An outsider's perspective—ecotaxis revisited: an integrative review of cancer environment, iron and immune system cells†
Maria
de Sousa
Iron Genes and the Immune System (IRIS) Lab, IBMC-Porto and ICBAS, University of Porto, 823 Campo Alegre, 4150 Porto, Portugal. E-mail: mdesousa@ibmc.up.pt
First published on 22nd December 2010
Abstract
Lymphoid cell and tumor cell migration share similarities: 1. migration to specific microenvironments; 2. increased microvasculature with increased growth; 3. cell division. At the same time, contrasting aspects between the two merit attention: 1. failure of tumors to return to microvasculature quiescence; 2. failure of malignant cells to stop dividing; 3. failure of tumor cells to re-enter the circulation after returning to a non-activated phenotype. Analysis of these contrasting aspects leads to the reviewing of unexpected roles of immune cells in the tumor environment, recent work on ferroportin expression with lack of iron export by tumor cells, iron export by M2 macrophages, and deficient dendritic cells (DCs) in the tumor environment. DCs in lymph nodes have recently been found to bring lymph node vasculature to quiescence after antigen stimulation. Contrary to current dogma, the evidence is that some immune system cells in the tumor environment may be favoring regulators instead of diminishing tumor growth. In addition, recent data herein reviewed will make it difficult not to consider iron and iron gene expression as relevant components of the tumor environment. Finally, I conclude with wondering how much longer what I call the ‘Hunter Paradigm’ will dominate cancer research and immunology and how timely it is to acknowledge in the first decade of a new century, Mina Bissell as a pioneer in the change of that paradigm in Cancer Research.
“Suppose he'd listened to the erudite committee; He would have only found where not to look”
WH Auden
 Maria de Sousa | Maria de Sousa is Professor Emeritus of Porto University, a Fellow of the Royal College of Pathologists, Member of EMBO, and was awarded the Gold Medal of the Portuguese Ministry of Science in 2009. She provided early contributions to T and B lymphocyte mapping. Currently her research group focuses on clarifying the genetic and molecular basis of the crosstalk between the immune system and iron homeostasis. Recent contributions include: finding that one HFE mutation provokes an UPR and diminishes MHC Class I expression, that the UPR influences iron gene expression and that ferroportin expression in lymphocyes and monocytes explains their differences in iron handling. |
Insight, innovation, integrationMaria de Sousa's perspective as an outsider from the cancer research field brings some insight into areas where she has been an insider for many years: the circulation of lymphocytes and the functional interactions between immune system cells and iron. Surprisingly, these 3 fields have had separate lives, as so often happens in modern Biology and Biomedicine. In general, few immunologists know about iron biology and reciprocally few iron biologists know the possible significance of their work to De Sousa's postulated view of the immune system in the surveillance of the potential iron toxicity associated with the red blood cell circulation. In addition, iron and iron genes discovered in the last 10 years have been left out of the tumor environment and do not appear to be too well known by molecular oncologists. Two papers from Gaetano Cairo's Lab in Milan and Suzi and Frank Torti in North Carolina, published already in 2010, point to the potential importance of ferroportin, the only known iron exporter, in the tumor environment, linked to M2 macrophages by Cairo's Group (Recalcati et al. Eur. J. Immunol. 2010, 40:824–835) and to human breast tumor cells and metastasis free survival in breast cancer, by the Tortis's Group (Pinnix et al. Sci. Transl. Med.2, 43–56 (2010). This perspective from an outsider brings to integrative biology the three expected Is: 1. Innovation, from the review of these most recent data. 2. Insight, from a fresh view of the role of immune system cells and expression of iron genes in the tumor environment. 3. Integration, by bringing closer data living separate lives in separate journals directed to separate audiences. Put together, the data reviewed in de Sousa's perspective might add to the comprehension of the extent and relevance of Mina Bissell's “dynamic reciprocity” concept in cancer. |
No pioneers in their own time
The measure of time in Science is different from the measure of time in clocks. Recently, I had the opportunity of seeing correspondence from Alexis Carrell to Reynaldo dos Santos written by hand in the early 1900s1 that reminded me that Carrell had the Nobel Prize for Physiology and Medicine for his work on organ transplantation. Reading his Nobel Lecture delivered in 1912, I found out his pioneering view on the fact that the success of transplantation would not depend on perfecting surgery. “Thus, while the problem of the transplantation of organs has been solved from a surgical point of view, we see that this by no means suffices to render such operations of definite surgical practicability, and it will only be through a more fundamental study of the biological relationships existing between living tissues that the problems involved will come to be solved and thereby render possible the benefits to humanity which we hope to see accomplished in the future.”2 Acknowledgement of “the discoveries concerning genetically determined structures on the cell surface that regulate immunological reactions”, known to dictate the success of organ transplantation between different individuals, was recognized by the attribution of the Nobel Prize to Dausset, Benacerraf and Snell in 1980,3 36 years after the death of Carrell in 1944.I was to find reference to Carrell in the introduction and historical background of M.J. Bissell's CSH paper with Kenny and Radisky of 20054 and to the organ perfusion perfected by Carrell and Charles Lindbergh in one of the first papers of Judah Folkman on organ and tumor perfusion published in 1966.5 Nearly a century was thus vanishing as evidence of that magic fast passing of time for pioneers in Science, albeit unknowingly to themselves, for no one is a pioneer in his/her own time.6–11
Mina Bissell, from her early experiments with the Rous sarcoma virus, pioneered the view that oncogenic viruses did not suffice for tumor development.6 The environment of the tumor had a significant say in determining how a tumor was going to develop (see ref. 4 for review). Folkman pioneered the view that malignant transformation could signal the development of new blood vessels9,10 through the isolation of TAF, a tumor angiogenesis factor,11 foreseeing the eventual existence of molecules with anti-angiogenic properties that could come to be a valuable addition to chemotherapy.
Why ask for the contribution of an outsider?
Reading the early papers of those two giants on the shoulders of others that they always had the elegance to cite (see ref. 4 and 9), any one not working on cancer that was asked to contribute to this issue had to ask herself why? The answer must lie largely on the kindness of the editor and possibly partly on an old interest of mine on the lymphocyte circulation12 and the role of lymphoid tissue microenvironments in lymphoid cell positioning.13–15 That interest led later to considering a role for iron proteins in lymphoid cell positioning, leading to the postulate of a new surveillance role for the immune system.16,17 The latter considers that circulating immune system cells could have a surveillance role of the potential iron toxicity inherent in the red blood cell circulation.Coincidence has it, however, that as I started writing this review, a paper came out of the Lab of Suzy and Frank Torti reporting the results of an extensive study demonstrating a link between high ferroportin expression, grade of malignancy and metastasis free survival advantage in breast cancer patients.18
The puzzle
Before going on to the relevance of ferroportin expression in breast cancer, I will touch on a puzzle that has interested me for many years made of the similarities and differences between lymphocyte and tumor cell migration.Environments. Both cell types migrate and arrange themselves within well delineated microenvironments in the adult organism. The environment destination is therefore of importance in both cases. In the case of lymphoid cell migration, I thought the capacity of cells of different origins to migrate and arrange themselves in distinct areas of the peripheral lymphoid organs was so important that it should be “stamped” with a neologism: ecotaxis.14 Ecotaxis is a word built from the Greek oikos meaning house and tassein meaning to arrange. It seemed necessary in 1971, at a time when the immune system was seen (as it continues to be today) as the system capable of discriminating between self and non-self, with a principal function of defence against the invasion of external pathogens. It appeared important to remind ourselves that cells of the immune system also had the capacity to recognize self within self, a capacity that surely had to depend on interactions of the circulating cells with the extracellular matrix (ECM).15
Enhanced blood microcirculation constitutes another similarity between a growing tumor and a lymph node growing in response to an antigen challenge. Changes in the blood microcirculation were described by Peter Herman's group in 1972 in lymph nodes undergoing a primary immune response.19 One major difference distinguishes the changes in the microcirculation of the lymph node from the angiogenesis provoked by a tumor. In the case of the responding lymph node, between the 2nd and 5th days after antigen challenge, previously avascular areas became vascular with changes in diameter and density of capillaries in most lymph node zones. By 7 days after the antigenic stimulus, the lymph node microvasculature had returned to normal. A great many technical microscopy advances have occurred in the field of study of cell interactions and expansion of the lymph node microvasculature during an immune response.20 The appearance of many molecular markers of endothelial cells in lymph nodes and the introduction of real time imaging have transformed the field “molecularly” and visually but perhaps not so much substantially.
Indeed, regardless of the nature of the many technical advances, it was already assumed in 1972 that “redistribution of the capillaries and post capillary structures throughout the cortex of the lymph node facilitated increased interchange between the lymphatic tissue and the circulating blood”.19 Both in the case of an immune response and in tumor sites, novel technologies have illustrated beautifully the interactions between dendritic cells (DCs) and lymphocytes in lymph nodes,20 or tumor cells and cytotoxic cells within tumors.21 With 2-photon microscopy, cell–cell interactions can be seen as never imagined but to the resolution of the puzzle that I want to introduce in this review, it is another recent study that seemed of greater interest and opportunity.22 This is a unique study from Te-Chen Tzeng and colleagues of the return to quiescence of the lymph node microvasculature after antigenic stimulation. Tzeng and colleagues identified a subset of dendritic cells CD11chiMHC class II (MHC II)med as the engineers of the quiescence reached by the microvasculature after an immune response.22CD11chiMHC class II (MHC II)med DCs accumulate later, and their short-term depletion in mice abrogates the re-establishment of vascular quiescence and stabilization. CD11chiMHC IImedcells promote endothelial cell quiescence in vitro. Culturing aortic rings in fibrin gels stimulated proliferative outgrowths of endothelial cells with a microvascular phenotype. Adding CD11chiMHCIImedcells to these cultures specifically reduced endothelial cell proliferation. In vivo, CD11chiMHCIImedcell depletion disrupted endothelial cell quiescence, at least in part, by mediating reduced lymph node vascular endothelial growth factor.22 Some data from the same study indicate that the subset of DCs in question may also have a role in the homeostasis of the microvasculature independently of antigen stimulation. The puzzle is that a tumor does not seem to have “learnt” from a lymph node to return its microvasculature to quiescence.
Could dendritic cells be important in that failure of cancer “to learn”?
In a study of the immune profile of 47 Sentinel and 104 Axillary Human Lymph nodes removed from breast cancer patients, Kohrt et al. found that lower numbers of DCs were generally found in tumor-involved axillary lymph nodes.23 Conversely, higher numbers were seen in tumor free nodes. Thus, DCs may enter the resolution of the puzzle with two unexpected actions: as possible regulators of the microvasculature in normal lymph nodes and relating to tumor load in nodes in breast cancer. CD11chi DCs are clearly becoming a new thread in the fabric of answers to the question of the role of microenvironment to tumor progression from other studies of the tumor environment (Lyden, personal communication).Tumor cells do not “know” when to stop. A second most important difference between lymphocytes responding to an antigen stimulus in the lymph node environment and malignant cells in a tumor environment is the fact that the latter “do not seem to know” when to stop. Dividing lymphocytes during an immune response “know”. Moreover, activated lymphocytes stop dividing, return to a small lymphocyte memory phenotype and move to re-enter the continuous blood to lymph circulation, disseminating the lasting information of a specific protective immune response. One is tempted to speculate that tumor cells are at a stage in evolution much behind lymphocytes. Or are DCs in the surrounding tumor environment dictating that they behave that way? There is evidence indicating that tumors secrete products that affect DCs differentiation (Lyden, personal communication).
Is the fact that tumor cells keep dividing an exclusive matter of genes? The consequence of the dynamic reciprocity with the environment that Mina Bissell tells us about? Or simply as part of the failure of that dynamic reciprocity malignant transformation makes the tumor cell loose the property of stopping angiogenesis, a function that might in turn be related to the scarcity and deficiency in dendritic cells found within the substance of tumors themselves? But again, very few people seem to be looking into that.24 After all, the dominating paradigm is still that the immune system is here to defend us from the attack of non-self threats and there still is very little reason to think that dendritic cells have physiological functions beyond their well established antigen presentation role and much expected decisive future in the development of cancer vaccines.25
It is perhaps noteworthy that real time imaging of tumor cell apoptosisin vivo using intravital 2-photon microscopy and a Förster resonance energy transfer-based (FRET-based) reporter of caspase 3 activity revealed, in real time, to the manifest surprise of the authors, that the killing of one target cell by an individual cytotoxic T lymphocyte (CTL) took an extended period of time, 6 h on average, showing therefore the inefficiency of the process for a much hoped for adoptive immune cell therapy in cancer.21
Why would ferroportin expression matter?
We return now to ferroportin. Ferroportin is the only known exporter of iron from cells, acting as a receptor for the peptide hormone hepcidin, presently acknowledge as the key regulator of systemic iron homeostasis.26 In the extensive breast cancer study from the Tortis Lab, Pinnix et al.18 compared ferroportin protein abundance in three pairs of mammary epithelial cell types with variable malignant potential. Examination of ferroportin in those cells revealed that protein abundance was reduced in all aggressive breast cancer cell lines when compared to their counterparts with little or no malignant potential, a finding also reported recently by Jiang et al. in MCF 7 cells, one of the aggressive cell lines examined by Pinnix et al.18,27 To test whether ferroportin concentrations were also altered in the tissue of breast cancer patients, Pinnix et al. moved to verify the presence of ferroportin by immunohistochemical analysis in tissue derived from a single patient that contained areas of normal epithelium, ductal carcinoma in situ and invasive breast cancer. Staining intensity decreased with increasing malignant potential, with the highest expression in normal ductal structures and the lowest expression in invasive tissue.18 The decrease observed in ferroportin protein abundance in malignant breast tissue and the association of decreased ferroportin gene expression with molecular subtypes of breast cancer with poor prognosis led them to test whether ferroportin concentrations were related to breast cancer outcome. Four large patient cohorts with accompanying gene expression profiling data from studies of long-term followed-up breast cancer patients were therefore compared (for details see ref. 18). Low ferroportin gene expression was associated with a statistically significant and clinically substantial reduction in metastasis-free survival in all 4 groups [P value from log-rank test = 0.003 (Norway/Stanford), 0.0006 (NKI), 0.036 (Uppsala), and 0.007 (Stockholm)]. The most pronounced effect was seen in the Norway/Stanford study, where the 8-year disease-free survival rates were 77% for those with high ferroportin and 43% for those with low ferroportin.18Thus, ferroportin expression by tumor cells may indeed matter. Low expression goes with increased levels of intracellular free iron, and as also shown in Pinnix's study, such free iron becomes available for DNA synthesis and/or to cause DNA damage. Cell division, whether in a responding lymph node or in a tumor, requires iron for ribonucleotide reductase, a key iron enzyme that by converting ribonucleotides into deoxyribonuclotides is essential to the process of DNA synthesis, repair of DNA damage and cellular growth.28
Ferroportin could be just one more gene marker to add to the many that have been reported in breast cancer malignancy and prognosis, and the whole thing could be left at that. But the concern of this integrative review is to integrate cancer with environment, iron and immune system cells. Ferroportin in the systemic control of iron metabolism is expressed in enterocytes and in macrophages. Ferroportin expression occurs in macrophages in response to heme iron as part of their role in systemic iron homeostasis recycling iron from red blood cell phagocytosis,29 but macrophages do exactly the same in regional situations of increased blood flow such as in red blood cell migration to an inflammatory site or to a tumor.
Why should iron export by macrophages matter in the breast cancer environment?
The finding of macrophages in tumors started to be seen in the light of current immunological dogma, signifying that they were there as defensive “weapons”. That, however, does not seem to be the case and has been the cause of some surprise,30 much in the same way that the real time imaging of the adoptive transfer of cytotoxic cells in a tumor model showed the inefficiency of tumor cell killing by cytotoxic lymphocyte interactions.21 In the case of macrophages, overwhelming evidence has been accumulating to the contrary both in experimental models of breast cancer development, as in Ojalvo et al. recent paper,30 and in human breast cancer.31,32 In breast cancer patients, the presence of tumor associated macrophages (TAMs) goes with angiogenesis and the development of malignancy and does not provide a successful “defense”. To my knowledge there are no studies of iron gene expression in TAMs isolated from breast cancer tissue. There is, however, a recent relevant paper showing that M2 polarized macrophages export ironin vitro.33 Such macrophages, following an activation pathway triggered by the Th2 cytokines IL-4 or IL-13, have been implicated in the regulation of adaptive immunity, cell growth and tissue repair.34,35 In addition, gene profiling of macrophages isolated from solid ovarian tumors revealed recently that the tumor environment may itself contribute to the differentiation of macrophages into M2 polarized macrophages.36Iron release by M2 macrophages has been demonstrated separately as being due mainly to the upregulation of ferroportin and heme oxygenase (HO1) and the downregulation of H-ferritin.33 Most importantly, Recalcati and her colleagues showed that a tumor cell line grown in conditioned medium from M2 macrophagesin vitro resulted in a significant increase in the growth of the malignant cells when compared to the cells grown in conditioned medium from differentiated but non-polarized (M0) macrophages (Fig. 1).33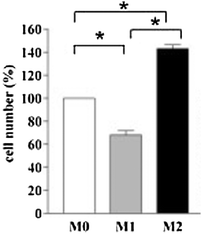 | ||
| Fig. 1 Proliferation of human renal clear carcinoma (RCC10) cells after incubation with conditioned media from M0, M1 and M2 macrophages 48 h after polarization. The values (mean ± 1SD, *p < 0.001, n = 4) are expressed as a percentage of the growth of cells incubated in medium alone. M0, macrophages differentiated with M-CSF; M1, M0 macrophages stimulated with LPS plus IFN-γ; M2, M0 macrophages stimulated with LPS plus IL-4 (modified with permission from ref. 33). | ||
If non-heme iron is important for the growth of tumor cells, if heme iron can dictate the iron exported by tumor macrophages to the benefit of the growth of a tumor, what else should a tumor do to ensure sustained growth? Angiogenesis becomes an obvious and indispensable element of tumor growth, for it is through an increased blood circulation that essential nutrients will reach it. But within that increased travel are not only red blood cells, but also immune system cells, including macrophages capable of donating further iron to malignant cells, and lymphocytes that can also synthesize relevant iron proteins in response to non-transferrin bound iron, including hepcidin and ferroportin.37 Much has been written about tumor environment, the role of macrophages in tumor growth and angiogenesis, etc., but to my knowledge Recalcati's study, an earlier study from the Tortis lab and one other paper from the same lab, are the three papers that have brought to light the significance of the molecular and gene expression basis of the presence of iron in a tumor environment.
The earlier study from the Tortis lab also seems of relevance, for it brings into play the presence of another iron protein, ferritin, as a component that should be considered in the characterization of a tumor environment.38HKa (cleaved high molecular weight kininogen) has been identified as an endogenous inhibitor of angiogenesis formed by the cleavage of kininogen on endothelial cells.39,40 Coffman et al. demonstrated that ferritin binds to HKa with high affinity (Kd = 13 nM) and in doing so antagonizes the antiangiogenic effects of HKa, enhancing the migration, assembly and survival of HKa-treated endothelial cells.38 In addition, they verified in vivo the effects of ferritin opposing HKa's antiangiogenic role in a human prostate cancer xenograft, restoring tumor dependent vessel growth. The results show therefore that ferritin could also contribute to the regulation of angiogenesis in the tumor environment.38
In summary, emerging results mean that from now on, it should be virtually impossible to speak of tumor environment without considering iron, iron-related gene expression and iron proteins beyond the transferrin receptor as significant functional components for tumor growth and possibly angiogenesis.
The return of the outsider: soils, rivers, buffalos and hunters
The demonstration that tumors or enlarged lymph nodes undergoing an immune response have the capacity to signal the appearance of a new microcirculation evoked the image of how much buffalos in time of drought might wish to be able to create new rivers and flood them with fresh water. Hunters may not find such an image funny or even appropriate. Hunters have spent most of their lifetime in the last hundred years thinking of killing the buffalo, and more recently trying to design molecules and/or monoclonal antibodies to target like magic bullets the molecule or molecules thought to be the key to the appearance of the rivers. The concept of magic bullets was first put forward by Paul Ehrlich at about the same time Alexis Carrell was writing to Reynaldo dos Santos 100 years ago. Strangely enough, to an outsider hunters seem to be much more interested in hitting targets in the margins rather than in the river flow or in the soil surrounding the river where buffalos actually find pasture. Yet, it appears to an outsider that the evidence points to success being more likely to lie in understanding better the soil surrounding the rivers. Macrophages delivering essential nutrients and/or releasing proteins that might enhance angiogenesis, dendritic cells that may have the new role of regulating the growth and the quiescence of the river beds, not excluding of course all that is known41 and much that continues to be hoped for by immunology.42Paul Ehrlich with his pioneering notion of magic bullets had the Nobel Prize for his work on Immunity in 1908. There was no such thing as an immune system then. But the notion that the immune system in vertebrates is a bellicose system that evolved all its immense complexity just to hit enemies when they come generally in the form of occasional pathogen invasions, stayed. Magic bullets became the ultimate conceptual expression for the action of hunters in microbiology, cancer research and immunology. Hunter paradigm inspired clinical trials attempting to magically hit the top candidate culprit of angiogenesis, VEGF, unfortunately do not seem to be fulfilling their promise.43 Interestingly, some success seems to have been reached in animal tumor models when feeding the macrophages with Shigella flexnery,44 taking us back to Coley's much earlier thoughts and actions based on evidence that infection could affect the development of different types of tumor, including breast cancer.45,46
The growing complexity of the cellular and molecular components of the immune system, as we begin to know it now, makes it a probable regulatory system with many more functions than just the fight against the military invasion of pathogens. It consists of cells recirculating continuously between blood and lymph12 and therefore ready and available to follow rapidly the red blood cell circulation to inflammatory sites or tumors. Exciting and relevant to cancer cell biology because there may be many lessons in the ecotaxis of lymphoid cells, in the appearance and return to quiescence of new microvasculatures in lymphoid aggregates, in control of lymphoid cell division, that if learnt across fields, in the full exercise of intellectual dynamic reciprocity, might turn out to be quite valuable to the understanding of cancer: of cancer as a process, not as an enemy. Many of the immune system subtypes like dendritic cells and macrophages are undoubtedly important for functions which we already know, but they may also have other relevant functions in cancer and angiogenesis beyond tumor antigen presentation or innate immunity.
An easier way to accept that and to put it to service of reading results dispassionately is to reframe theory. TAMs may prove to be invaluable allies for some of those that have been trying to do that. Immune system cells may have had protective roles against threats and dangers from within much before the appearance of threats or dangers coming from without. It seems more likely that the immune system is uniquely endowed with protection of the latter because physiologically it fulfils continuously the former. Particularly as the result of its role in iron homeostasis.
Concluding remark
As I use the hunter metaphor, I wonder if the fact that Science has for many centuries been the domain of hunters only makes this tribute to a woman scientist the more opportune at the turn of another century. Time again and alone will tell if she is a pioneer also in changing the Hunter Paradigm in Science or (more modestly) in Cancer Research. From what I have watched as an outsider the answer would be yes. But as a scientist I know better for:“what we know is only a small part of what we do not know”.47
Acknowledgements
Drs António Carvalho and Assunção Júdice are most gratefully acknowledged for leading me to the original letters of Alexis Carrell to Reynaldo dos Santos. Arlinda Brandão for putting me on to ref. 18 before I had time to catch a plane and see it on the other side of an ocean. Mary Helen Barcellos-Hoff deserves special thanks for having kindly invited me to participate in this issue. Susana João Oliveira made sure that the reviewers' remarks deserved all my attention and is gratefully acknowledged for the final review of the manuscript. The reviewers themselves merit my acknowledgement for the care they put in the reading of the paper. But in fairness the greatest acknowledgment should go to Mina Bissell herself for her uniquely inspiring inner force and courage not to betray it.References
- A. Carrell, 1905–08, Letters from Chicago and the Kockfeller Institute to Reynaldo dos Santos, from the personal archives of dos Santos PT/CMC-CRSIQS/RS (Casa Reynaldo dos Santos Irene Quilhó dos Santos/Fundo Reynaldo dos Santos).
- A. Carrell, 1912, Nobel Lecture.
- Nobel Laureates, 1980, The Nobel Foundation.
- M. J. Bissell, P. A. Kenny and D. C. Radisky, Microenvironmental regulators of tissue structure and function also regulate tumor induction and progression: The role of extracellular matrix and its degrading enzymes, Molecular Approaches to Controlling Cancer, 2005, 70, 343–356 Search PubMed.
- J. Folkman, P. Cole and S. Zimmerman, Tumor Behavior tin Isolated Perfused Organs - in Vitro Growth and Metastases of Biopsy Material tin Rabbit Thyroid and Canine Intestinal Segment, Ann. Surg., 1966, 164(3), 491–502 CrossRef CAS.
- M. J. Bissell, The differentiated state of normal and malignant cells or how to define a “normal” cell in culture, Int. Rev. Cytol., 1981, 70, 27–100 Search PubMed.
- M. J. Bissell, H. G. Hall and G. Parry, How Does the Extracellular-Matrix Direct Gene-Expression, J. Theor. Biol., 1982, 99(1), 31–68 CrossRef CAS.
- D. S. Dolberg and M. J. Bissell, Inability of Rous-Sarcoma Virus to Cause Sarcomas in the Avian Embryo, Nature, 1984, 309(5968), 552–556 CrossRef CAS.
- J. Folkman, M. Bach, J. W. Rowe, F. Davidoff, P. Lambert, C. Hirsch, A. Goldberg, H. H. Hiatt, J. Glass and E. Henshaw, Tumor Angiogenesis - Therapeutic Implications, N. Engl. J. Med., 1971, 285(21), 1182–1186 CAS.
- J. Folkman, K. Watson, D. Ingber and D. Hanahan, Induction of Angiogenesis during the Transition from Hyperplasia to Neoplasia, Nature, 1989, 339(6219), 58–61 CrossRef CAS.
- J. Folkman, E. Merler, C. Abernath and G. Williams, Isolation of a Tumor Factor Responsible for Angiogenesis, J. Exp. Med., 1971, 133(2), 275–288 CrossRef CAS.
- M. de Sousa, Lymphocyte Circulation. Experimental and Clinical Aspects, Wiley and Sons, Chichester and New York, 1981 Search PubMed.
- D. V. Parrott, M. A. De Sousa and J. East, Thymus-dependent areas in the lymphoid organs of neonatally thymectomized mice, J. Exp. Med., 1966, 123(1), 191–204 CrossRef CAS.
- M. de Sousa, Kinetics of the distribution of thymus and marrow cells in the peripheral lymphoid organs of the mouse: ecotaxis, Clin. Exp. Immunol., 1971, 9(3), 371–380 CAS.
- M. De Sousa, N. L. Tilney and J. W. Kupiecweglinski, Recognition of Self within Self - Specific Lymphocyte Positioning and the Extracellular-Matrix, Immunol. Today, 1991, 12(8), 262–266 CrossRef CAS.
- M. De Sousa, Lymphoid cell positioning: a new proposal for the mechanism of control of lymphoid cell migration, Symp. Soc. Exp. Biol., 1978, 32, 393–410 Search PubMed.
- M. de Sousa, A. Smithyman and C. Tan, Suggested models of ecotaxopathy in lymphoreticular malignancy. A role for iron-binding proteins in the control of lymphoid cell migration, American Journal of Pathology, 1978, 90(2), 497–520 CAS.
- Z. K. Pinnix, L. D. Miller, W. Wang, R. D'Agostino, Jr, T. Kute, M. C. Willingham, H. Hatcher, L. Tesfay, G. Sui, X. Di, S. V. Torti and F. M. Torti, Ferroportin and iron regulation in breast cancer progression and prognosis, Sci. Transl. Med., 2010, 2(43), 43ra56 Search PubMed.
- P. G. Herman, I. Yamamoto and H. Z. Mellins, Blood microcirculation in the lymph node during the primary immune response, J. Exp. Med., 1972, 136(4), 697–714 CrossRef CAS.
- P. Bousso, T-cell activation by dendritic cells in the lymph node: lessons from the movies, Nat. Rev. Immunol., 2008, 8(9), 675–84 CrossRef CAS.
- B. Breart, F. Lemaitre, S. Celli and P. Bousso, Two-photon imaging of intratumoral CD8+ T cell cytotoxic activity during adoptive T cell therapy in mice, J. Clin. Invest., 2008, 118(4), 1390–97 CrossRef CAS.
- T. C. Tzeng, S. Chyou, S. Tian, B. Webster, A. C. Carpenter, V. H. Guaiquil and T. T. Lu, CD11c(hi) dendritic cells regulate the re-establishment of vascular quiescence and stabilization after immune stimulation of lymph nodes, J. Immunol., 2010, 184(8), 4247–4257 CrossRef CAS.
- H. E. Kohrt, N. Nouri, K. Nowels, D. Johnson, S. Holmes and P. P. Lee, Profile of immune cells in axillary lymph nodes predicts disease-free survival in breast cancer, PLoS Med., 2005, 2(9), e284 CrossRef.
- A. M. Jubb, E. J. Soilleux, H. Turley, G. Steers, A. Parker, I. Low, J. Blades, J. L. Li, P. Allen, R. Leek, I. Noguera-Troise, K. C. Gatter, G. Thurston and A. L. Harris, Expression of vascular notch ligand delta-like 4 and inflammatory markers in breast cancer, Am. J. Pathol., 2010, 176(4), 2019–2028 CrossRef.
- R. M. Steinman, Dendritic cells in vivo: a key target for a new vaccine science, Immunity, 2008, 29(3), 319–324 CrossRef CAS.
- E. Nemeth, M. S. Tuttle, J. Powelson, M. B. Vaughn, A. Donovan, D. M. Ward, T. Ganz and J. Kaplan, Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization, Science, 2004, 306(5704), 2090–2093 CrossRef CAS.
- X. P. Jiang, R. L. Elliott and J. F. Head, Manipulation of Iron Transporter Genes Results in the Suppression of Human and Mouse Mammary Adenocarcinomas, Anticancer Research, 2010, 30(3), 759–765 CAS.
- G. Cairo, F. Bernuzzi and S. Recalcati, A precious metal: Iron, an essential nutrient for all cells, Genes Nutr., 2006, 1(1), 25–39 Search PubMed.
- C. Delaby, N. Pilard, G. Hetet, F. Driss, B. Grandchamp, C. Beaumont and F. Canonne-Hergaux, A physiological model to study iron recycling in macrophages, Exp. Cell Res., 2005, 310(1), 43–53 CrossRef CAS.
- L. S. Ojalvo, W. King, D. Cox and J. W. Pollard, High-Density Gene Expression Analysis of Tumor-Associated Macrophages from Mouse Mammary Tumors, Am. J. Pathol., 2009, 174(3), 1048–1064 CrossRef CAS.
- J. A. Zuk and R. A. Walker, Immunohistochemical analysis of HLA antigens and mononuclear infiltrates of benign and malignant breast, J. Pathol., 1987, 152(4), 275–285 CrossRef CAS.
- R. D. Leek, C. E. Lewis, R. Whitehouse, M. Greenall, J. Clarke and A. L. Harris, Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma, Cancer Research, 1996, 56(20), 4625–4629 CAS.
- S. Recalcati, M. Locati, A. Marini, P. Santambrogio, F. Zaninotto, M. De Pizzol, L. Zammataro, D. Girelli and G. Cairo, Differential regulation of iron homeostasis during human macrophage polarized activation, Eur. J. Immunol., 2010, 40(3), 824–835 CrossRef CAS.
- A. Sica, T. Schioppa, A. Mantovani and P. Allavena, Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: Potential targets of anti-cancer therapy, Eur. J. Cancer, 2006, 42(6), 717–727 CrossRef CAS.
- D. M. Mosser and J. P. Edwards, Exploring the full spectrum of macrophage activation, Nat. Rev. Immunol., 2008, 8(12), 958–969 CrossRef CAS.
- G. Solinas, S. Schiarea, M. Liguori, M. Fabbri, S. Pesce, L. Zammataro, F. Pasqualini, M. Nebuloni, C. Chiabrando, A. Mantovani and P. Allavena, Tumor-Conditioned Macrophages Secrete Migration-Stimulating Factor: A New Marker for M2-Polarization, Influencing Tumor Cell Motility, J. Immunol., 2010, 185(1), 642–652 CrossRef CAS.
- J. P. Pinto, V. Dias, H. Zoller, G. Porto, H. Carmo, F. Carvalho and M. de Sousa, Hepcidin messenger RNA expression in human lymphocytes, Immunology, 2010, 130(2), 217–30 CrossRef CAS.
- L. G. Coffman, D. Parsonage, R. D'Agostino, Jr, F. M. Torti and S. V. Torti, Regulatory effects of ferritin on angiogenesis, Proc. Natl. Acad. Sci. U. S. A., 2009, 106(2), 570–575 CrossRef CAS.
- J. C. Zhang, F. Donate, X. P. Qi, N. P. Ziats, J. C. Juarez, A. P. Mazar, Y. P. Pang and K. R. McCrae, The antiangiogenic activity of cleaved high molecular weight kininogen is mediated through binding to endothelial cell tropomyosin, Proc. Natl. Acad. Sci. U. S. A., 2002, 99(19), 12224–12229 CrossRef CAS.
- R. W. Colman, B. A. Jameson, Y. Z. Lin, D. Johnson and S. A. Mousa, Domain 5 of high molecular weight kininogen (kininostatin) down-regulates endothelial cell proliferation and migration and inhibits angiogenesis, Blood, 2000, 95(2), 543–550 CAS.
- R. Xu, A. Boudreau and M. Bissell, Tissue architecture and function: dynamic reciprocity via extra- and intra-cellular matrices, Cancer Metastasis Rev., 2009, 28(1–2), 167–176 CrossRef.
- K. Palucka, H. Ueno, L. Roberts, J. Fay and J. Banchereau, Dendritic Cells Are They Clinically Relevant?, Cancer J., 2010, 16(4), 318–324 CrossRef CAS.
- K. Miller, M. L. Wang, J. Gralow, M. Dickler, M. Cobleigh, E. A. Perez, T. Shenkier, D. Cella and N. E. Davidson, Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer, N. Engl. J. Med., 2007, 357(26), 2666–2676 CrossRef CAS.
- K. Galmbacher, M. Heisig, C. Hotz, J. Wischhusen, A. Galmiche, B. Bergmann, I. Gentschev, W. Goebel, U. R. Rapp and J. Fensterle, Shigella mediated depletion of macrophages in a murine breast cancer model is associated with tumor regression, PLoS One, 2010, 5(3), e9572 CrossRef.
- W. B. Coley, II, Contribution to the Knowledge of Sarcoma, Ann. Surg., 1891, 14(3), 199–220 Search PubMed.
- H. C. Nauts, G. A. Fowler and F. H. Bogatko, A review of the influence of bacterial infection and of bacterial products (Coley's toxins) on malignant tumors in man; a critical analysis of 30 inoperable cases treated by Coley's mixed toxins, in which diagnosis was confirmed by microscopic examination selected for special study, Acta. Med. Scand. Suppl., 1953, 276, 1–103 Search PubMed.
- G. Horta, Botanist, author of Coloquios dos Simples e Drogas Medicinais da India. 1563.
Footnote |
| † Published as part of an Integrative Biology themed issue in honour of Mina J. Bissell: Guest Editor Mary Helen Barcellos-Hoff. |
| This journal is © The Royal Society of Chemistry 2011 |
