Electroswitchable fluorescent thin film controlled by polyoxometalate†
Bin
Wang
,
Li-Hua
Bi
* and
Li-Xin
Wu
*
College of Chemistry, State Key Laboratory of Supramolecular Structure and Materials, Jilin University, Changchun, 130012, P. R. China. E-mail: blh@jlu.edu.cn; wulx@jlu.edu.cn
First published on 8th November 2010
Abstract
We have fabricated an organic/inorganic hybrid thin film based on a Dawson-type polyoxometalate P2W18O626− (P2W18) and a luminescent transition metal complex tris(2,2′-bipyridine)ruthenium [Ru(bpy)3]2+ (Rubpy) by using a layer-by-layer assembly technique, which functions as an electroswitchable fluorescent device operated by electrochemical stimulation.
Polyoxometalates (POMs) constitute an intriguing and distinctive class of inorganic metal–oxygen clusters.1 A large number of POMs with novel structures continue to be reported.2 In contrast, the development of POM properties or their application to the materials area is still limited.3 Therefore, to explore POM functional properties with well-defined structures for their extended application will be a challenge.
As is well known, POMs have become prominent candidates to be applied to materials. On one hand, thin film containing POMs have exhibited electrochromism, photochromism or fluorescence;4 on the other hand, POMs were also used as host components to encapsulate guest functional species into the materials.5 Recently, Liu and Tang prepared a photoswitchable fluorescence hybrid film based on photochromic POM K14[Na(H2O)P5W30O110] and luminescent core-shell CdSe@CdS NPs, which exhibited remarkably reversible and stable optical writing/erasing behavior under the control of UV light/visible light irradiation.6 This research suggested that POMs can be used as switches to modulate the physical and chemical properties of other molecular units by external stimulation.
Transition metal complex tris(2,2′-bipyridine)ruthenium [Ru(bpy)3]2+ (Rubpy) is an interesting functional molecule with excellent fluorescence properties, which has been the focus of extensive electrochemical and spectroscopic studies.7 Recently, Rubpy-based electrochemiluminescence sensors have been mostly studied including the immobilization of Rubpy on the electrode surface through different methods,8 among which POMs have been used as effective species to immobilize Rubpy onto the substrates via electrostatic interaction and the luminescent properties of Rubpy can be well maintained on the substrates.9,10 However, all the works on POMs and Rubpy are related to the studies of electrochemiluminescence sensors and luminescent properties as well as electrocatalytic properties.11 Strikingly, no attempt to perform a study on the electroswitchable fluorescence of Rubpy in a thin film controlled by POMs has been mentioned in the literature. Recently, we reported on an electroswitchable fluorescent thin film based on a luminescent polyoxometalate cluster.12 Herein, we firstly use POM without luminescent properties as a switch to electrochemically modulate the fluorescence of a thin film containing Rubpy for potential application in electronic devices.
P2W18 is well known to exhibit fast and reversible multi-electron redox transformations under an electric field without any significant structural alteration, which has resulted in reversible, high contrast electrochromic characteristics for thin films containing P2W18.13 In addition, the absorption band of the electroreduced P2W18 exhibits a favorable overlap with the luminescence band of Rubpy. Therefore, if both Rubpy and P2W18 are in the same thin film, the highly efficient energy transfer between luminescent Rubpy and electroreduced P2W18 will be expected to occur, which can profitably give rise to reversible luminescent switching controlled by the redox process of P2W18 under electrochemical stimulation.
With this motivation, we successfully fabricated a thin film containing P2W18 and Rubpy on ITO-coated glass slides (ITO) by layer-by-layer (LbL) assembly through alternately dipping ITO in poly(ethyleneimine) (PEI), P2W18, Rubpy and poly(styrenesulfonate) (PSS) solutions. As shown in Fig. S1,† comparing the UV-vis spectrum of the thin film with that of P2W18 and Rubpy in solution indicates that P2W18 and Rubpy have been fabricated on the thin film without any structural change. In addition, the absorbances at around 200 nm increased linearly with increasing number of layers, suggesting a nearly uniform growth of the thin film after each step. On the basis of UV-vis data, the average surface coverages (Γ) for P2W18 and Rubpy per layer are about 5.7 × 10−10 and 1.6 × 10−9 mol cm−2 respectively.4b The charges of P2W18 and Rubpy are formally −6 and +2, respectively. Thus, the Γ ratio of 2.8 and the charge ratio of 3 are similar, which signifies that the LbL process occurs stoichiometrically for each layer.
The cyclic voltammograms (CVs) of the thin film at different scan rates, P2W18 and Rubpy recorded in buffer solutions (pH = 3) are shown in Fig. S2.† It can be seen that the redox waves of the thin film are similar to those of P2W18 and Rubpy under the same conditions, which are assigned to W6+/5+ and Ru3+/2+redox processes respectively, suggesting that the electrochemical properties of P2W18 and Rubpy are fully maintained in the thin film.
The excitation spectra of solid Rubpy and {(PEI/P2W18)8(PEI/P2W18/Rubpy/PSS)5(PEI/P2W18)7} thin film on quartz are shown in Fig. 1 (curves 1 and 2). As can be seen from Fig. 1, the two spectra are identical and both have the most intense bands at ca. 460 nm assigned to the singlet metal-to-ligand (MLCT) d-π* transitions,7b indicating that the luminescent property of Rubpy is retained in the thin film. The emission spectra of the thin film and solid Rubpy are shown in Fig. 1 (curves 3 and 4), excited at 460 nm. The emission spectrum for the thin film is quite similar to that of solid Rubpy and it shows a characteristic broad band in the 550–700 nm wavelength range, originating from the π*-t2g ligand-to-metal transition of Rubpy.7b Importantly, the characteristic luminescence band of Rubpy shows a favorable overlap with the absorption band of the electroreduced species of P2W18 in the thin film (see Fig. 2), which can profitably lead to highly efficient energy transfer between Rubpy and P2W18.
 | ||
| Fig. 1 Excitation spectra of solid Rubpy (1) and the thin film (2) at 600 nm. Emission spectra of solid Rubpy (3) and the thin film (4) at 460 nm. | ||
 | ||
| Fig. 2 Absorption spectra of the thin film on ITO under open circuit (curve 1) and at applied potential of −0.9 V (curve 2) in 0.5 M Na2SO4 + H2SO4 (pH = 3); fluorescence spectrum of the thin film on ITO in 0.5 M Na2SO4 + H2SO4 (pH = 3) (curve 3). | ||
Initially, the thin film is perfectly transparent in the visible range of 400–750 nm (see curve 1 in Fig. 2). After electrochemical reduction, a new broad absorption band in the same visible range appears, which is assigned to the W5+ → W6+IVCT (intervalence charge transfer) in the thin film (see curve 2 in Fig. 2), meanwhile, the yellow thin film is changed to blue in color. According to the CV of the thin film, which undergoes multiple reversible redox steps (see Fig. S2†), the electroreduced species in the thin film were regulated by the different applied potentials and prolonged electroreduction time. It is clearly noticed from Fig. S3 and S4† that the absorbances of the reduced species increase with the increase of the reduced potentials, and the absorbance of the thin film can gradually reach saturation after sufficient electroreduction time. These observations suggest that the absorbance of the W5+ → W6+IVCT band changes in strength according to the number of absorbing species in the thin film, which can be controlled by applying different potentials or electroreduction time.
Fig. 3 shows fluorescence switching of the {(PEI/P2W18)8(PEI/P2W18/Rubpy/PSS)5(PEI/P2W18)7} thin film on ITO at an applied reduction potential of −0.9 V and oxidation of 0.9 V. It can be seen that the luminescent thin film at open circuit exhibits a fluorescence signal (curve 1) and the color of the thin film is yellow, originating from Rubpy. After reduction at an applied potential of −0.9 V for 120 s, the fluorescence of the thin film is dramatically quenched (curve 2) and the color of the thin film clearly turns to blue. Further, when the blue thin film is re-oxidized at an applied potential of 0.9 V for 120 s, the fluorescence of the thin film is recovered (curve 3) and the color of the thin film clearly returns to yellow.
 | ||
| Fig. 3 Fluorescence switching of the thin film from open circuit (curve 1), to an applied potential of −0.9 V (curve 2), to an applied potential of 0.9 V (curve 3). | ||
The stability and reversibility of recycling the potential-dependent color changes and switchable fluorescence of {(PEI/P2W18)8(PEI/P2W18/Rubpy/PSS)5(PEI/P2W18)7} thin film were investigated. It can be seen from Fig. S5,† both the response times for coloration and bleaching and the absorbances of the electroreduced species in the thin- film do not change noticeably even after 9 cycles. This observation demonstrates the stable electroreduced properties of the {(PEI/P2W18)8(PEI/P2W18/Rubpy/PSS)5(PEI/P2W18)7} thin film. In addition, as shown in Fig. 4, the changes of the fluorescence intensities of the thin film in double-potential steps are less than 2% in 9 cycles. Meanwhile, the stability of “on” and “off” fluorescence of the thin film under ambient conditions was investigated by exposure to air before the thin film was reduced and to N2 gas after the thin film was reduced. The experiment results showed that the fluorescence intensities of the “on” and “off” states of the thin film almost did not change for at least 1 month. It is confirmed that the {(PEI/P2W18)8(PEI/P2W18/Rubpy/PSS)5(PEI/P2W18)7} thin film has good stability, which is probably due to the chemical interaction between POM, PEI, Rubpy and PSS.
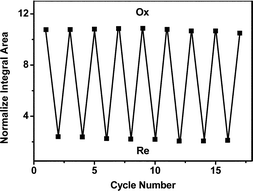 | ||
| Fig. 4 Normalized integral fluorescence area of the thin film in the double-potential cycles as a function of cycle number. All the bottom dots were detected after the thin film was reduced at an applied potential of −0.9 V, and all the top dots were measured after the thin film was oxidized at an applied potential of 0.9 V. | ||
Finally, the thin film was chosen for an electroswitching fluorescence study based on the following consideration: (i) the fluorescence intensity of the thin film can be adjusted by altering the number of Rubpy layers in the thin film; (ii) the optimization of the efficiency of electroswitching fluorescence can be manipulated by controlling the relative concentrations of P2W18 and Rubpy in the same thin film, namely, changing the relative number of P2W18 and Rubpy layers in the thin film. According to our attempt, a {(PEI/P2W18)8(PEI/P2W18/Rubpy/PSS)5(PEI/P2W18)7} thin film was finally chosen for our study. In addition, during our experiments we found that the concentration of P2W18 is an essential factor for the luminescence quenching of the thin film. Therefore, it is concluded that the dominant luminescence-quenching mechanism results from the intermolecular fluorescence resonance energy transfer (FRET) between the electroreduced species of P2W18 and luminescent Rubpy, which is similar to that of a photoswitchable fluorescence thin film containing CdSe@CdS NPs and K14[Na(H2O)P5W30O110].6
In summary, a luminescent Rubpy-based thin film of {(PEI/P2W18)8(PEI/P2W18/Rubpy/PSS)5(PEI/P2W18)7} on a solid substrate has been successfully fabricated by using the layer-by-layer self-assembly method and the electroactive and luminescent properties of Rubpy and P2W18 are well maintained in the thin- film. Further investigations showed the as-prepared thin film can be electroreduced resulting in a color change from yellow to blue, which is due to the formation of the colored reduced species of P2W18 in the thin film. Coloration and bleaching of the thin film occurred very quickly and were reversible with a long-time stability. In addition, the fluorescence of the thin film can be switched under electrochemical stimulation. At the same time, the changes of absorbences and luminescent intensities of the thin film were observed by repeated switching of the applied potentials. Therefore, the composite thin film shows electroswitchable fluorescence behavior under electrochemical stimulation, which provides a promising way to develop thin film materials with controlled functionality based on POMs.
The authors are grateful for the financial support from the National Basic Research Program of China (2007CB808003), the National Natural Science Foundation of China (21073076), the Technology of Jilin Province, China (20090592), the Open Projects of State Key Laboratory of Electroanalytical Chemistry of the Chinese Academy of Sciences (SKLEAC2010002), CAS, and 111 Project (B06009) for the visit, and helpful discussions with Prof. Ulrich Kortz at Jacobs University.
Notes and references
- (a) X. K. Fang, M. Speldrich, H. Schilder, R. Cao, K. P. O'Halloran, C. L. Hill and P. Kögerler, Chem. Commun., 2010, 46, 2760 RSC; (b) J. D. Compain, P. Mialane, A. Dolbecq, I. M. Mbomekallé, J. Marrot, F. Sécheresse, C. Duboc and E. Rivière, Inorg. Chem., 2010, 49, 2851 CrossRef CAS.
- (a) L. H. Bi, B. Wang, G. F. Hou, B. Li and L. X. Wu, CrystEngComm, 2010, 12, 3511 RSC; (b) B. Nohra, P. Mialane, A. Dolbecq, E. Rivière, J. Marrot and F. Sécheresse, Chem. Commun., 2009, 2703 RSC; (c) H. Y. Zhang, Y. Q. Lan, G. S. Yang, X. L. Wang, K. Z. Shao, G. J. Xu and Z. M. Su, CrystEngComm, 2010, 12, 434 RSC.
- (a) S. Q. Liu and Z. Y. Tang, Nano Today, 2010, 5, 267 CrossRef CAS; (b) Z. L. Wang, R. L. Zhang, Y. Ma, L. D. Zheng, A. Peng, H. B. Fu and J. N. Yao, J. Mater. Chem., 2010, 20, 1107 RSC; (c) C. M. Granadeiro, R. A. S. Ferreira, P. C. R. Soares-Santos, L. D. Carlos, T. Trindade and H. I. S. Nogueira, J. Mater. Chem., 2010, 20, 3313 RSC; (d) Z. L. Wang, R. L. Zhang, Y. Ma, A. D. Peng, H. B. Fu and J. N. Yao, J. Mater. Chem., 2010, 20, 271 RSC; (e) Y. Y. Zhao, H. M. Fan, W. Li, L. H. Bi, D. J. Wang and L. X. Wu, Langmuir, 2010, 26, 14894 CrossRef CAS.
- (a) L. H. Bi, W. H. Zhou, J. G. Jiang and S. J. Dong, J. Electroanal. Chem., 2008, 624, 269 CrossRef CAS; (b) S. Q. Liu, H. Mohwald, D. Volkmer and D. G. Kurth, Langmuir, 2006, 22, 1949 CrossRef CAS; (c) T. R. Zhang, S. Q. Liu, D. G. Kurth and C. F. J. Faul, Adv. Funct. Mater., 2009, 19, 642 CrossRef CAS; (d) P. Mialane, G. J. Zhang, I. M. Mbomekallé, P. Yu, J. D. Compain, A. Dolbecq, J. Marrot, F. Sécheresse, B. Keita and L. Nadjo, Chem. Eur. J., 2010, 16, 5572 CAS.
- (a) Z. X. Sun, L. Xu, W. H. Guo, B. B. Xu, S. P. Liu and F. Y. Li, J. Phys. Chem. C, 2010, 114, 5211 CrossRef CAS; (b) S. Y. Yin, H. Sun, Y. Yan, W. Li and L. X. Wu, J. Phys. Chem. B, 2009, 113, 2355 CrossRef CAS; (c) L. H. Bi, K. Foster, T. McCormac and E. Dempsey, J. Electroanal. Chem., 2007, 605, 24 CrossRef CAS.
- B. Qin, H. Y. Chen, H. Liang, L. Fu, X. F. Liu, X. H. Qiu, S. Q. Liu, R. Song and Z. Y. Tang, J. Am. Chem. Soc., 2010, 132, 2886 CrossRef CAS.
- (a) M. Goral, T. McCormac, E. Dempsey, D. L. Long, L. Cronin and A. M. Bond, Dalton Trans., 2009, 6727 RSC; (b) L. H. Bi, B. Li, S. Bi and L. X. Wu, J. Solid State Chem., 2009, 182, 1401 CrossRef CAS.
- (a) L. H. Zhang, F. Tsow, E. Forzani and N. J. Tao, Chem. Commun., 2010, 46, 3333 RSC; (b) L. B. Zhang, J. Li, Y. H. Xu, Y. M. Zhai, Y. H. Li and E. K. Wang, Talanta, 2009, 79, 454 CrossRef CAS.
- T. Dong, H. Y. Ma, W. Zhang, L. H. Gong, F. P. Wang and C. X. Li, J. Colloid Interface Sci., 2007, 311, 523 CrossRef CAS.
- H. Y. Ma, J. Peng, Y. H. Chen, Y. H. Feng and E. B. Wang, J. Solid State Chem., 2004, 177, 3333 CrossRef CAS.
- (a) L. H. Bi, H. Y. Wang, Y. Shen, E. K. Wang and S. J. Dong, Electrochem. Commun., 2003, 5, 913 CrossRef CAS; (b) L. H. Bi, W. H. Zhou, H. Y. Wang and S. J. Dong, Electroanalysis, 2008, 20, 996 CrossRef CAS.
- B. Wang, Z. D. Yin, L. H. Bi and L. X. Wu, Chem. Commun., 2010, 46, 7163 RSC.
- B. B. Xu, L. Xu, G. G. Gao and Y. N. Jin, Appl. Surf. Sci., 2007, 253, 3190 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: CVs of P2W18 and Rubpy containing solutions and thin film under the same experimental conditions. UV-vis spectra of P2W18 and Rubpy containing solutions and thin film under the same experimental conditions. Visible spectra of thin film at different applied potentials and times. Potential currents and absorbances at 627 nm of the thin film during subsequent double-potential steps from −0.8 to 0.8 V in pH 3 solution. See DOI: 10.1039/c0jm03140b |
| This journal is © The Royal Society of Chemistry 2011 |
