Liquid crystalline mesophases based on symmetric tetrathiafulvalene derivatives†
Lei
Wang
a,
Hyunduck
Cho
c,
Soo-Hyoung
Lee
b,
Changhee
Lee
c,
Kwang-Un
Jeong
*a and
Myong-Hoon
Lee
*a
aDeparment of Polymer/Nano Science and Technology, Chonbuk National University, Chonju, Chonbuk 561-756, Korea. E-mail: mhlee2@chonbuk.ac.kr; kujeong@chonbuk.ac.kr
bDivision of Environmental and Chemical Engineering, Chonbuk National University, Chonju, Chonbuk 561-756, Korea
cSchool of Electrical Engineering and Computer Science, Seoul National University, Seoul, 151-744, Korea
First published on 5th November 2010
Abstract
A series of new symmetric tetrathiafulvalene derivatives were designed and synthesized. These compounds have highly ordered lamello-columnar liquid crystalline phases in addition to their crystalline phases at lower temperatures. Charge transport in the organic thin-film-transistor devices is faster in the liquid crystalline phase by two orders of magnitude than that in the crystalline phase due to the decreased defects.
Liquid crystalline (LC) π-conjugated materials have attracted a lot of attention as promising organic charge-transfer candidates in optoelectronic devices.1–5 First, flexible alkyl chains chemically attached at the periphery of rigid aromatic LC mesogens can increase the solubility in common organic solvents, and make them solution-processable, which is technically favorable.1 Additionally, due to the self-assembling and self-healing capabilities, LCs can minimize defects such as the grain boundary, and line and point defects, which are inevitable drawbacks for charge transportation in the crystalline materials.2 It has been reported that columnar and smectic LCs show good charge carrier mobility in their mesophases, in which self-assembled 2D columns are well organized in ordered structures over a macroscopic domain. The lamello-columnar structures6–9 can be achieved by the self-assembly and subsequent self-organization process of board-like molecules whose molecular shapes are between discs and rods. Because of their unique molecular shapes, board-like molecules are recognized as discotic molecules which simultaneously behave like rod-like molecules to form a lamellar structure (smectic phase). Consequently, this results in higher charge mobility compared with those of ordinary smectic phases constructed from conventional rod-like molecules.3
Tetrathiafulvalene (TTF) and its crystalline derivatives have been extensively studied, and proven to be promising molecular building blocks for organic optoelectronic applications due to their excellent electronic and optical properties.10–15TTF derivatives having a LC phase should be more promising semiconductor materials because the ordered molecular structures in the LC phase will generate fewer structural defects on the macroscopic scale.16 However, the research of TTF-based LCs has been restricted, mainly due to the synthetic limitations.17–22
Recently, we reported a new series of TTF derivatives with an asymmetric structure of the TTF mesogenic core with long alkyl peripheries.23 Some of these compounds show mesomorphic states at wide temperature ranges including room temperature. To the best of our knowledge this was the first report of a TTF-based compound with a LC phase at room temperature. By utilizing the combined techniques of differential scanning calorimetry (DSC), polarized optical microscopy (POM), 1D/2D wide angle X-ray diffraction (WAXD), selected area electron diffraction (SAED), and solid state 13C NMR, it was determined that these asymmetric TTF molecules form a highly ordered LC phase.24 However, 2D WAXD analyses revealed that the molecular packing of these asymmetric TTF molecules in the columnar structure are synclinically tilted by approximately 42° away from the long axis of the columns, which restricts the maximum overlap of the aromatic cores and consequently results in a low charge carrier mobility. In order to solve this problem and maximize the intermolecular π–π stacking, we designed and synthesized a new series of TTF-based LC compounds which posses a symmetric molecular structure as shown in Scheme 1.
 | ||
| Scheme 1 Synthetic route of symmetric TTF derivatives. | ||
For designing a symmetric board-like TTF-based mesogenic structure, a new TTF core fused with two naphthalene rings was chosen, and four flexible alkyl chains having a different number of carbons (n = 7, 9, 11, or 13) were introduced at the sides of the mesogenic core using ester linkages. A large board-like π-conjugated mesogenic core with high symmetry could promote the formation of highly ordered smectic mesophases and consequently enhance the charge-transport capability due to the stronger π–π interaction. The four flexible alkyl chain peripheries are attached not to the ends but to the sides of the TTF-based mesogenic core, which could lead to the efficient columnar packing of board-like molecules. Additionally, the ester linkage was adopted to increase the air/light stability of the TTF core by decreasing the HOMO levels of TTF with its electron-withdrawing effect.25 Instability of TTF at ambient conditions has been one of the main drawbacks of using TTF-based molecules for practical applications.
TTF-based LC compounds were synthesized via 3-step reactions as shown in Scheme 1. The cross-coupling reaction of 4 at 120 °C for 4 h yielded a symmetric TTF core structure with yields of 58.5, 89.4, 85.1 and 78.1% for 5a to 5d, respectively. Chemical structures and purities of 5a–5d were confirmed by 1H NMR, 13C NMR, elemental analysis and high-resolution mass spectrometry, and the results are given in the supporting information (ESI†).
The phase structural evolutions of the TTF derivatives 5a–5d were investigated by the combined techniques of DSC, POM and 1D WAXD. Thermal transition temperatures and enthalpy values were obtained from thermal analyses of DSC as summarized in Table 1. The reported temperatures are the onset temperatures of the first cooling (Fig. S1, ESI†). Compounds 5a–5d showed LC phases below the isotropic temperature (Ti). There were three phase transitions for compounds 5a and 5b, while 5c and 5d exhibited two ordered phases: a LC phase and a crystal phase. The melting point decreased from 184 to 165 °C when the number of carbons in the flexible alkyl chains was increased.26–29 Compared with the asymmetric TTF series, this symmetric series showed much higher Ti, probably due to the higher packing density, the bigger size of aromatic core, and the stronger π–π interaction30 of symmetric geometry.
| Compound | K1 | T/°C | E/kJ mol−1 | K2 | T/°C | E/kJ mol−1 | ColL | T/°C | E/kJ mol−1 | I |
|---|---|---|---|---|---|---|---|---|---|---|
| a K1 = crystal phase 1, K2 = crystal phase 2, ColL = lamello-columnar phase, I = isotropic phase, • = phase exists, T = phase transition temperatures (onset), E = enthalpies. | ||||||||||
| 5a | • | 101 | 55.6 | • | 156 | 17.6 | • | 184 | 23.8 | • |
| 5b | • | 117 | 113.4 | • | 154 | 0.2 | • | 179 | 28.1 | • |
| 5c | • | 115 | 143.1 | • | 174 | 37.3 | • | |||
| 5d | • | 110 | 12.5 | • | 165 | 124.6 | • |
The POM images of compounds 5a–5d displayed similar textures in their LC phases (Fig. S2, ESI†). The domain sizes of these LC phases were larger than those of the asymmetric TTF series with a single crystal like uniform molecular orientation, which suggests a more favorable charge carrier mobility with fewer domain boundaries. Especially, the lamellar domains from 5a (Fig. S3, ESI†) created in the LC phase during the first cooling at a rate of 2 °C min−1 are up to a few millimetres in size with uniform birefringence, indicating a homogeneous molecular alignment.31 However, the cracks were found when the sample was cooled down to a crystalline phase (Fig. S3. ESI†). The generation of cracks could be attributed to the anisotropic shrinkage of the volume during the crystallization process. It was possible to orient molecules in a specific direction to form a macroscopic domain by applying external forces in the LC phase below the isotropic temperature (Fig. S4, ESI†). When the sheared sample of 5c was aligned 45° to the direction of a polarizer or an analyzer, the optical birefringence was maximized, while it became minimal when the shear direction was parallel to the polarizer. This observation clearly indicates that the molecules in the sheared sample are well aligned along the shear direction with the π–π stacking of the molecules perpendicular to the shear direction, which is often observed in columnar phases.32
To identify the structure of the LC phase and to monitor the structural evolutions during the thermal transitions, 1D WAXD experiments were conducted at different temperatures, and their results were combined with those of the POM. 1D WAXD pattern data of 5a are shown in Fig. 1. At 180 °C, in the small-angle region of the 1D WAXD of 5a, a sharp reflection peak at 2θ = 4.46° was detected, from which the d-spacing was estimated to be 1.98 nm. This value corresponds to the spacing between the layers. Its second- and third-order reflections (1.98/n: n = 2 and 3) appeared at 1.00 and 0.67 nm, respectively. These X-ray reflections in the low angle region clearly indicate that 5a generates a layer structure. The diffused scattering halo at 2θ = 22.28° (d-spacing = 0.40 nm) and several sharp Bragg reflections in the wide-angle region suggest that this phase is not a typical SmA LC phase but a highly ordered lamello-columnar structure.6–9 This speculation can be supported by the observation of a small but sharp diffraction at 2θ = 24.22° (d-spacing = 0.37 nm), which can come from the face-to-face π–π packing of TTF mesogens. The strong layered structure in this highly ordered lamello-columnar LC phase is schematically illustrated in Fig. 1b. The continuous cooling below the thermal transition at 156 °C induced the formation of a crystalline structure (K2). This judgment is based on several sharp X-ray diffractions not only in the low angle region at 2θ = 5.24° (d-spacing = 1.68 nm) but also in the wide angle region at 2θ = 19.77, 20.86 and 22.01°, which correspond to 0.45, 0.42 and 0.40 nm, respectively. When the temperature reached 101 °C there was a crystalline–crystalline transition. This new crystalline structure at the lower temperature was abbreviated as K1. The 1D WAXD data also indicated that the d-spacings of layered structure of the lamello-columnar LC and crystalline phases suddenly decreased during the phase transitions, which is one of the main reasons for the crack formation in the crystalline phase. This explanation could be confirmed by a certain crack direction (Fig. S3, ESI†) which is related to the dimensions of the unit cell of ordered structures. However, more detailed LC and crystalline structures and the molecular packing of these TTF-based molecules should be further investigated.
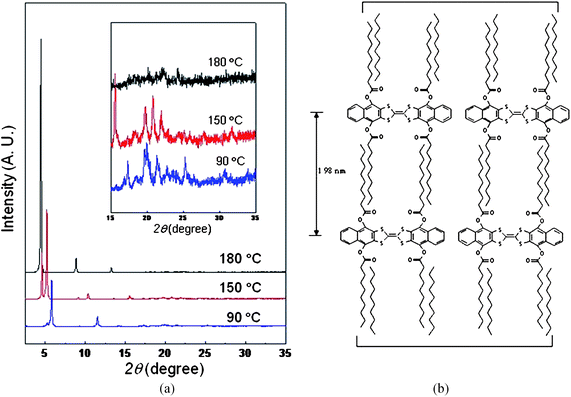 | ||
| Fig. 1 (a) 1D WAXD of 5a at 180, 130 and 60 °C. (b) A proposed molecular arrangement of the liquid crystals of 5a in the lamello-columnar phase. | ||
Utilizing UV/vis spectroscopy, the optical properties of the symmetric TTF molecules (5a–5d) were investigated in chloroform and the data were tabulated in Table 2 (also shown in Fig. S5, ESI†). The solubility of compounds 5a–5d decrease with increasing alkyl chain length. The absorption maxima of 5a–5d in chloroform were all found to be ∼384 nm irrespective of the alkyl chain length. The optical band gap energies were calculated to be 2.87–2.88 eV from the absorption tail, which are significantly higher than that of pentacene (2.2 eV), suggesting better air stability.33
| E1a/V | E2/V | UV/nm | Egb/eV | HOMOc/eV | |
|---|---|---|---|---|---|
| a E1 and E2 mean the onset of the oxidation potentials. b Eg = hc/λ0.1max. Eg means the optical band gap energy, and λ0.1max means wavelength at which the absorption coefficient drops to 10% of the peak value.39 c EHOMO = −(Eoxonset + 4.4) eV, where Eoxonset is the onset potential of the oxidation.40 | |||||
| 5a | 1.13 | 1.41 | 385 | 2.88 | −5.41 |
| 5b | 1.11 | 1.40 | 383 | 2.87 | −5.42 |
To evaluate the electrochemical properties, the cyclic voltammetry (CV) measurements were performed in a dry chloroform solution of Bu4NBF4 (0.1 M) at a scan rate of 10 mV s−1 at room temperature. The CV of 5a (Fig. S6, ESI†) exhibited two irreversible redox processes, corresponding to the formation of radical cations and dications, respectively. The oxidation potential of 5a was higher than those of previously reported asymmetric TTF-based molecules.23 This could be due to the presence of two ester groups substituted at the naphthalene ring. While 5a and 5b showed similar redox properties as estimated by CV, the electrochemical properties of 5c and 5d could not be measured by a solution method due to their limited solubility in chloroform. Since the alkyl chains do not significantly influence the oxidation processes,23 however, it can be assumed that 5c and 5d would exhibit similar oxidation potentials. The HOMO levels of 5a and 5b were estimated from the onset of the oxidation potentials (Table 2) to be −5.41 and −5.42 eV, respectively, which makes them suitable for use as p-type organic semiconductors.34 Low HOMO levels and larger energy gaps, in addition to the highly ordered LC property, suggest that 5a–5d can be promising hole transporting organic materials. Additionally, 5a–5d exhibited good air/light stability so they can be kept in ambient conditions for several months without any signs of oxidation.
The field-effect measurement was carried out by using bottom-contact thin film transistor (TFT) geometry. TFT devices with a channel length (L) of 40 μm and a channel width (W) of 1800 μm were fabricated on thermally oxidized highly n-doped silicon substrates. The dielectric SiO2 gate was 300 nm in thickness. Gold source/drain electrodes (60 nm) were evaporated through a shadow mask. Compound 5a was chosen and coated on the prefabricated TFT structure by solution (0.5 wt% solution in chloroform) drop casting owing to good solubility. After the evaporation of the solvent, there was no field-effect transistor characteristics observed for compound 5a at room temperature. This is attributed to the highly limited carrier mobility by domain boundary effect in the polycrystalline film as prepared from solution drop casting.35 The device was heated to 180 °C, and then cooled down to room temperature at 2 °C min−1, and TFT characteristics were measured at every 10 °C. Carrier mobility was calculated from the transfer characteristics following TFT equations in the linear regime.36 The device in the highly ordered lamello-columnar LC phase showed a charge mobility of about 10−5 cm2 V s−1. The mobility suddenly decreased to 10−7 cm2 V s−1 during the phase transition from the highly ordered lamello-columnar LC to K2 phase. There was no obvious change during the phase transition between K2 to K1. These coincide with the result of 1D WAXD and POM experiments performed at various temperatures. In the highly ordered lamello-columnar LC phase, compound 5a forms a uniform lamellar structure in the macroscopic domain. The TFT characteristics can be measured because of charge transport through the self-assembled 2D structure. When compound 5a transforms from the highly ordered lamello-columnar LC to K2 phase during cooling, the anisotropic structural shrinkage resulted in the creation of cracks, significantly high barriers for electrons to overcome. The decrease in electron mobility should be directly related with these cracks, which block the movement of charge carriers from source to drain. The more cracks formed during the K2–K1 transition do not affect the mobility significantly because the cracks generated during the LC–K2 transition are more than enough to disconnect the charge transport pathway. The field-effect measurement during a subsequent heating process was also in agreement with the results obtained during the cooling process. The mobility stayed at 10−7 cm2/Vs until the K2–LC transition, and then increased rapidly up to 10−5 cm2/Vs in the LC phase (Fig. 2 and Fig. S7 and S8, ESI†). These results confirm that the charge mobility in the LC phase can be 100 times higher than that in the crystalline phase because of fewer defects in the LC phase as a result of the self-healing process.37,38
 | ||
| Fig. 2 Temperature dependence of field effective mobility for (●) decreasing temperature from 180 °C to room temperature and (□) increasing temperature from room temperature to 180 °C. | ||
In summary, a series of new symmetric TTF derivatives were designed and synthesized. 1D WAXD and POM experiments confirmed that these symmetric TTF-based compounds have a highly ordered lamello-columnar LC phase in addition to crystalline phases at lower temperatures. Large single domains, up to a few millimetres without defects, were observed in the LC phase, which enabled us to fabricate organic TFT devices by using compound 5a as a p-type semiconductor. Measurements of TFT characteristics with respect to the phase transitions of compound 5a revealed that the charge transport in the LC phase is faster by two orders of magnitude than that in the crystalline phase due to fewer defects. Further study on the detailed structural evaluation of the LC phases and optimization of the TFT devices are in progress.
Acknowledgements
This work was supported by a grant from the Fundamental R&D Program for Core Technology of Materials funded by the Ministry of Knowledge Economy, Republic of Korea, and BK-21 Polymer BIN Fusion Research Team. LW would like to acknowledge the 2008 Post-doc Program of CBNU.References
- T. Sakurai, K. Shi, H. Sato, K. Tashiro, A. Osuka, A. Saeki, S. Seki, S. Tagawa, S. Sasaki, H. Masunaga, K. Osaka, M. Takata and T. Aida, J. Am. Chem. Soc., 2008, 130, 13812–13813 CrossRef CAS.
- S. Sergeyev, W. Pisula and Y. H. Geerts, Chem. Soc. Rev., 2007, 36, 1902–1929 RSC.
- Y. Shimizu, K. Oikawa, K. I. Nakayama and D. Guillon, J. Mater. Chem., 2007, 17, 4223–4229 RSC.
- M. O'Neill and S. M. Kelly, Adv. Mater., 2003, 15, 1135–1146 CrossRef.
- I. O. Shklyarevskiy, P. Jonkheijm, N. Stutzmann, D. Wasserberg, H. J. Wondergem, P. C. M. Christianen, A. P. H. J. Schenning, D. M. de Leeuw, Z. Tomovic, J. S. Wu, K. Mullen and J. C. Maan, J. Am. Chem. Soc., 2005, 127, 16233–16237 CrossRef CAS.
- A. El-ghayoury, L. Douce, A. Skoulios and R. Ziessel, Angew. Chem., Int. Ed., 1998, 37, 1255–1258 CrossRef CAS.
- C. W. Struijk, A. B. Sieval, J. E. J. Dakhorst, M. van Dijk, P. Kimkes, R. B. M. Koehorst, H. Donker, T. J. Schaafsma, S. J. Picken, A. M. van de Craats, J. M. Warman, H. Zuilhof and E. J. R. Sudholter, J. Am. Chem. Soc., 2000, 122, 11057–11066 CrossRef.
- G. Zucchi, B. Donnio and Y. H. Geerts, Chem. Mater., 2005, 17, 4273–4277 CrossRef CAS.
- A. Mori, M. Yokoo, M. Hashimoto, S. Ujiie, S. Diele, U. Baumeister and C. Tschierske, J. Am. Chem. Soc., 2003, 125, 6620–6621 CrossRef CAS.
- Y. B. Dong, L. Wang, J. P. Ma and R. Q. Huang, Inorg. Chem., 2006, 45, 9157–9159 CrossRef CAS.
- N. Martin, L. Sanchez, M. A. Herranz, B. Illescas and D. M. Guldi, Acc. Chem. Res., 2007, 40, 1015–1024 CrossRef CAS.
- M. Mas-Torrent and C. Rovira, J. Mater. Chem., 2006, 16, 433–436 RSC.
- L. Wang, B. Zhang and J. P. Zhang, Inorg. Chem., 2006, 45, 6860–6863 CrossRef CAS.
- X. K. Gao, Y. Wang, X. D. Yang, Y. Q. Liu, W. F. Qiu, W. P. Wu, H. J. Zhang, T. Qi, Y. Liu, K. Lu, C. Y. Du, Z. G. Shuai, G. Yu and D. B. Zhu, Adv. Mater., 2007, 19, 3037–3042 CrossRef CAS.
- O. Aleveque, P. Frere, P. Leriche, T. Breton, A. Cravino and J. Roncali, J. Mater. Chem., 2009, 19, 3648–3651 RSC.
- M. Katsuhara, I. Aoyagi, H. Nakajima, T. Mori, T. Kambayashi, M. Ofuji, Y. Takanishi, K. Ishikawa, H. Takezoe and H. C. C. M. Hosono, Synth. Met., 2005, 149, 219–223 CrossRef CAS.
- R. Andreu, J. Barbera, J. Garin, J. Orduna, J. L. Serrano, T. Sierra, P. Leriche, M. Salle, A. Riou, M. Jubault and A. Gorgues, J. Mater. Chem., 1998, 8, 881–887 RSC.
- R. Andreu, J. Garin, J. Orduna, J. Barbera, J. L. Serrano, T. Sierra, M. Salle and A. Gorgues, Tetrahedron, 1998, 54, 3895–3912 CrossRef CAS.
- R. A. Bissell, N. Boden, R. J. Bushby, C. W. G. Fishwick, E. Holland, B. Movaghar and G. Ungar, Chem. Commun., 1998, 113–114 RSC.
- A. Gonzalez, J. L. Segura and N. Martin, Tetrahedron Lett., 2000, 41, 3083–3086 CrossRef CAS.
- X. Gao, W. Wu, Y. Liu, S. Jiao, W. Qiu, G. Yu, L. Wang and D. Zhu, J. Mater. Chem., 2007, 17, 736–743 RSC.
- I. C. Pintre, J. L. Serrano, M. B. Ros, J. Ortega, I. Alonso, J. Martinez-Perdiguero, C. L. Folcia, J. Etxebarria, F. Goc, D. B. Amabilino, J. Puigmarti-Luis and E. Gomar-Nadal, Chem. Commun., 2008, 2523–2525 RSC.
- L. Wang, K. U. Jeong and M. H. Lee, J. Mater. Chem., 2008, 18, 2657–2659 RSC.
- L. Wang, S.-J. Park, S.-H. Lee, Y.-J. Kim, Y.-B. Kook, S.-W. Kuo, R. M. Van Horn, S. Z. D. Cheng, M.-H. Lee and K.-U. Jeong, Chem. Mater., 2009, 21, 3838–3847 CrossRef CAS.
- J. Naraso, D. Nishida, S. Kumaki and Y. TokitoYamashita, J. Am. Chem. Soc., 2006, 128, 9598–9599 CrossRef.
- S. H. Hwang, S. J. Park, H. Y. Kim, S. W. Kuo, S. H. Lee, M. H. Lee and K. U. Jeong, J. Phys. Chem. B, 2009, 113, 5843–5854 CrossRef CAS.
- K. U. Jeong, A. J. Jing, B. Mansdorf, M. J. Graham, D. K. Yang, F. W. Harris and S. Z. D. Cheng, Chem. Mater., 2007, 19, 2921–2923 CrossRef CAS.
- K. U. Jeong, A. J. Jing, B. Monsdorf, M. J. Graham, F. W. Harris and S. Z. D. Cheng, J. Phys. Chem. B, 2007, 111, 767–777 CrossRef CAS.
- K. U. Jeong, B. S. Knapp, J. J. Ge, S. Jin, M. J. Graham, H. M. Xiong, F. W. Harris and S. Z. D. Cheng, Macromolecules, 2005, 38, 8333–8344 CrossRef CAS.
- P. Jonkheijm, F. J. Hoeben, R. Kleppinger, J. van Herrikhuyzen, A. P. Schenning and E. W. Meijer, J. Am. Chem. Soc., 2003, 125, 15941–15949 CrossRef CAS.
- M. Melucci, L. Favaretto, C. Bettini, M. Gazzano, N. Camaioni, P. Maccagnani, P. Ostoja, M. Monari and G. Barbarella, Chem.–Eur. J., 2007, 13, 10046–10054 CrossRef CAS.
- Y. Che, A. Datar, K. Balakrishnan and L. Zang, J. Am. Chem. Soc., 2007, 129, 7234–7235 CrossRef CAS.
- H. Meng, F. Sun, M. B. Goldfinger, G. D. Jaycox, Z. Li, W. J. Marshall and G. S. Blackman, J. Am. Chem. Soc., 2005, 127, 2406–2407 CrossRef CAS.
- A. R. Murphy and J. M. Frechet, Chem. Rev., 2007, 107, 1066–1096 CrossRef CAS.
- T. J. Shin, H. Yang, M. M. Ling, J. Locklin, L. Yang, B. Lee, M. E. Roberts, A. Mallik and Z. Bao, Chem. Mater., 2007, 19, 5882–5889 CrossRef CAS.
- M.-C. Um, J. Kwak, J.-P. Hong, J. Kang, D. Y. Yoon, S. H. Lee, C. Lee and J.-I. Hong, J. Mater. Chem., 2008, 18, 4698–4703 RSC.
- J. C. Maunoury, J. R. Howse and M. L. Turner, Adv. Mater., 2007, 19, 805–809 CrossRef CAS.
- M. Funahashi, F. Zhang and N. Tamaoki, Adv. Mater., 2007, 19, 353–358 CrossRef CAS.
- D. A. M. Egbe, C. Bader, E. Klemm, L. Ding, F. E. Karasz, U.-W. Grummt and E. Birckner, Macromolecules, 2003, 36, 9303–9312 CrossRef CAS.
- D. M. de Leeuw, M. M. J. Simenon, A. R. Brown and R. E. F. Einerhand, Synth. Met., 1997, 87, 53–59 CrossRef.
Footnote |
| † Electronic Supplementary Information (ESI) available: Experimental and Fig. S1–S8. See DOI: 10.1039/c0jm02357d/ |
| This journal is © The Royal Society of Chemistry 2011 |
