A sensitive sequential non-chromatographic speciation analysis of chromium in natural/wastewaters by inductively coupled plasma optical emission spectrometry
Noorbasha N.
Meeravali
,
K.
Madhavi
and
Sunil Jai
Kumar
*
National Center for Compositional Characterization of Materials (NCCCM), Bhabha Atomic Research Centre, ECIL Post, Hyderabad, 500 062, India
First published on 5th November 2010
Abstract
A highly sensitive and novel sequential non-chromatographic speciation procedure has been developed for selective pre-concentration and separation of Cr(III) and Cr(VI) from natural and wastewaters. In this procedure, Triton X-114 micelle and cetylpyridinium bromide-Triton X-114 mixed-micelle are used sequentially for the extraction of hydrophobic Cr(III)-trifluoropentanedione and hydrophilic Cr(VI), respectively. Inductively couple plasma optical emission spectrometry (ICP-OES) is used for its determination. The parameters affecting the extraction process are optimized. Under the optimized conditions, the pre-concentration factors obtained are 50 and 15 while limits of detection (LOD) are 0.02 and 0.05 ng mL−1 for Cr(III) and Cr(VI), respectively. These LODs are better than those of IC-ICP-MS and comparable to those of IC-ICP-DRC-MS methods. The recoveries are in the range of 95 to 99% at 10 to 40 ng mL−1 with relative standard deviation of 2–4%. The accuracy of the procedure is validated by comparing the sum of the concentrations of each individual chromium species obtained from sequential extraction with total chromium in BCR certified reference materials such as Effluent-713, Influent-714 and Industrial effluent-715. The method was then applied to various water samples collected locally.
Introduction
Quality and composition of the ground and surface waters are major concerns to human beings worldwide because they cause direct impact on human health.1 In recent years, due to extensive use of chromium in industrial processes large quantities are discharged mostly to natural water bodies.2 The concentration of Cr(VI) in industrial wastewaters ranges from 0.5 to 270000 μg mL−1, and the environmental protection agency's (EPA) tolerance limits for these discharges into surface and potable waters are 0.1 and 0.05 μg mL−1, respectively.3,4 In order to ensure these limits, it is essential to monitor chromium species in the aquatic environment.5Over the decades, ion chromatographic (IC) separation followed by post column diphenylcarbazide (DPC) spectrophotometer detection has been the most widely used differential speciation approach for the determination of Cr(VI) and total chromium after oxidation of Cr(III). The separation of Cr(VI) has been achieved by either anionic or cationic exchangers.6–9 The main difficulty observed in these procedures is overloading of the columns with high levels of anionic and cationic species present in the sample and effect of dissolved organics that prevents Cr(VI) reaction with DPC. Recently, the combination of IC with inductively coupled plasma mass spectrometry detection is more frequently used simultaneous speciation approach for the determination of both the species Cr(III) and Cr(VI). The main drawbacks of this approach are isobaric interferences due to chloride and organic content if present in the sample, high instrumental operation time, and high cost make it difficult to facilitate these procedures in routine analysis. In some natural water samples the level of these species are below the limits of detection of these methods.10–12 To alleviate these problems, simultaneous matrix separation and pre-concentration procedure is an effective alternative, hence, non-chromatographic speciation procedures such as solid phase micro extraction and cloud point extraction are being developed.13,14 These are simple, fast and cheaper methods that provide high pre-concentration, which facilitates the use of even ICP-OES for the determination.
At present various cloud point extraction (CPE) procedures have been reported for speciation of chromium using differential approach.15–24 The differential approach often yields highly imprecise values, especially when the concentration of one species is far higher than the other. Therefore a method where both the species are determined sequentially is desirable.
In this paper, for the first time, we describe a novel sequential micelle and mixed-micelles cloud point extraction procedure for the separation and pre-concentration of Cr(III) and Cr(VI) from natural and wastewaters using ICP-OES. Triton X-114 micelles are used for selective extraction of Cr(III)-trifluoropentanedione (TFPD) in the presence of Cr(VI). The supernatant containing Cr(VI) is subjected to similar extraction produce after addition of cetylpyridinium bromide CPB-Triton X-114 mixed-micelle. The accuracy of the procedure is verified by analyzing the BCR certified reference materials such as Effluent-713, Influent-714 and Industrial effluent-715.
Experimental
Instrumentation
An inductively coupled plasma optical emission spectrometer (ICP-OES), Jobin Yvon, model No. JY-2000 equipped with a 40.68 MH rf generator was used for the determination of chromium in dissolved surfactant rich phases. The operating conditions are given in Table 1.| ICP-OES instrument | JY-2000 |
| Rf power/W | 1000 |
| Plasma gas flow/L min−1 | 12 |
| Auxiliary gas flow/L min−1 | 1.1 |
| Nebulizer gas flow/L min−1 | 0.5 |
| Slit width/μm | 20/20 |
| Wavelength/nm | 267.7 |
| Monochromator/m | 0.64 |
| Number of groves/mm | 2400 |
| Type of monochromator | Czerny Turner |
Reagents and standard solutions
Ultra pure water (18.2 MΩ cm) was obtained from a Milli-Q (Bedford, MA, USA) system. All reagents used were at least of analytical reagent grade (Merck, Darmstadt, Germany) unless otherwise stated. Hydrochloric and nitric acids were used after purification with quartz sub-boiling distillation system. Stock standard solutions (0.1 mg mL−1) of Cr(III) and Cr(VI) were prepared using 1 mg mL−1Cr(III) solution (Sigma-Aldrich, Steinheim, Germany) and respective amount of K2Cr2O7 (Merck, Darmstadt, Germany) in 0.2% v/v nitric acid and ultra pure water, respectively. Working standards were prepared daily by subsequent dilution. The solutions of 10% m/v Triton X-114, CPB and NH4SCN were prepared in ultra pure water and 20% m/v TFPD (Sigma-Aldrich, Steinheim, Germany) was prepared in methanol. Interference study was carried out by using 1 mg mL−1 of Ca2+, Mg2+, Ni2+, Cd2+, Pb2 Al3+, Fe2+, Fe3+, Mn2+ and sulfate solutions.Sample collection and storage
Wastewater samples were collected from local domestic drainage, pharmaceuticals and electronics industrial areas in to cleaned plastic containers initially rinsed three times with the same water. These wastewater samples were filtered through a 0.45 μm membrane filter paper and kept in a refrigerator at 4 °C until use.Micelles and mixed-micelles cloud point extraction procedure
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture CH3OH and HNO3, respectively and analyzed for Cr(III) and Cr(VI) using ICP-OES. Procedural blanks were also prepared in a similar manner.
1 mixture CH3OH and HNO3, respectively and analyzed for Cr(III) and Cr(VI) using ICP-OES. Procedural blanks were also prepared in a similar manner.
Results and discussion
Clouding phenomenon in CPB-Triton X-114 mixed-micelles with NH4SCN
The clouding phenomenon in mixed-micelles mostly depends up on the dominating electrostatic repulsion between charged head groups, which varies with concentration of ionic surfactants added to nonionic surfactants. These electrostatic repulsions in mixed-micelles are controlled by addition of electrolytes which changes the Cloud Point Temperature (CPT).25,26 Hence, the CPT variation of mixed-micelles of 1% m/v Triton X-114 micelles with CPB monomers and micelles in the concentration range of 0–0.5% m/v in presence of 1% v/v HCl was studied in presence and absence of 1% v/v NH4SCN electrolyte. The results are shown in Fig. 1. They indicate that the CPT of monomer (CPB)-micelle (Triton X-114) interaction in the presence of NH4SCN is higher than the CPT of the same system in the absence of electrolyte. It is mainly due to the effect of SCN−, which increases solubility of mixed aggregates that increases the CPT.27 When the concentration of CPB is above CMC, micelle (CPB)-micelle (Triton X-114) interaction takes place, which increases and decreases the CPT in the absence and presence of NH4SCN, respectively. Beyond 0.5% CPB instead of liquid–liquid phase separation, liquid–solid phase separation was observed in the presence of NH4SCN. The increase in CPT above CMC of CPB in the absence of electrolyte is due to the increase in the electrostatic repulsion between the charged head groups, which prevents the formation of mixed-micelle aggregates. The same trend is observed till a CPB concentration of 0.2%, which increased the CPT to 100 °C. In the presence of NH4SCN, the charge of the mixed-micelle formed by micelle–micelle interaction is neutralized slightly, which subsequently increases the hydrophobic nature and decreases the CPT drastically from 30 °C to below 5 °C in the CPB concentration from CMC to 0.5%. After 0.5% CPB, the formation of solid indicates the complete neutralization of available positive charge of the mixed-micelles. The acquired positive surface charge of the mixed-micelles formed in the monomer-micelle and micelle–micelle regions were used for extraction of chromate.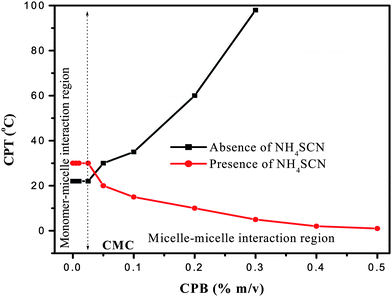 | ||
| Fig. 1 Mixed-micelles CPT of 1% m/v Triton X-114 micelles with CPB monomer (<CMC) and micelles (>CMC) with and without 1% v/v NH4SCN in presence of 1% v/v HCl. | ||
Optimization of micelles and mixed-micelle cloud point extraction parameters
The parameters affecting the sequential cloud point pre-concentration of Cr(III) and Cr(VI) using the Triton X-114 micelles and CPB-Triton X-114 mixed-micelles formed by monomer-micelle and micelle-micelle interaction were studied. Optimization was carried out by spiking 20 ng mL−1 of both the species to aqueous as well as wastewater samples.The effect of pH on the sequential micelles and mixed-micelles separation and pre-concentration of Cr(III) and Cr(IV) was investigated. Hence, the effect of pH in the range of 1–6 on the recovery of Cr(III) and Cr(VI) were studied. Results are shown in Fig. 2. As can be seen in Fig. 2(a), in the pH range of 4.2–6.0, the recovery of Cr(III)-TFPD was 98–102%, whereas only 4–5% recovery of Cr(VI) was observed at the whole pH range studied using the micelles extraction system. Thus, pH 5 was selected for selective extraction of Cr(III)-TFPD in presence of Cr(VI). As can be seen in Fig. 2(b), in the pH range 1.5–3.5, the recovery of Cr(VI) was 92–96%, and then decreased to 40% on further increase in pH of the solution to 6, whereas only 5–6% recovery of Cr(III) was observed in the whole studied pH range under these conditions. Thus, pH 2.5 was used for selective extraction of Cr(VI) in the presence of Cr(III), by using mixed-micelles in the presence of NH4SCN.
 | ||
| Fig. 2 Effect of pH on the recovery of Cr(III) and Cr(VI) using sequential (a) micelles (in presence of 0.3% m/v TFPD and 0.3% m/v Triton X-114) and (b) mixed-micelle (in presence of 0.3% m/v CPB, 0.8% m/v of Triton X-114 and 0.6% v/v NH4SCN). The error bar indicates the standard deviation at each measurement (n = 3). | ||
Optimizations of concentration of chelating agents such as TFPD for Cr(III) and CPB for Cr(VI) are necessary especially in wastewater samples for quantitative extraction using micelle and mixed-micelle, respectively. The preliminary results indicated that the concentration of TFPD and CPB required for quantitative extraction of chromium species from aqueous solution was quite different compared to real wastewater sample. Therefore, the effect of the concentrations of TFPD and CPB on the recovery of Cr(III) and Cr(VI) spiked aqueous solutions and wastewaters were evaluated carefully in the concentration range of 0–0.5% m/v. The results showed that 100 and 96% recovery of Cr(III) and 94 and 92% recovery of Cr(VI) were obtained from aqueous and wastewaters by addition of 0.08% and 0.20% TFPD, and 0.1 and 0.2% CPB, respectively. Above these concentrations of TFPD and CPB the recoveries are unchanged. The different behaviors at lower concentrations of TFPD and CTAB may be due to differences in the concentration of metal ions available to compete with chromium species reaction with chelating agents. Hence, a concentration of 0.3% m/v TFPD and 0.3% m/v CPB were selected, respectively for micelles and mixed-micelles.
Optimization of Triton X-114 concentration on the recovery of Cr(III) and Cr(VI) spiked aqueous and wastewaters were evaluated carefully in the range of 0–1% m/v. The results showed that the recovery of Cr(III)-TFPD increased with increase in concentration of Triton X-114 and reached 98 and 100% recovery at 0.1 and 0.2% respectively, in wastewater and aqueous solutions. Not much change in the Cr(III)-TFPD recovery was observed by further increase in Triton X-114 concentration up to 1%. Hence, 0.3% Triton X-114 concentration was selected. In case of mixed-micelles, clear phase separation in the supernatant solution was obtained at and above 0.4% Triton X-114 concentration added to 0.3% CPB in the presence of NH4SCN. At 0.4% Triton X-114, the recovery of Cr(VI) from aqueous and wastewaters were 60 and 75%, respectively. These recoveries increased further by increase in concentration of Triton X-114 up to 0.7 and 0.6%, respectively, for aqueous and wastewaters and reached maximum 95% recovery in both the matrices. This recovery of Cr(VI) was constant by further increasing the Triton X-114 concentration up to 1%. Hence, a 0.8% Triton X-114 concentration was selected.
As shown in Fig. 1, the NH4SCN electrolyte is playing a very important role in achieving the cloud point phase separation in CPB-Triton X-114 mixed micelles. Hence, the effect of NH4SCN on Cr(VI) recovery was studied in the range of 0 to 1.5% m/v. These results show that without electrolytes no phase separation was observed and no recovery of Cr(VI). At 0.2% NH4SCN, the surfactant-rich phase (SRP) was observed on top of aqueous solution. In between 0.2 to 0.3%, SRP was floating on the solution. On further increase in the concentration of NH4SCN to 0.4%, the SRP was settled at bottom of the solution and showed 80% recovery of Cr(VI). This recovery was reached 97% at 0.5% NH4SCN and remained constant up to 1.5%. Hence, a 0.6% m/v NH4SCN concentration was selected.
The optimization of incubation temperature and time is necessary, especially for complete complexation and quantitative extraction of inert Cr(III) and water soluble Cr(VI) species sequentially from aqueous solution into pre-concentrating micelle and mixed-micelle SRP. The volume of these SRP decides the phase volume ratio (PVR) and pre-concentration factor (PCF). The PVR is the ratio of the final volume of the SRP to that of the aqueous phase. The PCF is the ratio of the analyte concentration in the final SRP to that of the initial aqueous phase. Therefore, the effect of incubation temperature on SRP volumes and recovery of Cr(III) and Cr(VI) were studied in the range 30 to 90 °C using 30 min incubation time. The recoveries of chromium species along with corresponding SRP volumes are shown in Fig. 3. These results indicate that the extraction of reactive anionic Cr(VI) was independent of temperature in the studied range. But in the case of Cr(III), temperature is playing a very important role and the quantitative recovery (98%) was achieved only after 60 °C incubation temperature.
 | ||
| Fig. 3 Effect of incubation temperature on sequential micelle and mixed-micelle recovery of Cr(III) and Cr(VI) and SRP volumes. The error bars indicate the standard deviation at each measurement (n = 3). | ||
The incubation temperature is also playing an important role on the volume of SRP. The SRP volumes of 1000 and 100 μL were obtained for micelle and mixed-micelles respectively in the range of 30–50 °C temperature, and further increase caused decrease in the volumes of SRP and reached constant of 300 and 50 μL in between 70-90 °C. Therefore an 80 °C incubation temperature was selected and it has provided that the lowest SRP volume and quantitative recoveries. The optimization of incubation time was carried out in the range 10 to 60 min. These experiments showed that 98% and 95% recovery of Cr(III) and Cr(VI) was observed after 15 min of incubation time. Therefore, 20 min incubation time was selected. Under these conditions, the achieved PCF for Cr(III) and Cr(VI) were 50 and 15, respectively.
Interferences and recovery studies
The effects of common interfering ions on the sequential CPE of Cr(III) and Cr(VI) were investigated. Interference may occur due to natural redox species and also due to anionic species present in the wastewaters. The tolerance limit of various interfering ions spiked with 50 ng mL−1 in all species were found, keeping the relative error between ±10%, to be 600 mg L−1 of Ca2+, Mg2+ and sulfate; 200 mg L−1 of Mn2+; 100 mg L−1 of Al3+ and Fe2+; 50 mg L−1 of Ni2+and 20 mg L−1 of Cd2+, Pb2+ and Fe3+ for micelle extraction and 1200 mg L−1 of Ca2+ and Mg2+; 600 mg L−1 of sulfate; 300 mg L−1 of Al3+; 200 mg L−1 of Ni2+ and Mn2+; 100 mg L−1 of Cd2+ and Pb2+ and Fe3+ and 0.2 mg L−1Fe2+ for mixed-micelle extraction, respectively. These results showed that this procedure can be applied to any water samples containing interfering ions at these levels.The recovery studies were performed after spiking BCR certified reference materials (BCR 713, 714 and 715) and wastewater samples collected locally from various industrial areas. The spiking concentrations were approximately similar to allowed concentrations of these species in natural waters, for better demonstration of the performance of the procedure. Therefore, BCR certified reference materials and wastewater samples were spiked with Cr(III) and Cr(VI) in the range 10 to 200 ng mL−1 and at different concentrations, and the recoveries were measured. As shown in Table 2, the recoveries were between 95 to 99%, which indicated that these matrices have no effect on sequential recoveries of chromium species.
| Matrices | Spiked/ng mL−1 | Recovery (%) | ||
|---|---|---|---|---|
| Cr(III) | Cr(VI) | Cr(III) | Cr(VI) | |
| a Values are means of four measurements ± standard deviation. b Not detected. | ||||
| BCR-713 | 10 | 0 | 97.2 ± 2.5 | NDb |
| 0 | 20 | NDb | 95.8 ± 3.2 | |
| 10 | 20 | 96.8 ± 2.9 | 96.8 ± 3.5 | |
| BCR-714 | 30 | 40 | 97.8 ± 1.9 | 97.8 ± 2.5 |
| 40 | 30 | 98.6 ± 2.2 | 98.2 ± 2.8 | |
| BCR-715 | 100 | 200 | 99.8 ± 2.3 | 98.8 ± 1.9 |
| 200 | 100 | 99.6 ± 1.6 | 97.2 ± 2.8 | |
| Real samples | ||||
| Electronic industry | 20 | 10 | 97.6 ± 2.3 | 95.2 ± 2.8 |
| Pharmaceuticals | 10 | 10 | 98.8 ± 1.9 | 94.8 ± 3.6 |
| Domestic drainage | 10 | 0 | 95.6 ± 2.6 | NDb |
| 0 | 20 | NDb | 97.3 ± 2.9 | |
| 10 | 20 | 94.8 ± 2.4 | 95.8 ± 3.1 | |
| 20 | 10 | 98.6 ± 2.3 | 96.8 ± 3.4 | |
Analytical figures of merit
Under the optimized experimental conditions, the calibration curve was obtained by pre-concentrating the successively spiked standards of Cr(III) and Cr(VI) in the range of 5–1500 and 10–1000 ng mL−1, respectively. The correlation coefficients were 0.9994 and 0.9932, respectively for Cr(III) and Cr(VI). Quantifications have been performed by using external aqueous standards prepared in 0.2% HCl. The limits of detection (LOD) calculated based on three times the standard deviation of ten measurements of procedural blanks were 0.02 and 0.05 ng mL−1, respectively for Cr(III) and Cr(VI). As shown in Table 3, these LODs obtained by the present procedure are better than those of reported micelle extraction, CPE10–17 and IC-ICP-MS6–8 and comparable to that of IC-ICP-MS28–30with dynamic reaction cell and IC-DPC7,9 procedures.| Speciation approach | Separation scheme | Instrument | LOD/ng mL−1 | Ref. | |
|---|---|---|---|---|---|
| Cr(III) | Cr(VI) | ||||
| a Cr(III) and total chromium using differential speciation approach. b Diphenylcarbazide. c Spectrophotometer. d Simultaneous determination of Cr(III) and Cr(VI). e Dynamic reaction cell. f Sequential determination of Cr(III) and Cr(VI). | |||||
| Cr(III) & Cr (T) Da | Micelle | GFAAS | 0.021 | — | 16 |
| Cr(III) & Cr (T) Da | Micelle | FAAS | 0.32 | — | 17 |
| Cr(III) & Cr (T) Da | Micelle | GFAAS | — | 0.01 | 18 |
| Cr(III) & Cr (T) Da | Micelle | FAAS | — | 0.6 | 20 |
| Cr(VI) | IC-DPCb | SP c | — | 0.05 | 7 |
| Cr(VI) | IC-DPC | SP | — | 0.018 | 9 |
| Cr(III) & Cr(VI) Sd | IC | ICP-MS | 0.3 | 0.5 | 10 |
| Cr(III) & Cr(VI) Sd | IC | ICP-MS | 0.2 | 0.1 | 12 |
| Cr(III) & Cr(VI) Sd | IC | ICP-DRC-MS e | 0.09 | 0.06 | 28 |
| Cr(III) & Cr(VI) Sqf | Micelle and mixed-micelle | ICP-OES | 0.02 | 0.05 | Present method |
Validation of the method and analysis of real samples
The accuracy of the proposed procedure was validated by analyzing the BCR certified reference materials (CRM'S) such as final effluent-713, initial influent-714 and industrial effluent-715. The results are shown in Table 4. These results showed that the sums of the concentrations of individual chromium species were found to be in good agreement with the certified total chromium concentrations, which indicates the accuracy of the procedure. The samples collected from various industrial areas were also analyzed along with CRM'S and showed Cr(VI) as the major species. The sum of the values obtained for Cr(III) and Cr(VI) sequentially in samples were agreed with total chromium determined using earlier reported CPE procedure.20| Matrices | Measured values a/ng mL−1 | Certified values/ng mL−1 | |||
|---|---|---|---|---|---|
| Cr(III) | Cr(VI) | Total Crb | Total Crc | ||
| a Mean of six determinations ± standard deviation. b Values are obtained by addition of Cr(III) and Cr(VI) values. c Values are obtained from CPE procedure with chelating agent ref. 20. d Values are in μg mL−1. | |||||
| BCR-713 final effluent | 16.7 ± 1.1 | 4.9 ± 1.4 | 21.6 ± 1.8 | 21.4 ± 2.5 | 21.9 ± 2.4 |
| BCR-714 initial influent | 77.4 ± 5.5 | 47.2 ± 6.2 | 126.6 ± 8.3 | 123.3 ± 4.7 | 123 ± 10 |
| BCR-715 Industrial effluentd | 0.55 ± 0.05 | 0.48 ± 0.03 | 1.03 ± 0.06 | 0.99 ± 0.08 | 1.00 ± 0.09 |
| Analysis of real samples | |||||
| Electronic industry | 2.1 ± 0.2 | 8.2 ± 0.5 | 10.3 ± 0.5 | 10.5 ± 1.1 | — |
| Pharmaceuticals | 4.4 ± 0.1 | 7.4 ± 0.4 | 11.8 ± 0.4 | 14.9 ± 0.8 | — |
| Domestic drainage | 2.5 ± 0.2 | 6.8 ± 0.5 | 9.3 ± 0.5 | 10.3 ± 0.9 | — |
Conclusion
A novel sequential non-chromatographic speciation procedure has been reported for the determination of Cr(III) and Cr(VI) in natural and wastewaters. In this procedure, the specific reactive solubilizing sites of micelle and mixed-micelle have been used for selective extraction of hydrophobic and hydrophilic chromium species sequentially. This non-chromatographic speciation procedure provides the advantages of pre-concentration of each individual species and uses the minimum instrumental operation time, improves sensitivity and sample throughput, hence can be considered as a cheaper alternative speciation option to application laboratories. The method is simple and fast and adopts the green chemistry principle and generates less waste compared to existing methods.References
- U.S. Environmental Protection Agency Polluted Runoff (Nonpoint Source Pollution). Available from http://www.epa.gov/OWOW/NPS/facts/point1.htm.
- J. O. Nriagu and E. Nieboer, Chromium in Natural and Human Environment, Wiley Interscience, New York, 1988 Search PubMed.
- J. W. Patterson, Industrial wastewater treatment technology, 2nd edn, Butterworth- Heinemann, London, 1985 Search PubMed.
- Environnemental Protection Agency Environnemental pollution control alternatives, EPA/625/5-90/025, EPA/625/4-89/023, Cincinnati, US, 1990 Search PubMed.
- V. Gómez and M. P. Callao, TrAC, Trends Anal. Chem., 2006, 25, 1006–1015 CrossRef CAS.
- U.S. Environmental Protection Agency, USEPA, 1991, Method 218.6, Cincinnati, OH.
- J. W. Ball and R. B. McCleskey, U.S. Geological Survey, 2003, Report 4018, http://wwwbrr.cr.usgs.gov/projects/GWC_chemtherm/pubs/WRIR%2003-4018.pdf.
- U.S. Environmental Protection Agency, USEPA, 1996, Method 7199, http://www.epa.gov/wastes/hazard/testmethods/sw846/pdfs/7199.pdf.
- Dionex Corporation, 2003, Application Update 144, Sunnyvale, CA. http://www.dionex.com/en-us/webdocs/4242-AU144_V18.pdf.
- M. P. Kallio and P. K. G. Manninen, Anal. Chim. Acta, 1996, 318, 335–343 CrossRef CAS.
- M. P. Kallio and P. K. G. Manninen, J. Chromatogr., A, 1996, 750, 89–95 CrossRef CAS.
- Z. Chen, M. Megharaj and R. Naidu, Talanta, 2007, 72, 394–400 CrossRef CAS.
- M. A. Vieira, P. Grinberb, C. R. R. Bobeda, M. N. M. Reyes and R. C. Campos, Spectrochim. Acta, Part B, 2009, 64, 459–476 CrossRef.
- A. Gonzalvez, S. Armenta, M. L. Cervera and M. de la Guardia, TrAC, Trends Anal. Chem., 2010, 29, 260–268 CrossRef CAS.
- J. Lu, J. Tian, H. Wu and C. Zhao, Anal. Lett., 2009, 42, 1662–1677 CrossRef CAS.
- P. Liang and H. Sang, J. Hazard. Mater., 2008, 154, 1115–1119 CrossRef CAS.
- Z. Sun and P. Liang, Microchim. Acta, 2008, 162, 121–125 CrossRef CAS.
- Y. Li, B. Hu, Z. Jiang and Y. Wu, Anal. Lett., 2006, 39, 809–822 CrossRef CAS.
- X. Zhu, B. Hu, Z. Jiang and M. Li, Water Res., 2005, 39, 589–595 CrossRef CAS.
- E. K. Paleologos, C. D. Stalikas and M. I. Karayannis, Analyst, 2001, 126, 389–393 RSC.
- G. D. Matos, E. B. dos Reis, A. C. S. Costa and S. L. C. Ferreira, Microchem. J., 2009, 92, 135–139 CrossRef CAS.
- E. K. Paleologos, C. D. Stalikas, S. M. T. Karayanni, G. A. Pilidis and M. I. Karayannis, J. Anal. At. Spectrom., 2000, 15, 287–291 RSC.
- E. K. Paleologos, A. G. Vlessidis, M. I. Karayannis and N. P. Evmiridis, Anal. Chim. Acta, 2003, 477, 223–231 CrossRef CAS.
- N. N. Meeravali and S. J. Jiang, Talanta, 2009, 80, 173–178 CrossRef CAS.
- N. N. Meeravali and S. J. Jiang, J. Anal. At. Spectrom., 2008, 23, 555–560 RSC.
- T. Gu and P. A. G. Gomez, Colloids Surf., A, 1995, 104, 307–312 CrossRef CAS.
- K. S. Sharma, S. R. Patil and A. K. Rakshit, Colloids Surf., A, 2003, 219, 67–74 CrossRef CAS.
- R. E. Wolf, J. M. Morrison and M. B. Goldhaber, J. Anal. At. Spectrom., 2007, 22, 1051–1060 RSC.
- H. J. Wang, X. M. Du, M. Wang, T. C. Wang, H. O. Yang, B. Wang, M. T. Zhu, Y. Wang, G. Jia and W. Y. Feng, Talanta, 2010, 81, 1856–1860 CrossRef CAS.
- L. Xing and D. Beauchemin, J. Anal. At. Spectrom., 2010, 25, 1046–1055 RSC.
| This journal is © The Royal Society of Chemistry 2011 |
