Arsenic speciation in wheat and wheat products using ultrasound- and microwave-assisted extraction and anion exchange chromatography-inductively coupled plasma mass spectrometry†
Marilena
D'Amato
,
Federica
Aureli
,
Silvia
Ciardullo
,
Andrea
Raggi
and
Francesco
Cubadda
*
Department of Food Safety and Veterinary Public Health, Istituto Superiore di Sanità, Viale Regina Elena 299, 00161, Rome, Italy. E-mail: francesco.cubadda@iss.it; Fax: +39 06 49902540
First published on 1st December 2010
Abstract
Wheat appears to be the major contributor to the intake of inorganic arsenic in countries where the diet is not rice-based. Ultrasound- and microwave-assisted extraction of arsenic in wheat and wheat based food using different solvents or enzymes was investigated in terms of extraction yield and species stability. Four extraction procedures were selected for the study of arsenic speciation in wheat and wheat products by anion exchange HPLC-ICP-MS using a PRP-X100 column with 10 mM NH4H2PO4, 10 mM NH4NO3, and 2% CH3OH at pH 5.5 as the mobile phase. Total arsenic in the samples ranged from 8.6 to 29.8 ng g−1 dry weight. About 95% of the arsenic was found to be present in inorganic form with AsIII as the most abundant species, whereas the remainder was mainly DMA. Microwave-assisted extraction with HNO3 was the most effective in liberating the arsenic species, which were then satisfactorily recovered from the chromatographic column. The LODs achieved, i.e., 0.35–0.46 ng g−1 dry weight, were suitable for the determination of arsenic species at the low levels found in sample extracts.
Introduction
Inorganic As is an environmental and food-chain contaminant, which is carcinogenic to humans following chronic oral exposure.1 Concerns about dietary exposure to inorganic As led to recent assessments of As in food by the European Food Safety Authority (EFSA)2 and the Joint FAO/WHO Expert Committee on Food Additives (JECFA).3 As a result, the provisional tolerable weekly intake (PTWI) of 15 μg kg−1 b.w. previously established by JECFA was withdrawn as data had shown that inorganic As causes cancer of the lung and urinary bladder in addition to skin, and that a range of adverse effects had been reported at exposures lower than those reviewed at the time the PTWI was established. Based on dose-response data from epidemiological studies, a benchmark dose lower confidence limit (BMDL) for a 0.5% increased incidence of lung cancer of 3.0 μg kg−1 b.w. per day was computed by the JECFA,3 whereas the EFSA identified a range of BMDL values between 0.3 and 8 μg kg−1 b.w. per day for 1% extra risk of cancers of the lung, skin and bladder, as well as skin lesions.2 It was recommended that dietary exposure to inorganic As should be reduced and both the EFSA and the JECFA called for more speciation data for different food commodities to support dietary exposure assessment.For populations not exposed to As-contaminated drinking water, food is the major contributor to the intake of inorganic As.2,3 Rice attracted a lot of interest as a source of inorganic As owing to the relatively high As levels and As speciation in rice has been extensively investigated.4–13 Whereas rice contributes substantially to the human intake of inorganic As in a rice-based diet, in Europe and other Western countries the diet is mainly based on wheat, which represents at least 70% of total cereal consumption compared to ∼5% of rice.14,15 Cereal grains and cereal based products excluding rice have been identified as largely contributing to the inorganic As daily exposure in the general European population, followed by food for special dietary uses, bottled water, coffee and beer, rice grains and rice based products, fish and vegetables.2 However, due to the lack of speciation data, exposure assessment was made assuming various inorganic-to-total As ratios, with 70% of inorganic As considered as the most likely scenario. If this inorganic-to-total As ratio can be a reasonable assumption for rice, which typically has 50–60% of the total As in inorganic form2—even though wide variations are observed depending on sample provenance16—As speciation in wheat has not yet been thoroughly investigated. Inorganic As only was found to be present in a study that focused exclusively on contaminated samples owing to the limited detection power of the analytical method.17 In an earlier study on wheat with ‘normal’ As levels (<0.030 μg As g−1), we did detect methylated species at low concentrations in some samples, but inorganic As accounted for 95% of the chromatographed As on average with AsIII as the dominating species.18 Limited and conflicting information for wheat based-products is available in the literature, with a wide range of speciated As reported to be in inorganic form (28–100%).17,19–21
Owing to its detection power, HPLC-ICP-MS appears to be a suitable analytical method for the determination of As species in wheat grain and related products, which has total As levels about ten times lower than rice.17,18 Both anion exchange4,5,7,9–13 and ion-pairing8,22 have been reported for the chromatographic separation of the charged water-soluble species present in cereal grains before on-line selective detection of As by ICP-MS.
AsIII is likely to be bound to thio groups in peptides or proteins in the wheat kernel, as recently shown for rice.23 Extraction of As species from cereal grains and derived products has been carried out with different chemical and enzymatic methods. Use of mixtures of methanol–water along with sonication is a mild method, which however requires long times and use of high reagent volumes to achieve good extraction efficiencies.5 Forcing extraction methods have been developed to maximise extraction yields and shorten sample treatment. Trifluoroacetic acid has been used in several studies to extract inorganic As species from rice.4,6 This treatment can at least partially reduce AsV to AsIII, so a combined inorganic As value has been reported in those studies. Other acids21 and especially microwave-assisted extraction with HNO3 have been increasingly used subsequently.8,9,12,13 Enzymatic extractions have been also used in many studies, in most cases with ultrasound- and microwave-assisted procedures.6,7,10,22 However, systematic studies on wheat and products have not yet been carried out with any of these methods.
The aim of the present study was two-fold. Firstly, to identify conditions for sample treatment that are sufficiently forcing to extract the majority of the As in the sample without degrading the As species. Secondly, to study As speciation by anion exchange HPLC-ICP-MS in view of the relevance of wheat and wheat products for risk assessment of dietary inorganic As.
Experimental
Instrumentation
An Elan DRC II ICP mass spectrometer (PerkinElmer, Norwalk, CT, USA) was used for total As determinations and as element selective detector for As speciation. Chromatographic separations were performed using a PerkinElmer Series 200 metal-free HPLC system. The exit of the column was directly connected by means of PEEK capillary tubing to the Meinhard quartz concentric nebulizer of the ICP-MS, used in conjunction with a PC3 Peltier-cooled quartz cyclonic spray chamber (Elemental Scientific Inc., Omaha, NE) set at 2 °C.Sample handling was carried out in a laminar flow box (Spetec GmbH, Erding, Germany). Wheat grain and pasta were ground using a RM100 automatic agate pestle mill (Retsch GmbH & Co., Haan, Germany) for 2 h and 30 min, respectively. White bread was homogenized in a B-400 mixer (BÜCHI, Flawil, Switzerland), lyophilized at −50 °C using a LyoLab 3000 system (Heto-Holten A S−1, Alleroed, Denmark), and then ground with the automatic agate pestle mill for 30 min. Wholemeal flour obtained from wheat grain was sieved through a 500 μm mesh sieve (Retsch GmbH & Co., Haan, Germany). Residual humidity was determined using a DL-53 oven with natural convection (VWR International, Milan, Italy) in order to express analytical results on a dry weight basis. Microwave assisted extraction and digestion of samples were carried out by means of a Milestone Ethos E microwave labstation (FKV, Bergamo, Italy) equipped with a high-pressure 10-vessel rotor and high-purity quartz Q-20 rotor (FKV, Bergamo, Italy), respectively. Ultrasound assisted extractions were carried out by means of either an ultrasonic bath (VWR International, Milan, Italy) or a Sonopuls HD3200 ultrasonic probe (Bandelin Electronic GNBH & Co. KG, Berlin, Germany). A Zymark TurboVap II Workstation (FKV, Bergamo, Italy) was used to concentrate sample extracts.
Samples
A composite sample of wheat grain was obtained by pooling individual samples (n = 8) of different varieties grown in Italy. White bread and pasta samples were similarly obtained by pooling individual samples collected at retail (n = 6 and 3, respectively). Wheat- and rice-based certified reference materials were RM 8436 Durum Wheat Flour and SRM 1568a Rice Flour, obtained by NIST (Gaithersburg, MD, USA), and BCR CRM 189 Wholemeal flour, obtained by IRMM (Geel, Belgium).Chemicals and standard substances
Analytical reagent grade chemicals and ultrapure deionized water obtained by a Milli-Q Element System (Millipore, Molsheim, France) were used throughout unless stated otherwise. Ultrapure grade nitric acid (70% v/v) (Carlo Erba Reagenti, Rodano, Italy) and Suprapur® hydrogen peroxide (30% v/v) (Merck KGaA, Darmstadt, Germany) were used. Calibrants and the rhodium internal standard solution used for total As measurements were obtained from standard certified solutions of 1 mg mL−1 (High Purity Standard, Charleston, SC) by dilution with acidified (HNO3) water as necessary. Stock solutions of 1 mg mL−1, expressed as As, were prepared by dissolving in water adequate amounts of disodium hydrogen arsenate heptahydrate, dimethylarsinic acid (both from Fluka, Dorset, UK), and methylarsonic acid (from Tri Chemical Laboratories Inc., Yamanashi, Japan). Arsenic trioxide (Fluka, Dorset, UK) was dissolved in KOH (0.5% w/w). Stock solutions were stored at −20 °C and the exact concentrations were ascertained by ICP-MS analysis. Working standard solutions were prepared daily and their purity for speciation analysis was checked by HPLC-ICP-MS. For enzymatic extractions, α-amylase from Bacillus subtilis (Fluka, Seelze, Germany) and bacterial protease type XIV from Streptomyces griseus (Sigma-Aldrich, St. Louis, MO) were used. For the preparation of the mobile phase ammonium dihydrogen phosphate (99.99% Suprapur®), ammonium nitrate (pro analysis), ammonia solution (25% Suprapur®), and methanol (HPLC grade) (all from Merck KGaA, Darmstadt, Germany) were used. The mobile phase was degassed and filtered (0.22 μm) before use.Procedures
Procedure A (CH3OH–H2O extraction with sonication) – Samples were added with 5 mL of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) methanol–water mixture and sonicated at 50 °C for 2 h in an ultrasonic bath. The extraction procedure was repeated five times5 including an overnight step performed under mechanical agitation. After each extraction, samples were centrifuged (10 min, 8000 rpm, 4 °C). The supernatants were combined, concentrated to 5 mL (40 °C) and filtered through a 0.22 μm filter.
1 (v/v) methanol–water mixture and sonicated at 50 °C for 2 h in an ultrasonic bath. The extraction procedure was repeated five times5 including an overnight step performed under mechanical agitation. After each extraction, samples were centrifuged (10 min, 8000 rpm, 4 °C). The supernatants were combined, concentrated to 5 mL (40 °C) and filtered through a 0.22 μm filter.
Procedure B (H2O extraction with ultrasonic probe) – Samples were added with 10 mL of water and treated with an ultrasonic probe for 3 min at 95 W. The extraction procedure was repeated three times. The extracts were centrifuged, concentrated and filtered as described in procedure A.
Procedure C (Microwave-assisted HNO3 extraction) – Samples were added with 10 mL of 0.16 M HNO3 and left to stand overnight. Microwave irradiation was performed with the following temperature profile: 3 min ramp to 55 °C, 10 min at 55 °C, 2 min ramp to 75 °C, 10 min at 75 °C, 2 min ramp to 95 °C, 30 min at 95 °C. The extracts were centrifuged (10 min, 8000 rpm, 4 °C) and the supernatants filtered through a 0.22 μm filter.
Procedure D (Microwave-assisted enzymatic extraction) – Samples were added with 10 mL of water and 20 mg of α-amylase. The microwave program was as follows: 3 min ramp to 37 °C, 40 min at 37 °C. The extracts were treated as described above.
| Chromatographic conditions | |
| Column | PRP-X 100, 250 × 4.6 mm, 5 μm, PEEK (Hamilton Company, Reno, Nevada, USA) |
| Temperature | 23 °C |
| Injection volume | 100 μL |
| Mobile phase | 10 mM NH4H2PO4, 10 mM NH4NO3, 2%(v/v) CH3OH, pH 5.5 adjusted with NH4OH |
| Flow rate | 1.0 mL min−1 |
| Elution | Isocratic, 12 min |
| ICP-MS parameters | |
| RF power | 1.5 kW |
| Plasma Gas flow, Ar | 15.5 L min−1 |
| Aux Gas flow, Ar | 1.20 L min−1 |
| Nebulizer Gas Flow, Ar | 1.02 L min−1 |
| Dwell time | 300 ms |
In the absence of a reference material with certified values of As species, SRM 1568a was used for quality control in speciation analysis too. The concentration of As species is not certified in this material, however it has been previously used in several speciation studies. The material was extracted with procedure C and found to contain 51.9 ng As g−1 of AsIII, 39.6 ng As g−1 of AsV, 187.6 ng As g−1 of DMA, 12.5 ng As g−1 of MMA and 1.6 ng As g−1 of an unknown compound, from which it could be calculated that 31% and 64% of the chromatographed As were present as inorganic As and DMA, respectively, in agreement with literature data.11,21 The sum of the As species (0.292 μg As g−1) amounted to 101% of the extracted As, as measured by analysing digested samples extracts, and compared well with the certified total As value (0.29 μg As g−1).
Results and discussion
Selection of the extraction procedures
Several extraction procedures were initially screened for their ability to dissolve the As present in the different wheat-based samples studied. Chemical and enzymatic extractions, either ultrasound-assisted or MW-assisted, were investigated (Table 2). Wheat contains ∼60% of starch and 12–13% of proteins on a fresh weight basis on average,24 hence α-amylase and protease XIV were considered for enzymatic extraction. Both enzymes were checked for As impurities and the analysed batches of protease XIV were all found to be severely contaminated with AsV (mean 0.48 μg g−1, range 0.32–0.56 μg g−1, n = 4). Therefore the use of the latter enzyme was excluded. Water, methanol–water, nitric acid were used as solvents in chemical extractions. For each extraction procedure, both the extracts and the residues were MW digested and analysed for total As in order to check mass balances. The procedures named A–D in Experimental showed extraction yields >70% for the majority of the samples and were thus selected for the study. Sample weight was also optimised at this stage and 0.35 g was chosen for all of the selected extraction procedures.| a 30 W, 75 W, 95 W tested. b 400 W, different programs tested with duration 38–57 min and maximum temperature 90–95 °C. |
|---|
| Ultrasound-assisted |
| CH3OH–H2O with sonication (5 steps, including 1 under mechanical agitation overnight) |
| H2O with ultrasonic probe (3–5 steps)a |
| CH3OH–H2O with ultrasonic probe (1 step H2O, 2 steps CH3OH)a |
| CH3OH–H2O + α-amylase with ultrasonic probe (1 step H2O + α-amylase, 2 steps CH3OH)a |
| Microwave-assisted |
| HNO3 extractionb |
| H2O + α-amylase |
Spike recovery with the selected extraction procedures
In order to further characterize the extraction procedures A–D, spiking experiments were carried out by adding AsIII, AsV, DMA, and MMA to a wheat flour sample before extraction. Duplicates of durum wheat flour (RM 8436) were spiked with 17.5 ng As of the As species. They were compared with duplicates of the same material without spikes in order to calculate the spike recovery and the results are shown in Fig. 1. Average recovery of the four As compounds with procedure A, C, and D was 100% (range 97–103%), 100% (98–102%), and 99% (95–103%), respectively, whereas it was 94% (range 92–95%) with procedure B (significantly different, p < 0.01). On average, the recoveries of AsIII, AsV, DMA and MMA with the four extraction procedures were 99% (range 95–103%), 98% (92–101%), 97% (94–101%), and 100% (95–103%), respectively.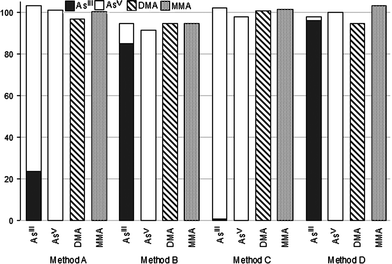 | ||
| Fig. 1 Recovery of AsIII, AsV, DMA and MMA added to wheat flour samples (RM 8436 durum wheat flour) (n = 3). | ||
Species interconversions in the spiking experiments
As shown in Fig. 1, added AsV, DMA and MMA were recovered as such after extraction and chromatography. However, all extraction procedures caused a more or less pronounced oxidation of AsIII to AsV. Redox transformation of AsIII and AsV was minimal with procedure D, following which 96% of AsIII was recovered as such and only 2% was oxidized to AsV. Procedure B yielded 85% AsIII and 10% AsV, whereas with procedures A and C only 24% and 0.5% of added AsIII remained unchanged, respectively.It has been recently reported that preservation of AsIII and AsV speciation during HNO3 extraction of rice grains occurs at a narrow range of acid concentrations, i.e., 0.28–0.70 M.13 Spiking experiments showed AsIII oxidation at HNO3 concentrations >0.70 M and AsV reduction at HNO3 concentrations <0.28 M, the latter attributed to free thiols released by acidic hydrolysis of rice.13 We did find oxidation of AsIII added to wheat flour during extraction using a HNO3 concentration of 0.16 M, which may be due to matrix differences between rice and wheat and most likely also to the potassium bromate present in the flour used as the source of material for RM 8436.25 Potassium bromate, a strong oxidizing agent, can be added to flour in some countries as flour improver. It has to be noted that interconversion of the inorganic As species was higher with the procedures A and C which implied a longer contact time between the matrix and the extractant.
Figures of merit of the HPLC-ICP-MS method
A chromatogram of a mixture of standards at a concentration of 1.5 ng mL−1 (as As) in Fig. 2 shows complete separation of the analytes within ∼10 min. The sensitivity of AsV, DMA and MMA was approximately the same, i.e., 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 cps per ng mL−1 (100 pg as As). For AsIII the sensitivity was higher, ca. 35
500 cps per ng mL−1 (100 pg as As). For AsIII the sensitivity was higher, ca. 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cps per ng mL−1, possibly due to coelution with components of the mobile phase giving rise to matrix effects resulting in signal enhancement.
000 cps per ng mL−1, possibly due to coelution with components of the mobile phase giving rise to matrix effects resulting in signal enhancement.
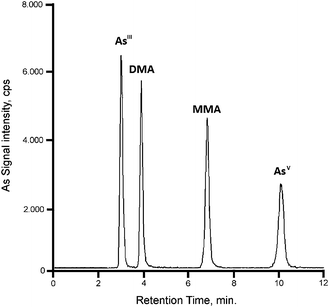 | ||
| Fig. 2 HPLC-ICP-MS chromatogram of a mixture of standards at a concentration of 1.5 μg L−1 as As. | ||
Precision as average intra-day repeatability was 2.7%–5.5% for the four As species, based on 3 consecutive injections of the same sample submitted to procedure A. Average inter-day repeatability was 4.9%–5.7%, based on measurements of the same sample (submitted to procedure A) on 3 consecutive days. Repeatability of the retention times was 0.09%–0.14%, calculated on the basis of six consecutive injections. Limits of detection (LODs), calculated as the mean of the background signal of ten method blanks at the retention time of each species plus three times the standard deviation, are shown in Table 3.
| Method | AsIII | AsV | DMA | MMA |
|---|---|---|---|---|
| A | 0.18 | 0.54 | 0.19 | 0.17 |
| B | 0.19 | 0.51 | 0.19 | 0.20 |
| C | 0.39 | 0.46 | 0.35 | 0.41 |
| D | 0.36 | 0.40 | 0.38 | 0.42 |
Quantification of As species in selected real samples was done using either external calibration or standard addition. The results compared well (within 1–8% for the four species), thus five point calibration curves obtained with external standards (0.25, 0.5, 1, 2.5, 5 ng mL−1, R2 ≥ 0.9999) were used for quantification.
Mass balance
Fig. 3 shows the HPLC-ICP-MS chromatograms obtained for the wheat grain sample extracted with the four different procedures A–D. A major peak corresponding to AsIII appears, followed by AsV as the next abundant species and DMA as a minor compound. Similar chromatograms were obtained for wheat flour, bread and pasta. The quantitative results are summarized in Table 4 along with data on chromatographed As, extracted As, total As and the percentage of the detected species in inorganic form.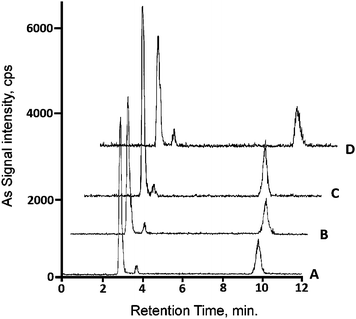 | ||
| Fig. 3 HPLC-ICP-MS chromatograms of extracts of the same wheat grain sample (composite sample of wheat grain obtained by pooling 8 individual samples) submitted to extraction procedures A–D. Chromatograms vertically and horizontally offset for easier evaluation of elution profiles. | ||
| Sample | Extraction procedure | Concentration/ng g−1 | i-As (%) | AsIII/AsV (%) | Extracted As/ng g−1 | Σ detected/Extracted As (%) | Total As/ng g−1 | Extracted As/Total As (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AsIII | AsV | DMA | MMA | Σ detected | ||||||||
| a Composite sample of wheat grain obtained by pooling 8 individual samples. b RM 8436 durum wheat flour. c Composite sample of white bread obtained by pooling 6 individual samples. d Composite sample of pasta obtained by pooling 3 individual samples. | ||||||||||||
| Wheat graina | A | 13.6 (0.7) | 6.50 (0.29) | 0.46 (0.06) | n.d. | 20.6 (0.9) | 98 | 68 | 22.8 (3.0) | 90 | 29.8 (0.7) | 77 |
| B | 10.7 (0.9) | 5.71 (0.45) | 1.01 (0.05) | n.d. | 17.4 (0.5) | 94 | 65 | 21.6 (0.4) | 81 | 73 | ||
| C | 18.3 (0.3) | 9.48 (0.31) | 0.83 (0.06) | n.d. | 28.6 (0,7) | 97 | 66 | 29.8 (1.0) | 96 | 100 | ||
| D | 8.25 (0.03) | 5.48 (0.20) | 0.39 (0.01) | n.d. | 14.1 (0.2) | 97 | 60 | 24.9 (2.0) | 57 | 84 | ||
| Wheat flourb | A | 1.21 (0.20) | 7.23 (0.08) | 0.51 (0.09) | 0.18 (0.04) | 9.12 (0.80) | 93 | 14 | 10.0 (0.3) | 91 | 13.2 (1.3) | 76 |
| B | 3.18 (0.09) | 2.70 (0.25) | 0.94 (0.19) | <LOD | 6.82 (0.60) | 86 | 54 | 7.77 (1.40) | 88 | 59 | ||
| C | <LOD | 10.9 (0.6) | 0.58 (0.10) | n.d. | 11.4 (0.7) | 95 | ∼0 | 13.1 (0.9) | 87 | 99 | ||
| D | 2.16 (0.44) | 1.69 (0.30) | 0.40 (0.08) | n.d. | 4.26 (0.79) | 91 | 56 | 5.58 (0.44) | 76 | 42 | ||
| Breadc | A | 2.52 (0.08) | 1.67 (0.09) | 0.24 (0.02) | n.d. | 4.43 (0.03) | 95 | 60 | 5.07 (0.23) | 87 | 8.57 (0.02) | 59 |
| B | 2.85 (0.02) | 1.89 (0.08) | 0.45 (0.10) | n.d. | 5.20 (0.21) | 91 | 60 | 8.60 (0.47) | 60 | 100 | ||
| C | 2.75 (0.20) | 2.52 (0.40) | <LOD | n.d. | 5.27 (0.42) | 100 | 52 | 7.67 (0.41) | 69 | 90 | ||
| D | 3.20 (0.62) | 1.74 (0.31) | n.d. | n.d. | 4.94 (0.98) | 100 | 65 | 8.04 (1.26) | 61 | 94 | ||
| Pastad | A | 4.94 (0.03) | 2.63 (0.14) | 0.22 (0.03) | n.d. | 7.79 (0.14) | 97 | 65 | 7.89 (0.31) | 99 | 8.60 (0.03) | 92 |
| B | 4.76 (0.28) | 2.90 (0.30) | 0.34 (0.04) | n.d. | 7.99 (0.62) | 96 | 62 | 8.58 (1.02) | 93 | 100 | ||
| C | 5.70 (0.05) | 2.90 (0.15) | <LOD | n.d. | 8.60 (0.18) | 100 | 66 | 8.67 (0.64) | 99 | 101 | ||
| D | 4.62 (0.44) | 2.39 (0.02) | n.d. | n.d. | 7.01 (0.35) | 100 | 66 | 8.15 (1.05) | 86 | 95 | ||
Overall, speciation analysis was simple for pasta in terms of both extraction and chromatographic detection, notwithstanding the low As concentration (see the ratios extracted/total As and detected/extracted As with the various extraction procedures, Table 4). Whole wheat grain was an item of intermediate difficulty, whereas wheat flour and bread posed more problems, probably due to their salt content resulting from food processing. As far as the different extraction procedures are concerned, the highest efficiency was obtained with procedure C, which solubilised 97% of total As on average. Furthermore, the extracted As eluted almost entirely form the chromatographic column, with the partial exception of bread. Methods A, B and D extracted on average 76%, 83% and 79% and showed average post-column recoveries of 92%, 81% and 70%, respectively. Therefore, the sum of the detected species accounted for 86% of total As with method C on average, whereas the corresponding figures with method A, B, and D were 70%, 66%, and 55%, respectively. These mass balances are lower than those obtained in the spiking experiments described above, which highlights that in speciation analysis the capability of a method entailing sample extraction and chromatographic detection can be assessed by spiking with standards only to a limited extent.
Arsenic species in wheat and wheat based food
By averaging the results obtained with the different extraction procedures, inorganic As was found to account for 97%, 91%, 96% and 98% of the sum of As species in whole grain, flour, bread and pasta, respectively (mean 96%). DMA was detected as a minor species in all samples, whereas traces of MMA where detected in the flour sample only. The percentage of As in inorganic form was calculated in a more meaningful way using the maximum amount of AsIII + AsV, DMA and MMA detected in each sample with the various extraction procedures and the sum of the species resulting thereof. With this approach, inorganic As accounted for 96%, 91%, 92% and 96% of the sum of As species in whole grain, flour, bread and pasta, respectively (mean 94%). The same calculation for DMA led to 4%, 8%, 8% and 4% of the sum of As species in whole grain, flour, bread and pasta, respectively (mean 6%). The finding that almost the entirety of arsenic in wheat and wheat based food is present in inorganic form is in agreement with the studies where extraction efficiency and species recovery were comprehensively documented; these studies investigated items covering a tenfold range of As concentrations.17,18,21AsIII is more toxic than AsV26 and was found to be the most abundant species in all samples, except RM 8436 (Table 4). For the latter sample interpretation is less straightforward, since almost only AsV was found with the extraction procedure C, which caused the greatest oxidation of AsIII to AsV in spiking experiments, whereas AsIII was predominant with extraction procedures B and D, which were less prone to AsIII oxidation but extracted far less As (42–59%). The different AsIII to AsV ratio in this sample compared to the others was most likely due to the presence of potassium bromate.25 In general terms, the finding that AsIII predominates over AsV in wheat and wheat based food is in agreement with the literature.17–19,21
Unlike a recent report that found AsV reduction during HNO3 extraction of rice grains with acid concentrations <0.28 M, we did not observe any obvious difference between procedure C and the other extraction methods as to the ratio of extracted AsIII and AsV in wheat and wheat products (except for the RM 8436, as explained above). As an example, for the pasta sample good extraction and chromatographic recoveries were obtained with all extraction methods and the AsIII/AsV ratio obtained with procedure C does not differ statistically from that of the other (milder) procedures (p > 0.05). Considered that a high acidity considerably shifts retention times and may damage the chromatographic column, the use of higher HNO3 concentrations in procedure C is not recommended on the basis of the results obtained therein.
Zhao et al. extracted wheat grain samples with a phosphate buffer solution containing EDTA in an attempt to preserve the inorganic species and found 77% (range 64–90%) of the chromatographed As to be AsIII.17 However, their extraction yield was low (65%), whereas for food safety purposes a good recovery of the inorganic species is essential since they are far more toxic than the organic ones. In this perspective, procedure C in this work was the most effective since it extracted ≥90% of the As present and the extracted As was generally satisfactorily recovered from the chromatographic column.
Implications for human health
Based on the results of the present study, it appears that ca. 95% of As is in inorganic form in wheat and wheat based products. Using this inorganic-to-total As ratio and assuming a daily consumption of wheat products of 0.2 kg per day,15,27 a daily intake of inorganic As of 9.9–13.5 μg can be estimated for average European adults from the lower bound and upper bound occurrence data reported by EFSA for total As in cereal and cereal products,2 recalculated excluding rice grains and rice based products. This can be compared to an estimated intake of inorganic As of 1.3–1.4 μg per day from rice and derived products calculated on the basis of the EFSA's occurrence data for total As, assuming a daily consumption of 15 g14,15 and 60% of As present in inorganic form. Even though the above are rough estimates, nevertheless they suggest that the contribution of wheat to the intake of inorganic As is about ten times that of rice in populations consuming a predominantly wheat-based diet. Therefore, more data are needed on As speciation in wheat and products in order to accurately estimate the dietary exposure to inorganic As of such populations.Conclusions
Arsenic speciation in wheat and wheat products was studied using different extraction procedures and anion exchange HPLC-ICP-MS as the analytical method for the determination of As species. By combining the results obtained with different extraction conditions, ca. 95% of As was found to be present in inorganic form in the samples with AsIII as the most abundant species, whereas the remainder was mainly DMA. The LODs achieved were suitable for determining the As species at the low levels found in sample extracts. However the MW assisted procedure with HNO3 was the most effective in extracting the As species from samples, which were generally satisfactorily recovered from the chromatographic column.If the inorganic As intake from wheat and products estimated for European adults in this study is compared with the median estimated intake from food and drinking water of 21.0–30.0 μg per day for average European consumers (70 kg body weight)2 or with the daily dietary exposures of up to 14.0 μg and 12.7 μg estimated for the United States and Canada, respectively,28 wheat appears to be the major contributor to the intake of inorganic As. We conclude that more data are needed on As speciation in wheat products in order to accurately estimate the dietary exposure to inorganic As of populations consuming a predominantly wheat-based diet.
Acknowledgements
For financial support the Italian Ministry of Health (Project ISS-Q25) is gratefully acknowledged.References
- IARC, Monographs on the Evaluation of Carcinogenic Risks to Humans, International Agency for Research on Cancer, Lyon, 1987, Vol. 7–42, suppl. 7 Search PubMed; IARC, A review of human carcinogens. C. Metals, arsenic, dusts and fibres, International Agency for Research on Cancer, Lyon, 2010 (in press) Search PubMed.
- EFSA Panel on Contaminants in the Food Chain (CONTAM), Scientific Opinion on Arsenic in Food, EFSA J., 2009, 7(10), 1351 Search PubMed , http://www.efsa.europa.eu/en/scdocs/scdoc/1351.htm.
- Joint FAO/WHO Expert Committee on Food Additives, Seventy-second meeting: summary and conclusions, Geneva, 2010 Search PubMed.
- D. T. Heitkemper, N. P. Vela, K. R. Stewart and C. S. Westphal, J. Anal. At. Spectrom., 2001, 16, 299–306 RSC.
- M. D'Amato, G. Forte and S. Caroli, J. AOAC Int., 2004, 87, 238–243 CAS.
- A. H. Ackerman, P. A. Creed, A. N. Parks, M. W. Fricke, C. A. Schwegel, J. T. Creed, D. T. Heitkemper and N. P. Vela, Environ. Sci. Technol., 2005, 39, 5241–5246 CrossRef CAS.
- E. Sanz, R. Muñoz-Olivas and C. Cámara, Anal. Chim. Acta, 2005, 535, 227–235 CrossRef CAS.
- T. Narukawa, K. Inagaki, T. Kuroiwa and K. Chiba, Talanta, 2008, 77, 427–432 CrossRef CAS.
- Y. G. Zhu, G. X. Sun, M. Lei, M. Teng, Y. X. Liu, N. C. Chen, L. H. Wang, A. M. Care, C. Deacon, A. Raab, A. A. Meharg and P. N. Williams, Environ. Sci. Technol., 2008, 42, 5008–5013 CrossRef CAS.
- A. J. L. Guzmán Mar, L. H. Reyes, G. M. M. Rahman and H. M. S. Kingston, J. Agric. Food Chem., 2009, 57, 3005–3013 CrossRef.
- A. Raab, C. Baskaran, J. Feldmann and A. A. Meharg, J. Environ. Monit., 2009, 11, 41–44 RSC.
- G.-X. Sun, P. N. Williams, Y.-G. Zhu, C. Deacon, A.-M. Carey, A. Raab, J. Feldmann and A. A. Meharg, Environ. Int., 2009, 35, 473–475 CrossRef.
- J.-H. Huang, G. Ilgen and P. Fecher, J. Anal. At. Spectrom., 2010, 25, 800–802 RSC.
- World Health Organization, GEMS/Food Consumption Cluster Diets, http://www.who.int/foodsafety/chem/gems/en/index1.html (accessed August 10, 2010) Search PubMed.
- C. Leclercq, D. Arcella, R. Piccinelli, S. Sette, C. Le Donne and A. Turrini, Public Health Nutr., 2009, 12, 2504–2532 Search PubMed.
- A. A. Meharg, P. N. Williams, E. Adomako, Y. Y. Lawgali, C. Deacon, A. Villada, R. C. J. Cambell, G. Sun, Y.-G. Zhu, J. Feldmann, A. Raab, F.-J. Zhao, R. Islam, S. Hossain and J. Yana, Environ. Sci. Technol., 2009, 43, 1612–1617 CrossRef CAS.
- F.-J. Zhao, J. L. Stroud, T. Eagling, S. J. Dunham, S. P. McGrath and P. R. Shewry, Environ. Sci. Technol., 2010, 44, 5464–5468 CrossRef CAS.
- F. Cubadda, S. Ciardullo, M. D'Amato, A. Raggi, F. Aureli and M. Carcea, J. Agric. Food Chem., 2010, 58, 10176–10183 CrossRef CAS.
- R. A. Schoof, L. J. Yost, J. Eickhoff, E. A. Crecelius, D. W. Cragin, D. M. Meacher and D. B. Menzel, Food Chem. Toxicol., 1999, 37, 839–846 CrossRef CAS.
- O. P. Diaz, I. Leyton, O. Muñoz, N. Nuñez, V. Devesa, M. A. Suñer, D. Velez and R. Montoro, J. Agric. Food Chem., 2004, 52, 1773–1779 CrossRef CAS.
- M. N. Matos Reyes, M. L. Cervera, R. C. Campos and M. de la Guardia, Spectrochim. Acta, Part B, 2007, 62, 1078–1082 CrossRef.
- U. Kohlmeyer, E. Jantzen, J. Kuballa and S. Jakubik, Anal. Bioanal. Chem., 2003, 377, 6–13 CrossRef CAS.
- E. Lombi, K. G. Scheckel, J. Pallon, A. M. Carey, Y. G. Zhu and A. A. Meharg, New Phytol., 2009, 184, 193–201 Search PubMed.
- P. Gnagnarella, S. Salvini and M. Parpinel, Food Composition Database for Epidemiological Studies in Italy, Version 1. 2008, IEO European Institute of Oncology, http://www.ieo.it/bda Search PubMed.
- NIST Report of Investigation: Reference Material 8436 Durum Wheat Flour, National Institute of Standards & Technology, Gaithersburg, MD, 20899, 2008 Search PubMed.
- World Health Organization, Arsenic and arsenic compounds, Environmental Health Criteria 224, Geneva, 2001 Search PubMed.
- European Food Safety Authority, Concise European Food Consumption Database, http://www.efsa.europa.eu/en/datex/datexfooddb.htm (accessed August 10, 2010) Search PubMed.
- L. J. Yost, R. A. Schoof and R. Aucoin, Hum. Ecol. Risk Assess., 1998, 4, 137–152 CrossRef CAS.
Footnote |
| † This article is part of a themed issue highlighting outstanding and emerging work in the area of speciation. |
| This journal is © The Royal Society of Chemistry 2011 |
