Multiferroic and magnetodielectric properties of the Al1−xGaxFeO3 family of oxides
Ajmala
Shireen
,
Rana
Saha
,
P.
Mandal
,
A.
Sundaresan
* and
C. N. R.
Rao
*
New Chemistry Unit, Chemistry and Physics of Materials Unit and International Centre for Materials Science, Jawaharlal Nehru Centre for Advanced Scientific Research, Bangalore, 560064, India. E-mail: cnrrao@jncasr.ac.in; sundaresan@jncasr.ac.in; Fax: +91-80-22082766; Tel: +91-80-22082761
First published on 11th November 2010
Abstract
AlFeO3, GaFeO3 and Al0.5Ga0.5FeO3, all crystallizing in a non-centrosymmetric space group, are multiferroic and also exhibit magnetodielectric properties, with Al0.5Ga0.5FeO3 showing an unusually large magnetocapacitance at 300 K.
Multiferroic oxides are not common since it is difficult to satisfy the criteria required for magnetism and ferroelectricity in the same material.1–3 However, there are a few materials that show multiferroic properties due to mechanisms different from those in conventional ferroelectrics, such as BaTiO3. All multiferroic materials are not necessarily magnetoelectric because the latter property arises from the interaction between the magnetic and the electric order parameters.1–3 It has been recently reported that AlFeO3, which is derived from Fe2O3 by the substitution of one Fe3+ by Al3+, shows ferrimagnetic as well as ferroelectric properties, unlike the parent binary oxides, Al2O3 and Fe2O3.4–6AlFeO3 has an orthorhombic structure with the chiral, non-centrosymmetric Pna21 space group. GaFeO3 has a structure similar to that of AlFeO3 with a non-centrosymmetric space group.7GaFeO3 is also ferrimagnetic and ferroelectric.6–10 In both AlFeO3 and GaFeO3, there are four different cation sites Fe1, Fe2, A1 and A2 (A = Al, Ga), of which Fe1, Fe2 and A2 have an octahedral oxygen environment. The cation in the A1 site is in the tetrahedral oxygen environment. The octahedral environment of Fe1, Fe2 and A2 are slightly distorted, while the A1O4 tetrahedron is almost regular. The cause for this local deformation of the lattice is attributed to the difference in the octahedral radii of Fe3+ and A3+ ions and the disorder in the occupation of the octahedral sites. Low temperature magnetic ordering in these oxides is due to the cation–oxygen–cation superexchange antiferromagnetic interaction. Magnetodielectric properties of multiferroic AlFeO3 and Al0.5Ga0.5FeO3 are not established. In this communication, we present the multiferroic and magnetodielectric properties of Al1−xGaxFeO3.† More importantly, we have investigated the properties of Al0.5Ga0.5FeO3 since the nature of the A-cations as well as cation disorder would have a significant effect on dielectric and related properties.† We find that Al0.5Ga0.5FeO3 is not only multiferroic, but also shows remarkably large magnetocapacitance at 300 K, exceeding the performance of AlFeO3 and GaFeO3.
In Fig. 1, we show the X-ray diffraction patterns of AlFeO3, GaFeO3 and Al0.5Ga0.5FeO3 along with profile fits and difference patterns to show how they possess the same structure.†AlFeO3 is ferrimagnetic with a TN of 250 K and shows magnetic hysteresis at low temperature (Fig. 2a). GaFeO3 exhibits a ferrimagnetic TN of 210 K, while Al0.5Ga0.5FeO3 shows a magnetic behaviour similar to AlFeO3 and GaFeO3 with a TN of 220 K (Fig. 2b). We see divergence between the field-cooled (FC) and zero-field-cooled (ZFC) magnetization data just as in AlFeO3 or GaFeO3. We observe magnetic hysteresis at low temperatures (see inset of Fig. 2b). The value of saturation magnetization, remnant magnetization, and coercive field in the case of Al0.5Ga0.5FeO3 are 10.0 emu g−1, 19.1 emu g−1 and 10.3 kOe respectively, not very different from the values found in AlFeO3 and GaFeO3.
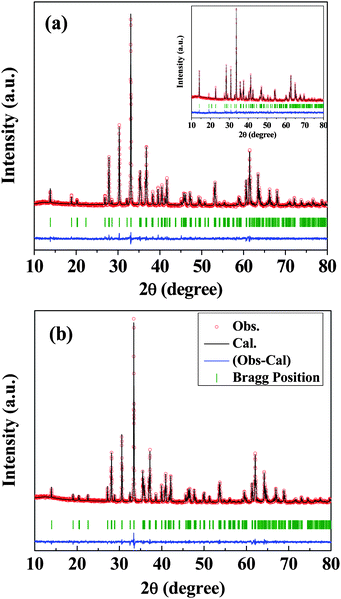 | ||
| Fig. 1 XRD patterns of (a) GaFeO3 and (b) Al0.5Ga0.5FeO3 along with profile fits, difference patterns and Bragg positions. Inset in (a) shows the XRD pattern of AlFeO3. | ||
 | ||
| Fig. 2 Temperature dependent magnetization of (a) AlFeO3 and (b) Al0.5Ga0.5FeO3 under field cooled (FC) and zero field cooled (ZFC) conditions. Magnetic hysteresis at 5 K is shown in the inset. | ||
We show the temperature variation of the dielectric properties of GaFeO3 and Al0.5Ga0.5FeO3 in Fig. 3(a) and (b), respectively. We see dielectric dispersion below TN and a significant increase in the dielectric constant above TN, behaviour similar to that found in AlFeO3. The dielectric behaviour shown in Fig. 3 is not unlike that of relaxor ferroelectrics. Al0.5Ga0.5FeO3 shows ferroelectric hysteresis at relatively low temperatures (<300 K) just as with AlFeO3 and GaFeO3, with the hysteresis loops reflecting the leaky nature of these materials. In Fig. 4, we show hysteresis loops in the case of GaFeO3 and Al0.5Ga0.5FeO3. The maximum (saturation) polarization (Pm) and remnant polarization (PR) are very much higher in Al0.5Ga0.5FeO3 than in GaFeO3 or AlFeO3, the values being Pm = 1.1 μC cm−2, PR = 0.5 μC cm−2 and coercive field (Ec) = 3.8 kV cm−1 at 200 K for an applied voltage of 500 V compared to Pm = 0.05(0.07) μC cm−2, PR = 0.02(0.04) μC cm−2 and Ec = 1.22(6.4) kV cm−1 found in AlFeO3 (GaFeO3). Clearly, Al0.5Ga0.5FeO3 is a multiferroic with better ferroelectric properties.
 | ||
| Fig. 3 The temperature variation of dielectric properties of (a) GaFeO3 and (b) Al0.5Ga0.5FeO3. The effect of 2 T is also shown for both (a) and (b). | ||
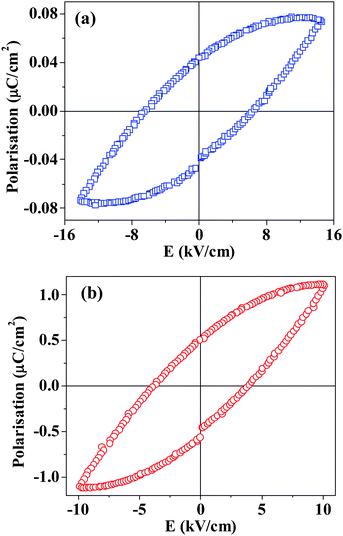 | ||
| Fig. 4 The ferroelectric hysteresis loops of (a) GaFeO3 and (b) Al0.5Ga0.5FeO3 at 200 K (1 kHz) showing leaky behaviour. | ||
A marked increase in the dielectric constant around TN signifies interaction between electric and magnetic order parameters. Application of a magnetic field has a marked effect on the dielectric properties of GaFeO3 and Al0.5Ga0.5FeO3, but the effect is considerably smaller in AlFeO3. We show the effect of magnetic field of 2 T on the properties of GaFeO3 and Al0.5Ga0.5FeO3 in Fig. 3. Magnetocapacitance in AlFeO3 reaches a value of around 20% at 300 K at 0.5 kHz and 2 T and does not vary significantly with frequency. The effect of magnetic field is however large in the case of GaFeO3 and Al0.5Ga0.5FeO3. Both these oxides show large magnetocapacitance and a significant variation of the magnetocapacitance with frequency, as shown in Fig. 5(a). Maximum magnetocapacitance is observed in these two oxides around 40 kHz and at this frequency, Al0.5Ga0.5FeO3 shows 60% magnetocapacitance at 2 T and 35% at 1 T. Such a large magnetocapacitance is indeed noteworthy. In Fig. 5(b), we show the variation of % magnetocapacitance with magnetic field for all three oxides. The marked variation of magnetocapacitance in GaFeO3 and Al0.5Ga0.5FeO3, is noteworthy, the value increasing with the magnetic field. We have examined the magnetoresistance properties of the three oxides. All the three oxides are insulators and show negligible magnetoresistance around 300 K, being 0.5% or less in the case of AlFeO3 and GaFeO3 at 2 T and 300 K. It is around 2.5% in the case of Al0.5Ga0.5FeO3. It therefore, appears that the origin of magnetodielectric effect observed by us is not entirely due to the magnetoresistance.
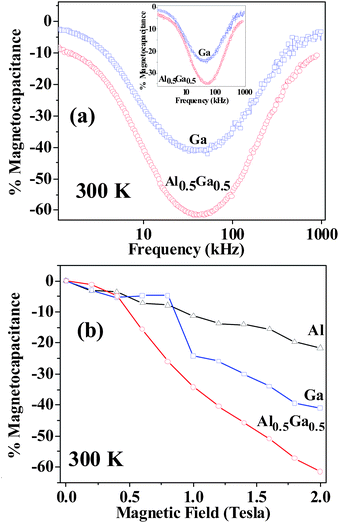 | ||
| Fig. 5 (a) The variation of % magnetocapacitance with frequency for GaFeO3 and Al0.5Ga0.5FeO3 at a magnetic field of 2 T. In the inset, the variation of % magnetocapacitance with frequency at 1 T is shown, (b) the variation of % magnetocapacitance with magnetic field for AlFeO3 (0.5 kHz, 300 K), GaFeO3 (40 kHz, 300 K) and Al0.5Ga0.5FeO3 (40 kHz, 300 K). | ||
In conclusion, it is significant that simple iron oxides of the type AFeO3 (A = Al, Ga) are both multiferroic and magnetodielectric. The large magnetodielectric effect exhibited by GaFeO3 and Al0.5Ga0.5FeO3, specially the latter, demonstrates the importance of the A-site cations. Displacements of the A cations and the disorder associated with their occupancies markedly affect the dielectric and magnetodielectric properties in a significant manner.6
Notes and references
- N. A. Hill, J. Phys. Chem. B, 2000, 104, 6694 CrossRef CAS.
- R. Ramesh and N. A. Spaldin, Nat. Mater., 2007, 6, 21 CrossRef CAS.
- C. N. R. Rao and C. R. Serrao, J. Mater. Chem., 2007, 17, 4931 RSC.
- F. Bouree, J. L. Baudour, E. Elbadraoui, J. Musso, C. Laurent and A. Rousset, Acta Crystallogr., Sect. B: Struct. Sci., 1996, B52, 217 CrossRef CAS.
- L. F. Cotica, S. N. De Medeiros, I. A. Santos, A. Paesano JR., E. J. Kinast, J. B. M. Da Cunha, M. Venet, D. Garcia and J. A. Eiras, Ferroelectrics, 2006, 338, 241 CrossRef CAS; L. F. Cotica, I. A. Santos, M. Venet, D. Garcia, J. A. Eiras and A. A. Coelho, Solid State Commun., 2008, 147, 123 CrossRef CAS.
- E. Kan, H. Xiang, C. Lee, F. Wu, J. Yang and M.-H. Whangbo, Angew. Chem., Int. Ed., 2010, 49, 1603 CAS.
- S. C. Abrahams, J. M. Reddy and J. L. Bernstein, J. Chem. Phys., 1965, 42, 3957 CrossRef CAS.
- K. U. Kang, S. B. Kim, S. Y. An, S.-W. Cheong and C. S. Kim, J. Magn. Magn. Mater., 2006, 304, c769.
- K. Sharma, V. R. Reddy, D. Kothari, A. Gupta, A. Banerjee and V. G. Sathe, J. Phys.: Condens. Matter, 2010, 22, 146005 CrossRef.
- V. B. Naik and R. Mahendiran, J. Appl. Phys., 2009, 106, 123910 CrossRef.
Footnote |
| † AlFeO3 and GaFeO3 were prepared by the co-precipitation method and sintered at 1350 °C. Al0.5Ga0.5FeO3 was prepared by heating the appropriate mixture of Al2O3, Ga2O3 and Fe2O3 at 1400 °C for 5 h. Al0.5Ga0.5FeO3 has a similar structure as AlFeO3 and GaFeO3. (a = 5.0292(1) Å, b = 8.6435(1) Å, c = 9.3155(1) Å). The lattice parameters of AlFeO3 (GaFeO3) are a = 4.9813(1) Å (5.0822(1) Å), b = 8.5516(2) Å (8.7447(1) Å), c = 9.2429(3) Å (9.3917(1) Å). |
| This journal is © The Royal Society of Chemistry 2011 |
