Distribution and orientation study of dyes intercalated into single sepiolite fibers. A confocal fluorescence microscopy approach†
Virginia
Martínez-Martínez
*,
Cecilia
Corcóstegui
,
Jorge
Bañuelos Prieto
,
Leire
Gartzia
,
Sandra
Salleres
and
Iñigo
López Arbeloa
*
Departamento de Química Física, Universidad del País Vasco, UPV/EHU, Apartado 644, 48080, Bilbao, Spain. E-mail: virginia.martinez@ehu.es; inigo.lopezarbeloa@ehu.es; Fax: +34 91 603 3500
First published on 21st October 2010
Abstract
Single sepiolite clay needles doped with dyes of different molecular dimensions, Rhodamine 6G (R6G), Pyronine Y (PY), Styryl 698 (LDS 698) and Styryl 722 (LDS 722), at low and high loadings, are studied by confocal fluorescence microscopy. The combination of fluorescence lifetime imaging (FLIM), polarized fluorescence intensity experiments and spectral resolution shows a straightforward method to study the molecular distribution inside the tunnels and/or at the external channels, the dye orientation (evaluated by the fluorescence dichroic ratio, D) and the formation of different dye species (monomer and aggregates). The ability of the dyes to penetrate into the Sep tunnels is R6G < LDS 698 < PY < LDS 722, analyzed by thermal analysis. As a result the alignment along the main axis of Sep increases in the same order, reaching fluorescence dichroic ratios of around 1.5, 6, 8 and 10 respectively for each dye.
Introduction
The encapsulation of dyes into nanostructured ordered systems is a good tactic to provide new functional materials with interesting optical, chemical and electrical properties.1–5 A great variety of solid matrices with different 1-, 2- or 3-dimensional arrays are suitable to induce a preferential orientation on the guest molecules. Based on these ordered hybrid materials, several applications have already been derived, such as antenna systems, light waveguides and dichroic filters, the development of solar cells, frequency-doubling devices and micro-solid dye lasers, among others.6–12 Therefore, nanotubes and nanostructured tubular systems with elongated pores can act as a powerful ordering framework in one dimension for the guest species. In the best case, dyes could individually fit into the pores with a preferential orientation along the main axis of the crystal with a negligible tendency to aggregate, which produces an important decrease of its fluorescence capacity.In this sense, sepiolite (Sep), Mg8Si12O30(OH)4·8H2O, is a very special magnesium silicate clay,13 with a similar composition to smectite-clay layered material but with a fibrous structure. In general a smectite-clay structure is formed by stacking an octahedral (O) MgO or Al2O3 sheet between two tetrahedral (T) SiO2 sheets, the so-called TOT layer. The isomorphic substitution of these cations by other cations with lower valence generates negative charge to the clay surface neutralized by inorganic exchangeable cations and therefore confers cation exchange capacity (CEC) to these systems. However, unlike smectites, TOT sheets in sepiolite are discontinued and the alternation of blocks gives rise to interior rectangular tunnel-like pores of 3.6 Å × 10.6 Å and external channels or open grooves along the c-axis (Scheme 1). Thus, individual particles of sepiolite have a needle-like morphology with a particle size in the range of 0.5–7 μm length and width of around 50–100 nm (Scheme 1).
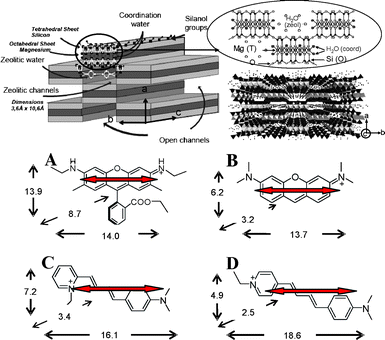 | ||
| Scheme 1 (Top) Schematic illustration of sepiolite clay structure; (Botton) dye dimensions in Å (AM1, Mopac): (A) R6G, (B) PY, (C) LDS 698 and (D) LDS 722. The transition moment (arrows) along the long molecular axis of the dye molecules (TD-DFT, Gaussian 09) is also included. | ||
It is worth noting that recently it has been reported that rod-like sepiolite clay suspensions can form liquid crystalline phases, in particular the nematic phase, showing a macroscopic alignment of the particles with a permanent birefringence.14 Therefore, the adsorption of photoactive molecules into the confined pores of sepiolite particles in order to achieve macroscopic alignment of the doped-Sep particles is of great interest. The highly ordered colloid liquid crystals will offer systems with a marked dichroism for potential applications.15 Alternatively, the elaboration of hybrid films with a preferred alignment of the dye/Sep fibers along the c-axis and are deposited parallel to the film plane by the Langmuir method, as recently reported.16 Therefore, the study of appropriate dye candidates for inclusion in sepiolite fibers is important to develop new functional materials with optical and electrical applications.
Although sepiolite has a very high adsorption capacity (higher than 300% of the CEC), it is usually used as an adsorbent in dyeing waste water;17 unlike smectites-type clays, sepiolite is a non-swelling clay and consequently will not allow the penetration of large molecules, the molecular size being the main limiting factor. Indeed, many studies have proved the adsorption of small polar molecules such as acetone, ammonia, ethylene glycol, methanol, and pyridine in the internal surface of sepiolite by thermal gravimetric analysis, Ar adsorption isotherms, FTIR, XRD and solid-state NMR techniques.18 Although some authors have reported the adsorption of larger molecules in a few dyes such as, methylene blue or indigo (constituent of the Maya Blue pigment) into the tunnels, its inclusion is still a matter for discussion.19
In this work, time-resolved confocal fluorescence microscopy is complementary to the above cited techniques, and can be used as a great tool for the characterization of dyes with different molecular dimensions such as Rhodamine 6G, Pyronine Y and Styryl 698 and 722 (Scheme 1) in Sep particles. Indeed, the combination of the FLIM technique (fluorescence lifetime imaging microscopy), along with polarized fluorescence intensity experiments and spectral resolution show a straightforward method to study the dye orientation, the molecular distribution inside the tunnels and/or at the external channels and the formation of different dye species (monomers and aggregates).
Results and discussion
Scheme 1 shows the optimized molecular structures (AM1) of the dyes used in this work. On the one hand, Pyronine Y (PY) and Styryl 722 (LDS 722) with totally linear and planar structures and Styryl 698 (LDS 698) with a mostly planar structure (aromatic ring slightly twisted due to steric hindrance by the ortho substitution) are dyes with molecular sizes which allow the access into the Sep internal channels. In fact, PY has very similar molecular dimensions to methylene blue dye (MV), whose accessibility into the pores have already been proposed.19 On the other hand and for the sake of comparison, Rhodamine 6G (R6G), with a large molecular size due to the carboxyl phenyl group almost perpendicular to the xanthene ring (Scheme 1), cannot penetrate into the small section of the tunnels, as has been studied in suspension.20An SEM image (Fig.1) shows the distribution of dye/Sep fibers along the glass substrate after the spin-coating process of a diluted suspension of dye/Sep particles. The distribution of the narrow needle-like dye/Sep particles is indicative of negligible agglomeration of clay particles as well as an undistorted morphology of Sep particles after the dye incorporation.
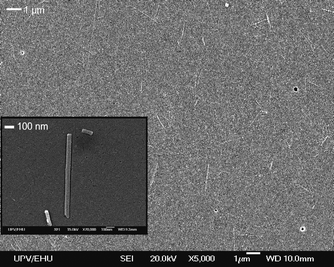 | ||
| Fig. 1 SEM images of dispersed single dye/Sep particles. | ||
Although confocal microscopy has no such spatial resolution, important spectroscopic information can be derived from the intensity and time-resolved images. In general, dye/sepiolite single crystals display a constant intensity profile over the whole particle for all dyes, confirming a homogeneous adsorption process over the whole crystals for all dye loadings (Fig. S1 in ESI†), without any agglomeration at the edges as other authors have detected in similar unidirectional systems (e.g.zeolite L).21 However, although FLIM images (the color of each pixel in the image corresponds to a lifetime value) reveal quite homogeneous profiles, fluorescence decay curves of dye/Sep particles generally need a bi-exponential function to be fitted, resulting in two lifetimes. Several lifetimes mean different dye species (monomer and aggregates) or dye in different environments (internal and external surfaces of the fiber). The combination of lifetime intensity polarization images and spectral resolution in single dye/Sep particles is a great tool for studying the distribution, orientation and spectroscopic characteristics of dye molecules in Sep fibers with submicron precision as will be discussed for each dye.
Because of its molecular dimensions, R6G dye cannot penetrate into the internal tunnels of the sepiolite needles and therefore a preferential order along the main axis of the Sep needle is not expected. Fig. 2 shows the corresponding lifetime image (FLIM), bi-exponential fitted, of a low loading R6G/Sep sample (∼15% CEC), with characteristic decay times of τ1 = 1.0 ns (45%) and τ2 = 3.5 ns (55%), distributed very homogeneously along the particle. The longer lifetime τ2 agrees well with that registered in diluted aqueous solutions of R6G22 and it is attributed to R6G monomers adsorbed on the external surface of sepiolite.
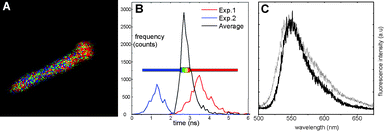 | ||
| Fig. 2 (A) FLIM image of 15% CEC R6G/Sep particle (length = 2.4μm); (B) histogram representing the average lifetime of the sample (central curve) and the two fitted lifetimes 1.0 ns and 3.5 ns (left and right curves, respectively); (C) emission spectra of 15% CEC R6G/Sep particle (dotted grey line) and R6G in aqueous solution (solid black line). | ||
On the other hand, it is well known that R6G molecules tend to aggregate in concentrated solutions and especially when they are adsorbed on surfaces, i.e.R6G intercalated in the interlayer space of smectite clays forms aggregates with a different geometry.22 Depending on the disposition of the monomers, the aggregates can be divided into two main types:23 H-aggregates, in which the monomers are in parallel planes as in a sandwich-like structure, are non-emissive and efficient quenchers for the fluorescent emission of the monomer (the hydrophobic character of R6G gives rise to these kind of associations in water);24 and J-aggregates, in which monomers arrange in a linear head-to-tail geometry and are characterized by red-shifted emission bands in respect to the monomeric fluorescent band (this more extended structure is observed for R6G in more hydrophobic media, such as ethanol).25 The spectrum in Fig. 2C does not show any new fluorescence band or shoulder bathochromically shifted with respect to that recorded in solution, which should discount the existence of J-aggregates. Although the fluorescence band of R6G adsorbed on sepiolite particles (maximum at 548 nm) is wider than that in liquid solution (Fig. 2C), this observation is a typical observable fact in solid-state dye samples, ascribed to changes in the vibronic structure of the electronic band in rigid media.26 Thus, τ1 is attributed to the lifetime of R6G monomers quenched by the presence of H-aggregates, probably dimers, due to an efficient energy transfer from the excited state of the monomers to the non-fluorescent low-energy excited state of H-type dimer.23
As has been cited before, R6G molecules are unlikely to penetrate into the internal tunnels and for this reason the maximum dye loading reached in R6G/Sep samples was around 50% of CEC (less than the other dyes studied in this work). Note that this mineral, besides adsorption by a cationic exchange process, can also accommodate molecules on neutral sites by other mechanisms, reaching up to 300% of CEC,17 since the proportion of external/internal surface areas in Sep is around 1/3, depending on the Sep fiber thickness.
Emission spectra of 50% CEC R6G/Sep particles remain identical to low-dye samples (data not-shown) and its FLIM image shows similar bi-exponential kinetics of τ1 = 0.9 ns (55%) and τ2 = 3.3 ns (45%), with a slightly higher quenching of the monomer lifetime (slight decrease of lifetime values and increase of the τ1 contribution) indicating a slightly higher amount of H-aggregates on the external surface. After the washing step of dye/Sep sample preparation procedure, R6G aggregates do not seem to be formed in a very high extent as the dye loading increases.
Polarization experiments, performed under nonpolarized excitation light to ensure that all the dye molecules are excited, were carried out in R6G/Sep samples. Images with fluorescence emission collected both nearly parallel and perpendicular to the c-long axis of the sepiolite fiber show similar fluorescence intensity counts (Fig. S2 in ESI†). Indeed, as a result of more than 10 particles measured in R6G/Sep samples, the dichroic ratios, D = I‖/I⊥, obtained after applying the isotropic factor G to correct the instrumental response to the polarized light (see Experimental section), were in the range of 1–2 and 1.5–2.5 for the 15% and 50% R6G loading into sepiolite samples, respectively. Since adsorption of dyes onto smectite-type clays (a disk-like morphology with layered structure of stacked TOT sheets) does not induce any preferential orientation in the xy-plane,22 images with an emission intensity in two perpendicular polarization orientations were also recorded in a R6G-doped laponite smectite clay particle to be compared with R6G/sepiolite. The experiment was performed in order to rule out undesirable effects produced from the clay particles (scattering, birefringence, etc.) which can lead to a misunderstanding of the results. In the R6G-doped laponite, the dichroic ratio obtained was near the unity, D ≈ 1, indicating a totally random distribution of the dye molecules with a negligible influence of the clay on the polarization experiments. Thus, the small difference in the D value with respect to unity in R6G/Sep samples suggests a slight alignment along the fiber axis attributed to the adsorption of the R6G molecules in the open channels distributed along the external surface of the clay. Although most external molecules could freely accommodate a random distribution conformation, these grooves can induce a slight preferential orientation, which is more evident for higher dye loadings.
On the other hand, the Styryl LDS 698 (Scheme 1), has molecular dimensions of 16.1 Å × 7.2 Å and a thickness of 3.4 Å, which is comparable to the height of 3.6 Å of the Sep tunnel and hence might be the limiting dimension for penetration into the tunnels. LDS 698/Sep samples of around 20% and 95% of CEC were prepared. The nearly 100% CEC value reached is preliminary evidence that LDS 698 dye molecules can penetrate into the internal Sep channels.
The adsorption of LDS 698 into the tunnels is supported by the TGA measurements, which have been performed for all dye/Sep samples with high dye loadings (Fig. 3). The weight losses between 25 and 120 °C correspond to the desorption of the zeolitic water inside the tunnels. The decrease of weight loss in this range is attributed to the substitution of the zeolitic water by the inclusion of dye molecules, for this reason, in the case of R6G dye, similar water content to that of Sep powder is registered. Note that this zeolitic water loss decreases according to LDS 698 < PY < LDS 722, which denotes an inverse increase in the ability of these dyes to penetrate into the Sep fibers.
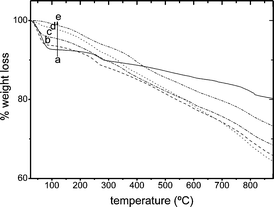 | ||
| Fig. 3 TG curves of dyes/Sep for high dye loadings: (a) pure Sep, (b) 50% CEC R6G/Sep, (c) 95% CEC LDS 698/Sep, (d) 80% CEC PY/Sep and (e) 90% CEC LDS 722/Sep. | ||
The dichroic ratio obtained from the polarized fluorescence intensity images are between 4–5 and 5.5–6.5 (Fig. 4A) for 20% CEC LDS 698 and the highest loading sample of 95% CEC, respectively. Therefore, D values indicate a preferential orientation of LDS 698 along the main axis of the fiber, which increase with the dye loading, suggesting the inclusion of dye molecules into the internal channels. In fact, by an analogous fluorescence polarization method, a similar anisotropy ratio (D ∼ 6) was exhibited by a needle crystal of native green fluorescence proteins (GPF) whose chromophores (inside the protein) were rigid and aligned with the long axis of the crystal.27
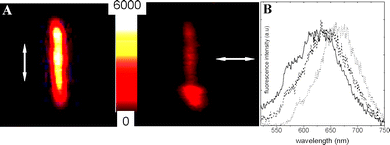 | ||
| Fig. 4 (A) Polarized intensity images of 95% CEC LDS 698/Sep particle 3.2 μm length (arrows indicate the direction of polarization of emission light detected). (B) Emission spectra of LDS 698/Sep particle of 20% and 95% CEC (solid and dash line) and LDS 698 in ethanolic solution (dotted line). | ||
Regarding FLIM images (Fig. S3 in ESI†), biexponential behavior was found in the LDS698/sepiolite particles, with characteristic decay times of τ1 = 2.1 ns (35%) and τ2 = 0.7 ns (65%) for 20% LDS 698/Sep CEC, which remains practically constant in the high dye loading sample (95% CEC), being in both cases homogeneously distributed along the particle (Fig. S3 in ESI†). Taking into account the low tendency of this dye to aggregate even in a concentrated solution (Fig. S4 in ESI†), the two different lifetimes should be attributed to dye molecules adsorbed in different parts of the sepiolite particle, i.e. internal and external channels. In general, radiative emission from styryl dyes (LDS), with polymethene chains connecting donor–acceptor aromatic rings, takes place from a ICT (internal charge transfer) state and conformational changes (i.e. twisted intramolecular charge transfer and cis-transisomerization) are the main channels of deactivation (increasing non-radiative decays).28 First of all, note that both lifetimes of LDS 698 in Sep are longer than that recorded in solution (τ1 = 0.36 ns, Table S1 in ESI†), which is due to the rigidity imposed by the clay structure reducing internal twisting motions and accordingly non-radiative paths of deactivation.29 Consequently, an increase in the fluorescence quantum yield is produced, as has been reported in polymeric matrixes (Table S1). For this reason, τ1 = 0.7 ns and τ2 = 2.1 ns are assigned to LDS 698 molecules adsorbed in the open external channels and into the internal tunnels, respectively. This assignment is based on those internal dye molecules (τ2) possessing a lower degree of freedom compared with external species (τ1) and consequently non-radiative processes should be further reduced (τ2 > τ1) and also because similar τ2 lifetimes have been obtained for LDS dyes embedded into polymeric matrices (Table S1 and Fig. S5 in ESI†). In fact, different dichroic ratios of 4 and 7 are obtained selecting two different time windows of analysis by considering fluorescence intensity count ratios at short lifetimes (first part of the decay curve corresponding to the lifetime value of around 0.7 ns) and at long lifetimes (tail fitting with a lifetime value of 2 ns), respectively, confirming the assignation of τ1 and τ2 (Fig. S6†). The confirmation of the external and internal LDS 698 species by the two different lifetimes might be in accordance with other previous studies reporting that first adsorption takes place on the external surface and then dye molecules start to penetrate into the less accessible pores (tunnels) by diffusion.30
Emission spectra (Fig.4B) of LDS 698/Sep samples barely change with the dye loading and there is no existence of new fluorescence species (i.e. J-aggregates) with characteristic bands in the less energetic part of the spectrum. On the other hand, the high blue shift registered in the emission spectra of LDS 698/Sep particles (band centered at 645 nm) with regard to ethanolic solution (maximum at 665 nm, Fig. 4B) is attributed to the rigidity imposed by the matrix to the dye and the emission occurs from a CT state with different geometry (not in the relaxed state) than that in solution.
More promising results are expected in the analog dye LDS 722 with dimensions of 18.6 Å × 4.9 Å due to its planarity and lower thickness of 2.5 Å with respect to LDS 698. The possibility of accommodating more molecules inside the tunnels will induce better alignment and consequently higher dichroic ratios.
For the LDS 722/Sep system and according to the TGA results (Fig. 3), for the 130% sample there is no sign of zeolitic water in highly concentrated samples, indicating that most of the water molecules have been replaced by dye molecules included inside the Sep tunnels; in fact, it is the sample with the highest % CEC reached.
For low dye loading samples (around 20% CEC of Sep), FLIM images were fitted to a bi-exponential function (Table 1), the two lifetimes being τ1 = 0.7 ns (70%) and τ2 = 2.6 ns (30%), homogeneously distributed along the particles. As described above for the case of the LDS 698/Sep sample, both values are higher than the lifetime of LDS 722 in ethanolic solution (0.58 ns, Table S1) and τ1 and τ2 are ascribed, for the same reasons, to the dye molecules absorbed outside and inside of Sep particle. Indeed, τ2 correlates with the lifetime recorded in a polymeric matrix (Table S1 in ESI†).
| Samples | R6G/Sep | LDS 698/Sep | LDS 722/Sep | PY/Sep | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 15% CEC | 50% CEC | 20% CEC | 95% CEC | 20% CEC | 90% CEC | 130% CEC | 6% CEC | 80% CEC | 50% CECb | |
| a Values in brackets correspond to the maximum of the emission spectra of diluted dye solutions recorded under the microscope in the same conditions. b High initial dye mother concentration but dye adsorption at the external surface. | ||||||||||
| τ/ns (% A) | ||||||||||
| τ1 (A1) | 1.0 (45%) | 0.9 (55%) | 0.7 (65%) | 0.7 (65%) | 0.7 (70%) | 0.7 (65%) | 0.5 (75%) | 1.2 (50%) | 0.7 (70%) | 0.15 (45%) |
| τ2 (A2) | 3.5 (55%) | 3.3 (45%) | 2.1 (35%) | 2.1 (35%) | 2.6 (30%) | 2.3 (35%) | 1.7 (25%) | 3.2 (50%) | 2.2 (30%) | 0.45 (50%) |
| 1.8 (5%) | ||||||||||
| λ em/nm | 547 (546)a | 645 (670)a | 663 (695)a | 560 (563)a | 560 | |||||
| 680 | ||||||||||
| D | 1–2 | 1.5–2.5 | 4–5 | 5.5–6.5 | 3.5–4.5 | 8–9 | 9–11 | 7.5–8.5 | 4–6 | 3.5–4.5 |
Regarding polarization experiments, dichroic ratios are in the range of 3.5–4.5 for 20% CEC LDS 722/Sep single particles but values up to 11 were reached for 130% CEC sample (Fig. 5). A maximum alignment along Sep fiber is reached for LDS722 molecules at the highest loadings, probably because its molecular structure is favored for the incorporation inside the tunnels.
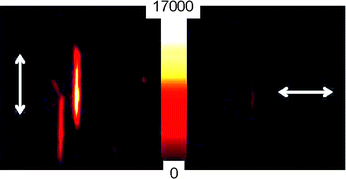 | ||
| Fig. 5 Polarized intensity images of 130% CEC LDS 722/particle: (left) emission detected along the long-axis (c-axis) of the Sep fiber; (right) orthogonal to the Sep c-direction. | ||
However, lifetimes for high loading (130% CEC) decrease, τ1 = 0.5 ns (75%) and τ2 = 1.7 ns (25%), suggesting that although LDS 722 dye has a very low tendency to aggregate at high concentration in solution (Fig. S7 in ESI†) H-aggregates and/or in-line J-aggregates with very low emission capacity might be formed. Fluorescence spectra of LDS722/Sep sample, in a similar manner to its analogue dye LDS 698, present a unique band placed at 663 nm (blue shifted compared to that registered in a diluted solution at 695 nm) which indicates the absence of J-aggregates (Table 1, Fig. S8 in ESI†).
Once more, an analysis at a different time window of the decay curve was performed and a dichroic ratio, D, of around 6 was obtained for short lifetime windows (0.5 ns) and D values ∼13 for long lifetimes (>1 ns), which verify the assignation of lifetimes (external and internal species). Although it is most probable that the majority of the LDS 722 aggregates are lodged at the external surface, their formation inside the tunnels cannot be discarded but they do not disrupt the good alignment of this sample.
With the goal of avoiding the formation of aggregates and trying to keep high dichroic ratios, an intermediate loading sample was prepared. Thus, 90% CEC LDS 722/Sep particles show lifetimes derived from the FLIM images of τ1 = 0.7 ns (65%) and τ2 = 2.3 ns (35%), near to those of low loadings (20% CEC) where the aggregation is considered negligible. According to the dichroic ratios obtained, D ∼ 8–9 (Table 1), they are substantially higher with respect to its analog dye LDS 698 at similar loading, indicating that LDS 722 is a good candidate to be included into Sep clay with a 1D preferential arrangement.
Finally, a similar study was carried out with Pyronine Y (PY). PY dye (14 Å × 6.2 Å × 3.2 Å) has the smallest long-axis of the dyes used in this work and similar molecular dimensions to methylene blue (MB), a dye for which different studies about its adsorption on the external and internal surfaces of sepiolite clay has already been reported.19
For low dye loading of around 6% CEC PY/Sep particles, FLIM images (Fig. S9 in ESI†) were fitted with bi-exponential decay functions giving lifetimes of τ1 = 1.2 ns (50%) and τ2 = 3.2 ns (50%), Table 1. In this case, τ2 = 3.2 ns agrees with that recorded in diluted solution21 and is attributed to PY monomers adsorbed in the Sep crystal. In this case and contrary to styryl dyes, a big change in the lifetime values for PY molecules adsorbed on external or internal surfaces of sepiolite is not expected because of the rigidity of its xanthene ring. For this reason and due to the ability of xanthene-type dyes to aggregate, τ1 could be attributed to the formation of aggregates. Since the emission spectra registered along the 6% CEC PY/Sep particles consists of an unique band at 560 nm, very similar to that registered in a dilute solution of PY (Fig. 6A), τ1 is ascribed to monomers quenched by the coexistence of H-aggregates, located probably at the external surface due to size restrictions. Although a very similar τ1 lifetime value has been assigned for PY J-aggregates21 adsorbed in zeolite L particles with a 20% loading, in this 6% CEC sample there is no evidences of the characteristic emission band placed in the 600–700 nm region and their contribution is ruled out. On the other hand, dichroic ratio values derived from the polarized intensity images were relatively high in this diluted sample, D ∼ 8 (Table 1), denoting a high 1D dye orientation as a consequence of dye internal adsorption, probably PY molecules in the monomeric state. The TGA curve of this diluted PY/Sep with lower zeolitic water with respect to pure Sep (Fig. 6B) agrees with this.
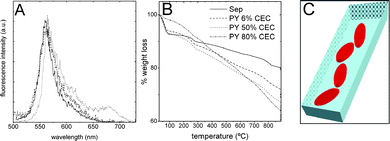 | ||
| Fig. 6 (A) Emission spectra of 10−6 ethanolic solution of PY (solid-line), 6% CEC PY/Sep particle (dashed-line) and 50% CEC PY/Sep (dotted-line) samples; (B) TG curves of PY/Sep samples with different dye loading; (C) schematic illustration of PY molecules intercalated into the Sep tunnels. | ||
In an attempt to increase the internal dye loading in the Sep tunnels and reach higher dichroic ratios, as obtained for the other dyes, a higher initial concentration of PY mother dye solution was used (10−4 M vs. 10−6 M). Surprisingly no more than a 50% CEC loading was achieved and mostly adsorbed at the external surface according to the TGA curve (Fig. 6B). It is attributed to a high aggregation process already in the PY mother solution where dimers, trimers or even higher-aggregates are formed and they cannot penetrate into the tunnels, as already stated for MB dye in a similar study.19
Indeed, FLIM images have to be fitted as 3-exponentials and much quenched lifetimes are derived in 50% PY/Sep sample (Table 1): τ1 = 0.15 ns (45%), τ2 = 0.45 ns (50%) and τ3 = 1.8 ns (5%), this is due to the agglomeration of dye molecules merely at the external area mainly in an aggregated state. In this sample the aggregation process is so pronounced that different types of aggregates are formed. Indeed, the emission band of J-aggregates was detected in the fluorescence spectrum with a characteristic shoulder at 680 nm (Fig. 6A). The predominance of short lifetimes (95%) derived from the FLIM image, τ1 = 0.15 ns (45%) and τ2 = 0.45 ns (50%), is indicative of a huge quenching by H-aggregates of the other emitting species present, i.e. monomer and J-aggregates.
Though most of the PY species are at the external surface, the dichroic ratio, D ∼ 4 (Table 1), is higher than that obtained for R6G molecules located on the Sep external surface, probably due to the Sep exterior grooves inducing PY molecules to be disposed with a larger preferential orientation because of its molecular planarity.
In order to achieve higher adsorption in the inner tunnels the adsorption process was carried out increasing the mixture time in high volumes of diluted mother solution of PY. In this way, up to an 80% CEC PY/Sep sample was obtained with a higher internal adsorption as shown in the TGA curves (Fig. 3 and Fig. 6B). The lifetimes derived from the FLIM images were τ1 = 0.7 ns (70%) and τ2 = 2.2 ns (30%). The decrease of the τ1 and τ2 values together with the increase in the contribution of τ1 over τ2 (Table 1) with respect to the 6% CEC sample, indicates higher quenching of PY monomers by the formation of further aggregates. The emission spectrum (data not shown) remains similar to the 6% CEC sample with no evidence of a new fluorescence band, indicating a higher formation of H-aggregates in this 80% CEC sample. The existence of a higher number of aggregates in this sample reduces the dichroic ratio values, which fluctuates around 4–6 values (Table 1). Moreover, the decrease in the dichroic ratio for high loading PY/Sep samples is also due to its short main molecular axis in comparison with LDS dyes because can be accommodated inside the Sep fiber with higher scattering or tilting angle (angle between the main molecular axis and the Sep long c-axis),31Fig. 6C,which disrupts the preferred 1D-orientation.
Therefore, dyes with low thickness (diffusion into the sepiolite tunnels favored), long main molecular axis (tilted angle reduced) and low aggregation tendency are the best candidates to get preferred 1D orientation along the Sep fiber. Accordingly, LDS 722 is the most suitable dye in this study, reaching dichroic ratios of D ∼ 9–11. Note, the large difference in the extent of the internal adsorption found in the case of styryl analogue dyes, LDS 722 and LDS 698, is a consequence of changes to their planarity (thickness) produced by the different positions, para and ortho, of the side chain substituent. Moreover, dyes with a long main-molecular axis and lower aggregation tendency, e.g. LDS dyesvs.PY, lead to higher unidirectional order along the internal tunnels of Sep needles for samples with high dye loadings
Conclusions
Confocal fluorescence microscopy by the combination of fluorescence intensity, polarization, lifetime measurements and spectral resolution are a good tool to study the adsorption of dyes into sepiolite fibers. It offers information about the distribution of the molecules, indicating in which area (external or internal) they are lodged and their orientation degree along the fibers. Thus, this technique can be extended to the study of other dyes into different unidirectional nanostructure systems with aligned nanopores.In dye/Sep systems, a homogeneous distribution of different dyes along the sepiolite fibers are obtained, without any agglomeration at the edges as has been detected in other nanochannel structured materials (e.g.zeolite L).
Due to the restricted cross section of Sep tunnels (3.6 Å × 10.2 Å), only dyes with the right molecular dimensions are able to penetrate them. A relatively high molecular thickness dimension of the dye can obstruct diffusion into the tunnels and molecules prefer to stay in the external area, producing a decrease of the dichroism of the systems. On the contrary, to favor internal over external adsorption in the Sep and therefore the alignment along the fiber direction, dyes with a small thickness and a long main molecular axis are the best candidates.
Dyes with a high tendency to form aggregates are not good candidates to be aligned one-dimensionally in Sep pores because aggregates usually disrupt the preferential order. Moreover, the dye adsorption process is recommended from diluted mother dye solutions and longer times for the dye/Sep mixture to avoid dye agglomeration on the external surface and favoring the inclusion of dye monomeric units into the internal tunnels with high unidirectional order degree (high dichroic ratio).
To summarize, sepiolite clay mineral with rod-like particles is a good host to get high 1D order for suitable dye/clay hybrid functional materials.
Experimental
Sepiolite clay (Sep) is supplied by Tolsa S.A. (90% pure) and used as received. It is a hydrated magnesium silicate with Mg8Si12O30(OH)4·8H2O as a unit cell formula, showing a microfibrous morphology with a particle size in the range of 0.5–7 μm length and a width of 50–100 nm containing open channels with dimensions of 3.6 Å × 10.6 Å along the axis of the particle. It has a very high surface area of about 300 m2 g−1 and a total cation exchange capacity (CEC) of around 15 meq per 100 g, with Mg, located on both external and internal surfaces, the main exchangeable cation. Cationic dyes Rhodamine 6G (R6G, Kodak, laser grade), Pyronine Y (PY, Acros Organics, High purity biological stain), Styryl 698 (LDS 698, Acros Organics) and Styryl 722 (LDS 722, Exciton), were used without further purification.Preparation of dye-doped sepiolite particles
Sepiolite clay powder (crushed before use) was suspended in ethanol (Merck) and stirred for one day, then ultrasonicated for 1 min to get a homogeneous suspension. The exact volume of the ethanolic dye solutions of different concentrations (from 10−6 to 10−4 M) was mixed with the clay suspension and stirred overnight in order to get lower CEC of around 10% and an even higher CEC than 100% samples, respectively. The mixture was centrifuged at 3000 rpm for 20 min, removing the supernatant and again washed with butanol, the process was repeated until colorless supernatant removed the excess of non-adsorbed dye. The dye/Sep sediment was dried at 60 °C in an airflow oven.Single dye-doped particles were obtained by spin-coating from a diluted aqueous dye/Sep suspension at 2500 rpm (30 s) onto a glass substrate.
Techniques
The morphology of the Sep particles (before and after the intercalation procedure) as well as the dispersion on the cover slide were checked by a Scanning Electron Microscope (SEM, LEOL JSM-6400 instrument).The amount of organic compound adsorbed on the clay surface was estimated by CHN elemental analysis (Euro EA 3000 Series Elemental Analyzer). Thermogravimetric measurements were conducted on a Nicolet TGA. Samples (∼10 mg) were heated from 25 °C to 850 °C at 10 °C min−1 under synthetic air flow (100 ml min−1).
Spectroscopic characterization of the samples was performed in a time-resolved fluorescence confocal microscope (model Micro Time 200, PicoQuant) for fluorescence lifetime imaging microscopy (FLIM). A 470 nm pulsed laser diode, with 70 ps pulses at 20 MHz repetition rate (LDH-P-C-470) whose polarization is changed to circular, polarized by a lambda/4 (Thorlabs, model WPQ05M-488), was used as the excitation source. The power of the laser beam was adjusted with a neutral density wheel around 0.1 μW at the entrance port of the microscope. It was directed into the oil-immersion objective (Olympus, 1.3 N.A., 100×, oil immersion) of an inverted microscope (Olympus IX70) by using a dichroic beam splitter (490DCXR, Chroma). The fluorescence signal was collected by the same objective, filtered with a 500 nm long-pass filter (Chroma) and focused (via a 50 μm pinhole) onto an avalanche photodiode detector (Micro-Photon-Devices MPD-APD), using the method of time-correlated single photon counting (TCSPC), with a FWHM photon timing resolution better than 100 ps.
The images were obtained by scanning the objective and recording the fluorescence intensity at each position, controlled by a 2D piezo scanner (Physik Instrument) with a scan range up to 80 × 80 μm with less than 10 nm resolution. The acquisition of data was done by a PicoHarp 300 TCSPC working in special time-tagged time resolved mode (TTTR), which stores all relevant information of a photon for further data analysis. Fluorescence lifetime images are processed with ShymPhotime software (Picoquant) by sorting all photons of one pixel into a histogram, then fitted to an exponential decay function to extract lifetime information; the procedure was repeated for every pixel in the image. The decay curves were adjusted normally to a sum of bi-exponential decays (i.e. as multi-exponentials) by means of:
Iflu(t) = A1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ1) + A2 exp(−t/τ1) + A2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ2) exp(−t/τ2) |
For polarization measurements the emission signal was divided by a polarizer beam splitter into two mutually perpendicular polarization orientation beams, which are simultaneously detected by two detector channels (MPD-APD). For all the samples, a dichroic ratio, D, was calculated by dividing the intensity counts registered at the channel parallel to the long axis of the Sep particle over the counts emitted at its perpendicular direction, previously corrected by an isotropic factor G to minimize the effect of the instrumental response to the different light polarization (different sensibility of detectors to the plane of polarization and other artifacts produced by the optic system) by means of (D)cor = I‖I⊥ × G. The correction factor G = (I⊥/I‖)iso was obtained by recording point measurements in both orthogonal emission channels of a diluted isotropic solution of each dye in the same conditions as the dye/particle samples (power laser, alignment, etc.) with the laser focused deep into the dye solution.
Spectra were recorded by directing the emission beam to an exit port, where a spectrograph (model Shamrock 300 mm) coupled to a CCD camera (Newton EMCCD 1600 × 200, Andor) were mounted.
For all the dye/Sep systems, results are derived from at least 10 dye/Sep particles measured.
Finally, quantum mechanical simulation was performed by AM1 semiempirical method implemented in the Mopac 2000 software. The molecular dimensions were obtained after optimization of the ground state geometry. The transition moment orientation was calculated by TD-DFT (Time Dependent-Density Functional Theory) method in the Gaussian 09 software.
Acknowledgements
This work is supported by a Saiotek project (S-PE09UN61) (Industry Program from the Basque Government). V. M. M. and C. C. thank the Basque Country University (UPV-EHU) and the Basque Government for research contract and fellowship, respectively, for the research contracts.Notes and references
- C. Sánchez, B. Julián, P. Belleville, M. Popall and B., J. Mater. Chem., 2005, 15, 3559 RSC.
- G. Schulz-Ekloff, D. Wöhrle, B. van Duffel and R. A. Schoonheydt, Microporous Mesoporous Mater., 2002, 51, 91 CrossRef CAS.
- M. Owaga and K. Kuroda, Chem. Rev., 1995, 95, 399 CrossRef CAS.
- S. Hashimoto, J. Photochem. Photobiol., C, 2003, 4, 19 CrossRef CAS.
- D. B. Mitzi, Chem. Mater., 2001, 13, 3283 CrossRef CAS.
- M. Ganschow, C. Hellriegel, E. Kneuper, M. Wark, C. Thiel, G. Schulz-Ekloff, C. Bräuchle and D. Wöhrle, Adv. Funct. Mater., 2004, 14, 269 CrossRef CAS.
- G. Calzaferri, S. Huber, H. Maas and C. Minkowski, Angew. Chem., Int. Ed., 2003, 42, 3732 CrossRef CAS.
- J. S. Moore, J. Am. Chem. Soc., 2008, 130, 12201 CrossRef CAS.
- B. V. Lotsch and G. A. Ozin, Adv. Mater., 2008, 20, 4079 CrossRef CAS.
- C. Brabec, V. Dyakonov, U. Scherf, ed., Organic Photovoltaics, Wiley-VCH, Weinheim, Germany, 2008 Search PubMed.
- Y. Geng, D. Gu and F. Gan, Opt. Mater., 2004, 27, 193 CrossRef CAS.
- J. Loerke and F. Marlow, Adv. Mater., 2002, 14, 1745 CrossRef CAS.
- J. Santarén, J. Sanz and E. Ruiz-Hitzky, Clays Clay Miner., 1990, 38, 63 CrossRef CAS.
- Z. X. Zhang and J. S. van Duijneveldt, J. Chem. Phys., 2006, 124, 154910 CrossRef CAS.
- N. Yasarawan and J. S. van Duijneveldt, Langmuir, 2008, 24, 7184 CrossRef CAS.
- Y. Umemura, E. Shinihara and R. A. Schoonheydt, Phys. Chem. Chem. Phys., 2009, 11, 9804 RSC.
- (a) H. Shariatmadari, A. R. Mermut and M. B. Benke, Clays Clay Miner., 1999, 47, 44 CrossRef CAS; (b) Y. Özdemir, M. Dogan and M. Alkan, Microporous Mesoporous Mater., 2006, 96, 419 CrossRef; (c) C. Bilgiç, J. Colloid Interface Sci., 2005, 281, 33 CrossRef CAS.
- (a) W. Kuang, G. A. Facey, C. Detellier, B. Casal, J. M. Serratosa and E. Ruiz-Hitzky, Chem. Mater., 2003, 15, 4956 CrossRef CAS; (b) C. Serna and T. Fernández-Alvarez, Anal. Quim., 1970, 70, 760; (c) G. Horwarth and K. Kawazoe, J. Chem. Eng. Jpn., 1983, 16, 470 CrossRef.
- (a) S. Ovalez, F. Giulieri, A.-M. Chaze, F. Delamare, J. Raya and J. Hirschinger, Chem.–Eur. J., 2009, 15, 11326 CrossRef CAS; (b) M. José-Yacamán, L. Rendón, J. Arenas and M. C. Serra Puche, Science, 1996, 273, 223 CrossRef CAS; (c) G. Chiari, R. Giustetto, J. Druzik, E. Doehne and G. Ricchiardi, Appl. Phys. A: Mater. Sci. Process., 2007, 90, 3 CrossRef; (d) E. Ruiz-Hitzky, J. Mater. Chem., 2001, 11, 86 RSC; (e) E. Fois, A. Gamba and A. Tilocca, Microporous Mesoporous Mater., 2003, 57, 263 CrossRef CAS.
- F. López Arbeloa, T. López Arbeloa and I. López Arbeloa, J. Colloid Interface Sci., 1997, 187, 105 CrossRef.
- M. Busby, C. Blum, M. Tibben, S. Fibikar, G. Calzaferri, V. Subramaniam and L. De Cola, J. Am. Chem. Soc., 2008, 130, 10970 CrossRef CAS.
- (a) V. Martínez Martínez, F. López Arbeloa, J. Bañuelos Prieto and I. López Arbeloa, Chem. Mater., 2005, 17, 4134 CrossRef; (b) F. López Arbeloa and V. Martínez Martínez, Chem. Mater., 2006, 18, 1407 CrossRef.
- M. Kasha, H. R. Rawls and M. A. El-Bayoumi, Pure Appl. Chem., 1965, 11, 371 CrossRef CAS.
- F. López Arbeloa, P. Ruiz Ojeda and I. López Arbeloa, J. Chem. Soc., Faraday Trans. 2, 1988, 84, 1903 RSC.
- F. López Arbeloa, P. Ruiz Ojeda and I. López Arbeloa, J. Photochem. Photobiol., A, 1988, 45, 313 CrossRef CAS.
- (a) P. Ballet, M. Van der Auweraer and F. C. De Schryver, J. Phys. Chem., 1996, 100, 13701 CrossRef CAS; (b) V. Martínez Martínez, F. López Arbeloa, J. Bañuelos Prieto and I. López Arbeloa, J. Phys. Chem. B, 2005, 109, 7443 CrossRef CAS.
- S. Inoué, O. Shimomura, M. Goda, M. Shribak and P. T. Tran, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 4272 CrossRef CAS.
- (a) S. Ferey-Forgues, M. T. Le Bris, J. C. Mialocq, J. Pouget, W. Retting and B. Valeur, J. Phys. Chem., 1992, 96, 701 CrossRef CAS; (b) D. Seth, S. Sarkar, R. Pramanik, C. Ghatak, P. Setua and N. Sarkar, J. Phys. Chem. B, 2009, 113, 6826 CrossRef CAS.
- (a) S. A. Lyapustina, A. V. Metelitsa, D. S. Bulgarevich, Y. E. Alexeev and M. I. Knyazhansky, J. Photochem. Photobiol., A, 1993, 75, 119 CrossRef CAS; (b) J. Kim, M. Lee, J.-H. Yang and J.-H. Choy, J. Phys. Chem. A, 2000, 104, 1388 CrossRef CAS.
- A. Rodríguez, G. Ovejero, M. Mestanza and J. García, Ind. Eng. Chem. Res., 2010, 49, 3207 CrossRef CAS.
- S. Megelski, A. Lieb, M. Pauchard, A. Dreschsler, S. Glaus, C. Debus, A. J. Meixner and G. Calzaferri, J. Phys. Chem. B, 2001, 105, 25 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Fluorescence intensity profiles of dye/Sep particles, polarized intensity images of R6G/Sep, FLIM images, fluorescence decay curves and histogram of lifetimes of LDS 698/Sep, LDS 722/Sep and Py/Sep particles, absorption and fluorescence spectra of LDS 698 and LDS 722 in diluted and concentrated dye solutions, fluorescence spectra and decay curves of LDS 698 in ethanol and in a polymer matrix. See DOI: 10.1039/c0jm02211j |
| This journal is © The Royal Society of Chemistry 2011 |
