Aqueous soft matter based photovoltaic devices†
Hyung-Jun
Koo
a,
Suk Tai
Chang
b,
Joseph M.
Slocik
c,
Rajesh R.
Naik
c and
Orlin D.
Velev
*a
aDepartment of Chemical and Biomolecular Engineering, North Carolina State University, Raleigh, NC 27695-7905, USA. E-mail: odvelev@unity.ncsu.edu; Fax: +1 (1)919 515 3465; Tel: +1 (1)919 513 4318
bSchool of Chemical Engineering and Materials Science, Chung-Ang University, 221 Heukseok-dong, Dongjak-gu, Seoul, 156-756, Republic of Korea
cMaterials and Manufacturing Directorate, Air Force Research Laboratory, Wright-Patterson AFB, Dayton, OH 45433, USA
First published on 21st September 2010
Abstract
We present a new type of photovoltaic systems based on aqueous soft gel materials. Two photosensitive ions, DAS− and [Ru(bpy)3]2+, were used as photoactive molecules embedded in aqueous gel. The hydrogel photovoltaic devices (HGPVs) showed performance comparable with or higher than those of other biomimetic or ionic photovoltaic systems reported recently. We suggest a provisional mechanism, which is based on a synergetic effect of the two dye molecules in photocurrent generation. We found an efficient replacement of the expensive Pt counter-electrode with copper coated with carbon materials, such as carbon nanotubes, carbon black or graphite. These Cu electrodes coated with carbon layers could drastically reduce the cost of such hydrogel devices without efficiency loss. Thus, a new class of low cost and flexible photovoltaic cells made of biocompatible matrix was demonstrated. Biologically derived photoactive molecules, such as Chlorophyll and Photosystem II, were successfully operated in aqueous gel media of such HGPVs.
Introduction
Photovoltaic cells attract considerable attention as one of the promising solutions to energy generation with minimized environmental pollution. The conventional types of photovoltaic cells are classified into three groups: inorganic solar cells,1,2 organic photovoltaics (OPVs)3–5 and dye sensitized solar cells (DSSCs).6–8 In addition to the research improving the performance of these conventional types of solar cells, new photovoltaic systems are being actively developed. Biomimetic or biocompatible solar cells, inspired by “artificial leaves”, are one example of such new classes of photovoltaics. Photoactive bio-complexes, e.g., Chlorophyll and photosynthetic reaction centers (Photosystem I and II), have been used for photosensitization of biomimetic solar cells prototypes. Lai et al. showed photocurrent generation by using Chlorophyll adsorbed on Au nanoparticle loaded photoelectrodes.9 Terasaki et al. and Ciesielski et al. reported a photoactive Au electrode functionalized with Photosystem I, a supramolecular enzyme involved in photosynthesis.10,11 The Photosystem I molecules have been immobilized by chemical or physical linker molecules and the photo-excited electrons in Photosystem I are transferred to the electrodevia the molecular linker. Biocompatible materials, such as water,12–14agarose gel15 or lipid membranes,16 have also been used as media for photovoltaic systems. For example, Murakami et al. reported water-based DSSCs with relatively high conversion efficiency by improving wettability of TiO2 photoelectrode.13Water could be a good alternative to the organic solvents used in photoelectrochemical cells that tend to be volatile and environmentally problematic. These new concepts of biomimetic photovoltaics could lower production cost and decrease their environmental footprint.Another class of new systems is based on ionic photovoltaics. Most of the conventional photovoltaic systems operate on electronic current rather than ionic current. Even though much effort has been invested into the study on ionic currents, especially in electrochemical cells,8,17–19 there has been little research on photovoltaics operating on photosensitive ions. This is because photosensitive ions mobile in the bulk are less efficient in electron transfer to electrodes than ones chemically bound to electrode surfaces. However, utilization of photosensitive ions in the bulk allows three-dimensional distribution of the photosensitizing sites, which are mostly limited to surfaces or interfaces in the conventional photovoltaic systems.20 Moreover, the photosensitive ionic compounds might provide the electrolyte medium by self-dissociation. In a pioneering work, Malliaras and coworkers have reported photovoltaic response in ionic rectifying junction.21 Two thin layers of oppositely charged photosensitive ions with mobile counter-ions are brought into contact. The diffusion of the counter-ions to the interface establishes built-in potential by which the HOMO–LUMO energy levels of two photosensitive ions are bent, leading to separation of the photogenerated electron-hole pairs and unidirectional current. This ionic junction device has established a new class of photovoltaic systems based on photosensitive ions in the bulk even if its photocurrent density has been low (0.15 μA/cm2).
Here, we demonstrate soft matter photovoltaic cells on the basis of hydrogel infused with photosensitive ions. The hydrogel, which contains 98 wt. % of water, could serve as a biocompatible and environmentally benign matrix for the new photovoltaic system. The “quasi” liquid material provides a medium where the electrochemical reaction rate and the mobility of ions are similar to those in liquid. We proved the device-building capabilities of similar water based gels earlier by demonstrating new types of gel diodes operating on ionic conductance.22,23 In the present study, two photosensitive dye molecules, DAS− and [Ru(bpy)3]2+, were used as ionic dopants of the aqueous gel bulk media of photovoltaic cells. We investigated the provisional mechanism of operation of the photovoltaic system, which includes a synergistic action of two dye molecules in photocurrent generation. To make low-cost flexible devices, an ITO coated plastic substrate and Cu electrode were used as anode and cathode, respectively. The Cu electrode was coated with carbon nanotubes, graphite and carbon black, for improving output voltage and facilitating charge transfer. Finally, a biomimetic photovoltaic cell was demonstrated by using bio-derived photosensitive materials, e.g., Photosystem II and Chlorophyll, which are well compatible with the aqueous gel based devices.
Experimental
The structure of the hydrogel based photovoltaic cells (HGPV cells) is illustrated in Fig. 1. The agarose gel matrix was prepared by the same process as in our previous publications.22,23 The gel contained 2 wt. % of agarose (biochemistry research grade, Acros Organics). Two ionic dyes were used as the photosensitive materials: 9,10-Dimethoxy-2-anthracenesulfonic acid sodium salt (DAS−Na+) and Tris(2,2′-bipyridine) dichlororuthenium(II) hexahydate ([Ru(bpy)3]2+ (Cl−)2) (Sigma Aldrich). Both dyes were dissolved in ethanol at a concentration of 5 mM. The solutions of the dyes were infused into the agarose gel layers followed by evaporation of the ethanol. The amounts of dyes were 2.5 × 10−8 mol (5 μL dye solution) per 0.009 cm3 gel layer in each of the experiments unless mentioned otherwise. The active area of the dye-infused hydrogel is ∼0.2 cm2 and the thickness of each gel layer is ∼450 μm in all devices measured. The dye-infused gel layers were sandwiched between the two electrodes. A fluorine-doped tin oxide glass (FTO glass, Pilkington) was used as the top transparent working electrode. The FTO glass was replaced with an indium tin oxide (ITO) coated poly(ethylene terephthalate) substrate (60 ohm/sq., Sheldahl) as a working electrode of a flexible device. A platinum electrode (Pt foil, Alfa Aesar) was used as a counter electrode in most of the experiments, but for low-cost devices, inexpensive Cu metal foil (3M) was used as counter electrode instead of Pt foil. | ||
| Fig. 1 Schematic of the hydrogel photovoltaic cells (HGPVs). The dyes were originally infused into two different agarose gel layers as shown here, but over time the dyes became redistributed because of diffusion in the aqueous gel. | ||
We tested multi-walled carbon nanotubes, carbon black (Cabot Corp.) and graphite (TIMCAL Ltd.) as modifiers of the Cu electrodes. The multi-walled carbon nanotubes were synthesized by a common chemical vapor deposition technique.24 All of the carbon materials were dispersed in ethanol by sonication for 90 min and coated on the Cu electrode by drop-casting. The bio-derived photosensitive materials, Chlorophyll (A type) and Photosystem II, were extracted and provided by Air Force Research Laboratory. Photosystem II was extracted and isolated from fresh spinach using a modified protocol.25 Deveined spinach was ground and washed twice in cold pH 7.4 buffer (50 mM potassium phosphate, 100 mM sucrose, and 200 mM sodium chloride). The extracted suspension was filtered and centrifuged at 12,300g for 25 min at 4 °C. The pellet was gently resuspended in cold pH 7.4 buffer. The suspension was centrifuged again and the pellet was suspended in a pH 6.9 buffer (50 mM potassium phosphate, 300 mM sucrose, and 50 mM sodium chloride). While on ice, 20% (w/v) Triton X-100 was slowly added to the suspension with gentle stirring in the dark. Photosystem II was then purified on a sucrose gradient following the procedure of Kiley et al.26 The separated Photosystem II was passed down a desalting column (Bio-rad Econo-Pac 10DG disposable column) to remove sucrose and concentrated using a microcon spin filter (Millipore, Ultracel YM-3). Chlorophyll was extracted by cold methanol from deveined freshly ground spinach leaves. The leaves were soaked in methanol for 1 h in the dark and then separated from the insoluble material by filtering with #3 Whatman filter paper. The concentration of Chlorophyll was determined by measuring the absorbance at 663 nm. The prepared Photosystem II and Chlorophyll were dissolved in water and methanol, respectively. The concentration of Chlorophyll solution was 76.44 μM and that of Photosystem II was 367.6 μM. Both were infused into the agarose gel by the same method as the dyes. The infused volume of the solution was 10 μL (7.6 × 10−10 mol of Chlorophyll and 3.6 × 10−9 mol of Photosystem II). The standard DSSC cells used for comparison in the mechanism studies were fabricated by following the process in the literature.27 The photoelectrode consisted of bi-layers of synthesized anatase TiO2 nanoparticles and large scattering particles. Ru[LL′-(NCS)2](N-719, L = 2,2′-bypyridyl-4,4′-dicarboxylic acid, L′ = 2,2′-bypyridyl-4,4′-ditetrabutylammoniumcarboxylate) was used as a dye.
The photocurrent response data as a function of time or bias voltage were collected by using a computer-controlled source meter (Keithley 2602, Keithley Instruments Inc.) under dark and illuminated conditions at room temperature. We used two different light sources: a fiber optic illuminator (∼180 mW/cm2, Fiber-Lite® High Intensity Illuminator series 180, Dolan-Jenner Industries, Inc.) for photocurrent measurement during bias sweep and a solar simulator with 300 W Xenon lamp (∼88 mW/cm2, Newport) for measurement of the photocurrent or photovoltage response as a function of time. The total intensities of the light sources were quantified using an optical power meter (model 1916-C, Newport) with thermopile detector (818P-001-12, Newport). The spectral intensity of the white light fiber optic illuminator was not verified.
Result and discussions
Initial evaluation of the photovoltaic properties
Typical current density–voltage (J–V) curves obtained from a prototype HGPV cell under dark and illuminated condition are shown in Fig. 2. The device illuminated for 12 min exhibits short circuit current density Jsc = 4.7 μA/cm2, open circuit voltage Voc = 0.41 V and fill factor FF = 0.37. The photovoltaic yield gradually increases with time. This delayed response is probably associated with the contribution of the dye ions in the bulk of the gel to the photocurrent generation. The ionic charge transport and the steady state current and voltage of photovoltaic HGPV cells are discussed in a later section. Even though the photocurrent generated by the HGPV cells is not comparable with that of the commercial Si-solar cells, the value is similar to or higher than those of other biomimetic or ionic photovoltaic systems reported recently.11,15,16,21 Moreover, the photovoltaic yield could be improved by increasing the dye concentration in the gel layers (data available in the electronic supplementary information (ESI†) Fig. S1). Our experiments indicate that the photovoltaic output is gradually saturated at dye amounts higher than 7.5 × 10−8 mol, which is presumably because above this concentration the ion transport through the gel and/or electrochemical reaction rate for dye regeneration are not rapid enough to utilize all dye molecules in the gel. | ||
| Fig. 2 Typical J–V curves of gel photovoltaic cell prototypes under dark conditions and after illumination for increasing times. The bias was swept from −0.9 V to 0.1V at a rate of 14 mV/s. The light source was a fiber optic illuminator. | ||
Provisional mechanism of photocurrent generation of HGPVs
Two dye ions, DAS− or [Ru(bpy)3]2+, may generate photocurrent by providing photoinduced electrons or holes to either FTO or Pt electrode. We investigated the effect of the dye location in the gels as a means of elucidating the mechanism by which the photosensitive materials generate current in the HGPVs. The initial location of each dye can be confined by inserting a thin film of concentrated DAS− or [Ru(bpy)3]2+ at either FTO/undyed-gel or undyed-gel/Pt interface of the HGPV device. The thin films containing concentrated dyes were prepared by drying the water and trace solvents from dye-infused gel layers overnight under ambient conditions. When these dried films situated between the undyed-gel and the electrode are rehydrated, we obtain devices where the photosensitive molecules are (initially) present in a thin gel layer near the surface of either electrode, FTO or Pt. Their photocurrent responses in terms of the position of dyes are shown in Fig. 3. Since the dyes slowly diffuse out of the concentrated subsurface layer facing the FTO or Pt into the bulk, the photocurrent at the early time stages results from the photosensitization of the dyes at the surface. Both dyes show higher photocurrent response on FTO/gel interface than on gel/Pt side, which means the dye molecules predominantly exchange ionic current at the FTO surface.20 Another interesting result is the different trend of the photocurrent response of the dyes at late time stages. The photocurrent at the late stages represents the contribution of the dyes in the bulk of the gel because of the redistribution from dye diffusion. When the dry gels containing either of the dyes are inserted at the FTO/gel interface, the photocurrent of the [Ru(bpy)3]2+ dye continuously decreases, whereas that of the DAS−dye gradually increases after 150 s (Fig. 3). Our first hypothesis related to this result is that the DAS−dye contributes to the photocurrent generation even when situated in the bulk. The excited DAS−dye may have a long enough lifetime to diffuse to the electrode and provide the electrons.20 Another explanation for the late increase of the photocurrent by the DAS−dye may be that the DAS−dye plays an additional role in the photoharvesting process such as a reducing agent for the regeneration of the oxidized dyes.![The photocurrent responses in terms of the original position of the dyes: (a) DAS− and (b) [Ru(bpy)3]2+. The light was turned on at time = 10 s. The currents level off as the diffusing dyes redistribute through the hydrogel.](/image/article/2011/JM/c0jm01820a/c0jm01820a-f3.gif) | ||
| Fig. 3 The photocurrent responses in terms of the original position of the dyes: (a) DAS− and (b) [Ru(bpy)3]2+. The light was turned on at time = 10 s. The currents level off as the diffusing dyes redistribute through the hydrogel. | ||
Based on the photocurrent responses in terms of the position of dyes, we formulated a provisional mechanism of device operation, which is presented in Fig. 4. The relative energy levels of the two dyes are estimated based on the UV-vis absorption spectra (plotted in the ESI Figure S2). DAS− has two absorption peaks at 380 nm (∼3.3 eV) and 400 nm (∼3.1 eV) and [Ru(bpy)3]2+ has a peak at 450 nm (∼2.8 eV). These data for the energy levels of [Ru(bpy)3]2+ dye are similar to the ones reported in the literature.21,28 Little research has been conducted on the energy levels of the DAS−dye. Even though the absolute energy levels of the DAS−dye are not firmly established yet, it is expected that the energy gap between the HOMO and LUMO of the DAS− is larger than that of the [Ru(bpy)3]2+ because of its shorter absorption wavelength. In the experiment on the effect of dye location, the dyes showed much higher photocurrent density at the FTO surface than the Pt surface. Both DAS− and [Ru(bpy)3]2+ dyes would generate photocurrent by absorbing light and directly injecting the excited electrons at the FTO surface (indicated by path (1) in Fig. 4). Moreover, the DAS−dye in the bulk of the gel probably contributes to the regeneration of the oxidized dye molecules by providing excited electrons (path (2) in Fig. 4). The excited DAS−dyes or the dye radicals may have relatively long lifetime, which enables the self-regeneration by the DAS−dyes in the bulk.20 The lower LUMO level of the [Ru(bpy)3]2+ dye than the DAS−dye might also facilitate the electron injection from the DAS−dye at the FTO electrode surface (path (3) in Fig. 4). The provisional mechanism suggests that the photosensitive ions work synergistically. Indeed, the HGPV devices with both DAS− and [Ru(bpy)3]2+ dyes showed photocurrent density nearly five times higher than those with either of the dyes alone (Fig. 5). Studies on spectral responses of HGPV cells and HOMO–LUMO energy levels of a DAS−dye are currently underway to verify the proposed provisional mechanism.
 | ||
| Fig. 4 Provisional mechanism for the operation of the hydrogel based photovoltaic devices. | ||
![Photocurrent densities as a function of time and original dye location. (a) DAS− was added to the device first, followed by [Ru(bpy)3]2+ addition. (b) [Ru(bpy)3]2+ was added to the device first, followed by DAS−. Both results show large increase of photocurrent densities when the other dye was introduced. The light was turned on at 10 s.](/image/article/2011/JM/c0jm01820a/c0jm01820a-f5.gif) | ||
| Fig. 5 Photocurrent densities as a function of time and original dye location. (a) DAS− was added to the device first, followed by [Ru(bpy)3]2+ addition. (b) [Ru(bpy)3]2+ was added to the device first, followed by DAS−. Both results show large increase of photocurrent densities when the other dye was introduced. The light was turned on at 10 s. | ||
Ionic mechanism of HGPV operation
The principle difference in the mechanism of operation of the HGPV cells as compared to e.g., dye-sensitized solar cells (DSSCs) is revealed by the transient current response to illumination. The slow current relaxation after external stimuli, such as bias or light, is a characteristic feature of devices operating on ionic transport in gels.22,23 To investigate the transient HGPVs response, we compared the photocurrent responses of HGPV cells with responses of standard DSSC cells (data shown in ESI,† Fig. S3). All of the dye molecules in the DSSCs are adsorbed on the electrode surface, and not present in the bulk. When the light is turned on, the DSSC current immediately rises in response to the light, while the photocurrent in the HGPV cells gradually increases after an initial current jump over periods as long as minutes. The same difference in the transient features of the two systems is also exhibited after the light is turned off. The delayed current response of the HGPV cells presumably results from the low mobility of the DAS− ions in the bulk. It takes time for the excited DAS− ions in the bulk to affect the photovoltaic properties by performing either or both functions of “photocurrent generating” and “photoreducing” agent expected in our mechanism. The delayed saturation of the photocurrent response of the DAS−dye is also observed in Fig. 5 (a).The bias sweep method, which is widely used for measurement of photovoltaic efficiency, might be imprecise in the new ion-based systems because the ionic equilibria are established slowly and the current could be affected by the rate of bias change during the measurement. To avoid the electrophoretic redistribution of the dye ions by the bias, photocurrent density and photovoltage of HGPVs were measured without application of external bias (short circuit) or external current (open circuit), respectively. Since the photovoltaic properties in HGPVs gradually increase, the current density and the voltage responses were recorded under continuous illumination until the values are saturated. As shown in Fig. 6, Jsc and Voc stabilized at 5.6 μA/cm2 and 0.4 V after 300∼400 s. Such current relaxation times are comparable to the characteristic diffusion times of ionic species through gel layers of similar thickness. Compared to the saturated Jsc and Voc values in Fig. 6, the result obtained by the bias sweep method in Fig. 2 (Jsc = 4.7 μA/cm2 and Voc = 0.41 V) seems reasonable even though the current was underestimated by ∼20%. Thus, the bias sweep method may not be highly precise, but could be still used to evaluate the photovoltaic efficiency of our HGPV cells.
 | ||
| Fig. 6 Examples of the transient characteristics of the photovoltaic properties of HGPVs: (a) photocurrent density at V = 0 V and (b) photovoltage at zero current. The arrows indicate the saturated values of Jsc and Voc. The light intensity was ∼ 88 mW/cm2. The photovoltage was recorded from four subsequent measurements under continuous illumination. The three spikes in (b) result from restarting the source meter and illustrate the slow establishment of the open circuit voltage. The light was turned on at 10 s for both measurement and was turned off at ∼400 s for Jsc measurement. | ||
Low-cost flexible HGPV devices with carbon coated copper counter electrode
The photoinduced voltage of our system depends on the energy gap between the Fermi level of the FTO glass and the work function of the counter electrode. The platinum work function of 5.65 eV is higher compared to other metals such as Ag (∼4.6 eV), Al (∼4.2 eV), Au (∼5.3 eV), Cu (∼4.7 eV). Due to its high work function, Pt turns out to be a suitable metal for the counter electrode of the HGPVs for generation of high output voltage. However, the high cost of the platinum (counter)electrodes would be a practical obstacle for the fabrication of low cost gel photovoltaic devices. To reduce the production cost, the platinum should be replaced with an inexpensive metal such as copper. This came out to be a nontrivial task. The photovoltaic performance of a device with pure copper counter electrode is compared to that with a platinum electrode in Fig. 7 (a). The Voc of the device decreased by about 0.1 V and the current density decreased more than 2× when the platinum was replaced by a copper electrode presumably because of the low work function of copper. Another disadvantage of the copper is the instability of the metal surface. The oxide layer forming on the surface of copper could increase interfacial resistance between the gel and electrode surface. The reason that Jsc of the device with the copper counter electrode is lower than that of the device with the platinum one is probably associated with higher surface resistance of the copper metal.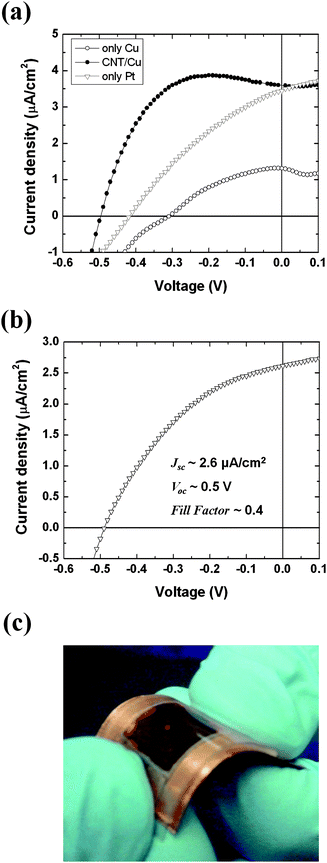 | ||
| Fig. 7 (a) Data comparison illustrating the effect of carbon nanotube coating on the photovoltaic performance compared to the one of uncoated Pt and Cu electrodes. The amount of the carbon nanotube coated on the Cu electrode was ∼0.03 mg/cm2. (b) J–V characteristics of a prototype flexible HGPV cell, where the Cu foil coated with graphite/carbon black mixture was used as a counter electrode. (c) Photograph of the real prototype flexible device with an active area ∼125 mm2. The light source was a fiber optic illuminator. All of the samples were characterized after illumination for 5 min. | ||
We found an efficient solution overcoming the Cu metal electrode drawbacks by coating the copper surface with a layer of carbon material, such as carbon nanotubes, carbon black or graphite. The coating of carbon (work function: ∼5 eV) may not only modify the work function of the copper counter electrode29,30 but may also play the role of catalyst assisting the redox reaction between the dye molecules and the counter electrode.31 The catalytic effect of the carbon could decrease the interfacial resistance by assisting the redox reaction and facilitating charge transfer at the interface. The effect of coating of multi-walled carbon nanotubes on the gel photovoltaic device characteristics is shown in Fig. 7(a). The device with the carbon nanotube coated copper counter electrode (Jsc = 3.6 μA/cm2, Voc = 0.49 V) exhibited better performance than that with the naked copper electrode (Jsc = 1.2 μA/cm2, Voc = 0.35 V). The photovoltage was improved by the modulation of the work function of copper by carbon coating. The increase of the photocurrent probably results from the catalytic effect of the carbon. The catalytic effect of the carbon could also contribute to the improvement of the photovoltage. The decrease of the charge-transfer resistance of the carbon-coated electrode could decrease the overvoltage loss on the counter electrode.32 The Voc of the device with the carbon-coated copper electrode was higher even than that with normal platinum electrode that has higher work function than the carbon. This may be explained by the additional catalytic effect of the carbon. Even though carbon nanotubes were used in the experiment, it was later confirmed that other carbon materials, including carbon black and graphite, also showed effects similar to the carbon nanotubes. Thus, carbon coatings over Cu foil electrodes could reduce the production cost without loss of the photovoltaic efficiency.
The replacement of Pt with carbon-coated copper allowed making prototypes of a new generation of inexpensive, flexible solar cells. The bulk of these HGPV devices is aqueous soft matter, agarose gel, which is mechanically flexible. The FTO glass used in the early prototypes could be easily replaced with a conductive plastic substrate. We constructed a prototype of flexible and inexpensive photovoltaic cells by using an ITO coated poly(ethylene terephthalate) substrate and a Cu/Carbon (carbon black and graphite 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture) counter electrode (Fig. 7 (b), (c)). These devices are made by a robust benchtop process, which involves mostly preparation of water-based agarose gels and is much simpler compared to the oxygen-less, ultra-clean environment that is needed to fabricate polymer solar cells. As described in the following section we could also replace the synthetic photosensitizers with biologically derived photoactive molecules.
1 mixture) counter electrode (Fig. 7 (b), (c)). These devices are made by a robust benchtop process, which involves mostly preparation of water-based agarose gels and is much simpler compared to the oxygen-less, ultra-clean environment that is needed to fabricate polymer solar cells. As described in the following section we could also replace the synthetic photosensitizers with biologically derived photoactive molecules.
Biomimetic photovoltaics with biologically derived dyes
The final goal of our work was to replace the artificial photosensitive molecules with light harvesting complexes of biological origin. The bio-complexes that we tested, such as Chlorophyll, Photosystem I and Photosystem II, are involved in photosynthesis, a “natural” solar energy conversion process. These bio-complexes are abundant in Nature, inexpensive and environmentally benign. Furthermore, the photoinduced charge separation in the bio-complexes is rapid and may exhibit high quantum efficiency.33 The water-based hydrogel of the HGPVs offers a media that might be uniquely suitable as a host of these bio-complexes. We tested Chlorophyll and Photosystem II to prove that water gel photovoltaic devices can be constructed on the basis of such biological photoactive molecules, which operate in an aqueous environment. Both bio-complexes generated comparable photocurrents of 0.2 μA/cm2, while the voltage developed by the Chlorophyll (0.16 V) was higher than the one of Photosystem II (0.07 V) (Fig. 8). Even though the photovoltaic performance of these prototype devices was not high, the yield could be improved easily in the future as the amount of the biomolecules we used was very small (7.6 × 10−10 mol of Chlorophyll and 3.6 × 10−9 mol of Photosystem II) and the devices were only first prototypes without any optimization. Such bio-complexes could be key components in environmentally benign photovoltaics and the water based HGPVs could be a promising platform for new generations of biomolecule-based photovoltaic systems. | ||
| Fig. 8 (a) Photocurrent densities and (b) photovoltage of gel-based cells operating on biological photosynthesizing complexes. The arrows indicate the time when the light turned on. | ||
Conclusions
We present a new type of photovoltaic systems based on aqueous soft gel matrix. Two ionizable dyes, DAS− and [Ru(bpy)3]2+, were infused into the water-based gel as photosensitive materials. The photosensitive ions are mobile in the “quasi” liquid HGPVs media. The provisional mechanism of the operation of the HGPVs suggests that the dye ions cooperatively work and contribute to the photocurrent generating process both on the surface of the working electrode and in the bulk of the gel. The contribution of the mobile dye ions in the bulk of the gel to the photocurrent generation resulted in a long-term transient photocurrent response. The saturated Jsc and Voc values were also measured without external bias or current to prevent the electrophoretic redistribution of the dye ions, confirming that the bias sweep method could be reasonably useful in such photovoltaic systems based on ionic currents. Overall, the photovoltaic performance of the HGPV prototypes was comparable to other biomimetic or ionic photovoltaic systems.We demonstrated that carbon-coated Cu electrodes could replace the expensive Pt counter electrodes and reduce the production cost without loss of efficiency. Photocurrent was also present when biologically derived photoactive molecules, such as Chlorophyll and Photosystem II, were embedded in aqueous gel media. Even though the photocurrent density of these devices is still low, there are many possible ways to improve the photocurrent generation. For example, working electrodes with rough surfaces provide larger area for the electron injection from the photosensitive ions.34,35 Semiconducting materials36–41 may be deposited on the electrode to improve the efficiency by radically decreasing the recombination at the photosensitive ions and the working electrode interfaces.20 Another strategy for improvement of such water-based devices is the use of medium of higher ionic conductivity. This can be achieved by using polyelectrolyte-doped gels as we recently demonstrated in diodes interfacing such gels and silica nanofilms.23 Further investigation of the precise operating mechanism of HGPV cells and improvement of performance of the biomimetic photovoltaic cells based on aqueous gel are in progress. Overall, solar cells based on such principles have the potential to be inexpensive, flexible, scalable and environmentally friendly.
Acknowledgements
This work was supported by grants from the Air Force Research Laboratory (F33615-03-D-5421, D0004) and DOE (08NT0001925). We thank Gregory Parsons and Jesse Jur for the helpful discussions.References
- D. M. Chapin, C. S. Fuller and G. L. Pearson, J. Appl. Phys., 1954, 25, 676 CAS
.
- K. L. Chopra, P. D. Paulson and V. Dutta, Progr. Photovolt.: Res. Appl., 2004, 12, 69 Search PubMed
.
- D. Wohrle and D. Meissner, Adv. Mater., 1991, 3, 129 CrossRef CAS
.
- H. Hoppe and N. S. Sariciftci, J. Mater. Res., 2004, 19, 1924 CAS
.
- C. J. Brabec, N. S. Sariciftci and J. C. Hummelen, Adv. Funct. Mater., 2001, 11, 15 CrossRef CAS
.
- B. O'Regan and M. Grätzel, Nature, 1991, 353, 737 CrossRef CAS
.
- M. Grätzel, Nature, 2001, 414, 338 CrossRef CAS
.
- M. Grätzel, Acc. Chem. Res., 2009, 42, 1788 CrossRef CAS
.
- W. H. Lai, Y. H. Su, L. G. Teoh and M. H. Hon, J. Photochem. Photobiol., A, 2008, 195, 307 CrossRef CAS
.
- N. Terasaki, N. Yamamoto, T. Hiraga, Y. Yamanoi, T. Yonezawa, H. Nishihara, T. Ohmori, M. Sakai, M. Fujii, A. Tohri, M. Iwai, Y. Inoue, S. Yoneyama, M. Minakata and I. Enami, Angew. Chem., Int. Ed., 2009, 48, 1585 CrossRef CAS
.
- P. N. Ciesielski, A. M. Scott, C. J. Faulkner, B. J. Berron, D. E. Cliffel and G. K. Jennings, ACS Nano, 2008, 2, 2465 CrossRef CAS
.
- P. Liska, N. Vlachopoulos, M. K. Nazeeruddin, P. Comte and M. Grätzel, J. Am. Chem. Soc., 1988, 110, 3686 CrossRef CAS
.
- T. N. Murakami, H. Saito, S. Uegusa, N. Kawashima and T. Miyasaka, Chem. Lett., 2003, 32, 1154 CrossRef CAS
.
- S. Mikoshiba, S. Murai, H. Sumino and S. Hayase, Chem. Lett., 2002, 31, 1156 CrossRef
.
- J. Matsui, K. Abe, M. Mitsuishi, A. Aoki and T. Miyashita, Langmuir, 2009, 25, 11061 CrossRef CAS
.
- K. Jiang, H. Xie and W. Zhan, Langmuir, 2009, 25, 11129 CrossRef CAS
.
- P. Wang, S. M. Zakeeruddin, P. Comte, I. Exnar and M. Grätzel, J. Am. Chem. Soc., 2003, 125, 1166 CrossRef CAS
.
- P. Wang, S. M. Zakeeruddin, I. Exnar and M. Grätzel, Chem. Commun., 2002, 2972 RSC
.
- K. Hara, T. Horiguchi, T. Kinoshita, K. Sayama and H. Arakawa, Sol. Energy Mater. Sol. Cells, 2001, 70, 151 CrossRef CAS
.
- R. Memming, Photochem. Photobiol., 2008, 16, 325 CrossRef
.
- D. A. Bernards, S. Flores-Torres, H. D. Abruna and G. G. Malliaras, Science, 2006, 313, 1416 CrossRef CAS
.
- O. J. Cayre, S. T. Chang and O. D. Velev, J. Am. Chem. Soc., 2007, 129, 10801 CrossRef CAS
.
- H.-J. Koo, S. T. Chang and O. D. Velev, Small, 2010, 6, 1393 CrossRef CAS
.
- R. Andrews, D. Jacques, D. Qian and T. Rantell, Acc. Chem. Res., 2002, 35, 1008 CrossRef CAS
.
-
M. Seibert, I. Yruela and R. Picorel, in Photosynthesis Research Protocols, Methods in Molecular
Biology, ed. R. Carpentier, Humana Press, Totowa, N.J., 2004, vol. 274, ch. 7, pp. 55–56 Search PubMed
.
- P. Kiley, X. Zhao, M. Vaughn, M. A. Baldo, B. D. Bruce and S. Zhang, PLoS Biol., 2005, 3, e230 CrossRef
.
- H.-J. Koo, J. Park, B. Yoo, K. Yoo, K. Kim and N.-G. Park, Inorg. Chim. Acta, 2008, 361, 677 CrossRef CAS
.
- G. C. Fiaccabrino, M. Koudelka-Hep, Y.-T. Hsueh, S. D. Collins and R. L. Smith, Anal. Chem., 1998, 70, 4157 CrossRef CAS
.
- H. Ago, K. Petritsch, M. S. P. Shaffer, A. H. Windle and R. H. Friend, Adv. Mater., 1999, 11, 1281 CrossRef CAS
.
- J. X. Geng and T. Y. Zeng, J. Am. Chem. Soc., 2006, 128, 16827 CrossRef CAS
.
- A. Kay and M. Grätzel, Sol. Energy Mater. Sol. Cells, 1996, 44, 99 CrossRef CAS
.
- G. Q. Wang, W. Xing and S. P. Zhuo, J. Power Sources, 2009, 194, 568 CrossRef CAS
.
- J. W. Lee, R. T. Collins and E. Greenbaum, J. Phys. Chem. B, 1998, 102, 2095 CrossRef CAS
.
- D. Bera, S. Patil, K. Scammon and S. Seal, Electrochem. Solid-State Lett., 2005, 8, D31 CrossRef CAS
.
- Y. Masuda, T. Ohji and K. Kato, Thin Solid Films, 2009, 518, 850 CrossRef CAS
.
- D. Kim, A. Ghicov, S. P. Albu and P. Schmuki, J. Am. Chem. Soc., 2008, 130, 16454 CrossRef CAS
.
- M. Zukalova, A. Zukal, L. Kavan, M. K. Nazeeruddin, P. Liska and M. Grätzel, Nano Lett., 2005, 5, 1789 CrossRef CAS
.
- J. Liu, Y.-T. Kuo, K. J. Klabunde, C. Rochford, J. Wu and J. Li, ACS Appl. Mater. Interfaces, 2009, 1, 1645 Search PubMed
.
- P. Ravirajan, A. M. Peiro, M. K. Nazeeruddin, M. Grätzel, D. D. C. Bradley, J. R. Durrant and J. Nelson, J. Phys. Chem. B, 2006, 110, 7635 CrossRef CAS
.
- L. Xu, Q. Chen and D. Xu, J. Phys. Chem. C, 2007, 111, 11560 CrossRef CAS
.
- A. B. F. Martinson, J. W. Elam, J. T. Hupp and M. J. Pellin, Nano Lett., 2007, 7, 2183 CrossRef CAS
.
Footnote |
| † Electronic Supplementary Information (ESI) available: Effect of dye concentration on performance, UV-Vis spectra of dyes and photocurrent responses of HGPVs and DSSCs. See DOI: 10.1039/c0jm01820a/ |
| This journal is © The Royal Society of Chemistry 2011 |
