Appl. Phys., 2006, 100, 013505 CrossRef.
- S. Reza, Q. T. Huynh, G. Bosman, J. Sippel-Oakley and A. G. Rinzler, J. Appl. Phys., 2006, 99, 114309 CrossRef.
- P. Dutta and P. M. Horn, Rev. Mod. Phys., 1981, 53, 497–516 CrossRef CAS.
- E. S. Snow, J. P. Novak, M. D. Lay and F. K. Perkins, Appl. Phys. Lett., 2004, 85, 4172–4174 CrossRef CAS.
- I. Heller, A. M. Janssens, J. Mannik, E. D. Minot, S. G. Lemay and C. Dekker, Nano Lett., 2008, 8, 591–595 CrossRef CAS.
- Z. C. Sanchez-Acevedo, J. Riu and F. X. Rius, Biosens. Bioelectron., 2009, 24, 2842–2846 CrossRef CAS.
- T. Sakata, M. Ihara, I. Makino, Y. Miyahara and H. Ueda, Anal. Chem., 2009, 81, 7532–7537 CrossRef CAS.
- W. Xia, Y. Li, Y. Wan, T. Chen, J. Wei, Y. Lin and S. Xu, Biosens. Bioelectron., 2010, 25, 2253–2258 CrossRef CAS.
- M. Portaccio, D. Di Tuoro, F. Arduini, M. Lepore, D. G. Mita, N. Diano, L. Mita and D. Moscone, Biosens. Bioelectron., 2010, 25, 2003–2008 CrossRef CAS.
Footnotes |
| † Published as part of a LOC themed issue dedicated to Korean Research: Guest Editors: Professor Je-Kyun Park and Kahp-Yang Suh |
| ‡ Electronic supplementary information (ESI) available: Supplementary Methods and Fig. S1. See DOI: 10.1039/c0lc00259c |
| This journal is © The Royal Society of Chemistry 2011 |