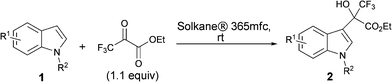
Catalyst-free and catalytic Friedel–Crafts alkylations of indoles in Solkane® 365mfc, an environmentally benign alternative solvent†
Xiu-Hua
Xu
,
Akihiro
Kusuda
,
Etsuko
Tokunaga
and
Norio
Shibata
*
Department of Frontier Materials, Graduate School of Engineering, Nagoya Institute of Technology, Gokiso, Showa-ku, Nagoya, 466-8555, Japan. E-mail: nozshiba@nitech.ac.jp; Fax: +81 52-735-5442
First published on 19th November 2010
Abstract
Solkane® 365mfc is a proven environmentally benign alternative solvent for catalyst-free Friedel–Crafts (F–C) alkylations of indoles with trifluoropyruvate and glyoxylate. Their enantioselective variants are also achieved by virtue of the high-affinity of fluorous cinchona alkaloids catalysts to Solkane® 365mfc to provide F–C adducts in excellent yields with good to excellent ees (up to 96% ee).
Introduction
The Friedel–Crafts (F–C) reaction and its variants are of key importance to practising synthetic organic chemists.1 The reaction consists of the electrophilic substitution of an aromatic nucleus with alkyl or acyl halides, or their equivalents, in the presence of catalysts such as Lewis acids. A combination of arenes and electrophiles is therefore capable of providing many structural variants of aromatic compounds for different purposes. These variants involve the addition of a heteroaromatic π nucleophile to a ketone or activated alkene electrophile.2 Among heteroaromatic systems, indoles have received much attention,3 since they are important structural motifs frequently encountered in biologically-active natural products, as well as in pharmaceuticals and agrochemicals. The most commonly used solvents for F–C alkylations of indoles are dichloromethane, toluene and ether. These solvents have some drawbacks such as toxicity and peroxide formation. Increasingly tight restrictions on the use of organic solvents in industrial synthesis4 has led us to search for an environmentally benign alternative solvent for the F–C reactions of indoles.5,6 Great efforts have been made to achieve the goal of making the F–C reaction of indoles a truly green process, while still retaining the high yield and selectivity, using green solvents, and, better still, by avoiding the use of any catalyst. The use of water or ionic liquids as solvents in environmentally friendly F–C reactions of indoles has generated considerable interest.7 The Kantam and Xiao groups have reported examples of ionic liquids used as both solvent and catalyst for the F–C alkylation of indoles.7k,l Following urgent demands by “green chemistry”, the development of new environmentally friendly solvents has created a fast-growing interest. Recently, we reported for the first time that Solkane® 365mfc, a hydrofluorocarbon (1,1,1,3,3-pentafluorobutane), developed by Solvay Fluor GmbH, was used as an environmentally benign alternative solvent for the nucleophilic trifluoromethylation of aldehydes, ketones and oxazolidinone.8a Indeed, as a green solvent, Solkane® 365mfc is non-toxic and has no impact whatsoever on the ozone layer.9 Although Solkane® 365mfc has a flash point below −27 °C, but is difficult to ignite. The minimum ignition energy is around 50 times higher than of n-pentane and is 10.8 mJ (25 °C, 8 vol% in air at 1 bar).9 It is used as an insulating and blowing agent for polyurethane foams, whose main uses are the thermal insulation of residential and industrial buildings, as well as in cold storage. Solkane® 365mfc is now produced in a pilot plant with a capacity of several hundred tons per year. As part of our ongoing fluorine chemistry project8 concerning the use of Solkane® 365mfc as a green medium in organic reactions,8a we herein show the catalyst-free F–C alkylation of indoles with trifluoropyruvate and glyoxylate in Solkane® 365mfc. Their enantioselective variants have also been achieved by virtue of the high-affinity of fluorous cinchona alkaloids catalysts to Solkane® 365mfc as a chiral source. Thus, the catalyst-free F–C reaction and the fluorous cinchona alkaloid-catalyzed asymmetric F–C reaction of indoles have been realized in Solkane® 365mfc for the first time (Scheme 1).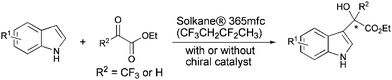 | ||
| Scheme 1 The catalyst-free F–C reaction and catalytic enantioselective F–C reaction of indoles in Solkane® 365mfc. | ||
Results and discussion
Catalyst-free Friedel–Crafts alkylation of indoles
Heterocyclic organofluorine compounds are receiving a great deal of attention in pharmaceutical, agrochemical and material sciences due to the unique features brought by the fluorine atom to the physical and chemical features of the parent molecules.10 Our initial investigation hence started with the F–C alkylation of indoles with ethyl trifluoropyruvate in Solkane® 365mfc. This transformation is usually accomplished under the catalysis of a Lewis and Brønsted acid.6b,11Török, Prakash and co-workers have reported that the reaction of indole (1a) with ethyl trifluoropyruvate proceeded in ether solution without using a catalyst.6b However, the reaction times were as long as 90 h and the reaction rates can be magnified by two orders in the presence of catalyst.6b,11e To our delight, when Solkane® 365mfc was used as the medium, this reaction was complete in 1 h at room temperature, without the addition of a catalyst, giving the desired product in 92% yield (Table 1, entry 1). The favorable outcome might be caused by the intimate interaction between fluorinated compounds and fluorinated solvents, i.e., ethyl trifluoropyruvate and Solkane® 365mfc,10f although it is not clear. This result encouraged us to apply this system to various substituted indoles (Table 1, entries 2–16). In all cases, the reactions were usually complete within 0.5–2 h, and high to excellent yields were achieved for various indole derivatives independent of the substituents at different positions of the indole ring. A wide range of functional groups, including electron-withdrawing, electron-donating and neutral groups, on the indole ring were well-tolerated. The presence of bulky substituents on the indole ring, such as phenyl and tert-butyl, also furnished the corresponding products in good to excellent yields (Table 1, entries 3, 14 and 16). It is noteworthy that this reaction proceeded with excellent yield with an N-methyl-protected indole (Table 1, entry 17). What is more, in a slightly large-scale preparation, the product was isolated by distillation to give 2a in 96% yield, while Solkane® 365mfc was recovered in 81% yield (Table 1, entry 18).|
|
||||||
|---|---|---|---|---|---|---|
| Entry | 1 | R1 | R2 | Time/h | 2 | Yield (%)a |
| a Isolated yield by silica-gel column chromatography. b The reaction was performed at a slightly large scale and product 2a was isolated by distillation. Solkane® 365mfc was recovered in 81% yield. See ESI. | ||||||
| 1 | 1a | H | H | 1.0 | 2a | 92 |
| 2 | 1b | 2-Me | H | 0.5 | 2b | 91 |
| 3 | 1c | 2-Ph | H | 0.5 | 2c | 84 |
| 4 | 1d | 4-Me | H | 2.0 | 2d | 83 |
| 5 | 1e | 5-Me | H | 0.5 | 2e | 96 |
| 6 | 1f | 5-OMe | H | 0.5 | 2f | 96 |
| 7 | 1g | 5-F | H | 1.0 | 2g | 90 |
| 8 | 1h | 5-Cl | H | 2.0 | 2h | 91 |
| 9 | 1i | 5-Br | H | 2.0 | 2i | 89 |
| 10 | 1j | 5-I | H | 1.0 | 2j | 95 |
| 11 | 1k | 6-Me | H | 0.5 | 2k | 99 |
| 12 | 1l | 7-Me | H | 0.5 | 2l | 99 |
| 13 | 1m | 7-Et | H | 0.5 | 2m | 97 |
| 14 | 1n | 7-But | H | 1.0 | 2n | 94 |
| 15 | 1o | 7-Br | H | 1.0 | 2o | 85 |
| 16 | 1p | 7-Ph | H | 2.0 | 2p | 96 |
| 17 | 1q | H | Me | 0.5 | 2q | 91 |
| 18b | 1a | H | H | 0.25 | 2a | 96 |
Solkane® 365mfc was also found to be useful for the catalyst-free F–C alkylation of indoles with ethyl glyoxylate at room temperature to give the products in good to high yields (Table 2), although a longer reaction time was required compared to the reaction with ethyl trifluoropyruvate, in particular, the indole containing an electron-withdrawing carboxylate group (Table 2, entry 10).
|
|
|||||
|---|---|---|---|---|---|
| Entrya | 1 | R | Time/h | 3 | Yield (%)b |
| a Commercially available ethyl glyoxylate (∼50 wt% in toluene) was used. b Isolated yield by silica gel column chromatography. | |||||
| 1 | 1a | H | 1.0 | 3a | 73 |
| 2 | 1b | 2-Me | 4.0 | 3b | 89 |
| 3 | 1d | 4-Me | 3.0 | 3d | 56 |
| 4 | 1e | 5-Me | 1.0 | 3e | 66 |
| 5 | 1f | 5-OMe | 1.5 | 3f | 79 |
| 6 | 1g | 5-F | 1.0 | 3g | 56 |
| 7 | 1h | 5-Cl | 1.5 | 3h | 54 |
| 8 | 1i | 5-Br | 2.0 | 3i | 82 |
| 9 | 1j | 5-I | 16.0 | 3j | 84 |
| 10 | 1r | 5-CO2Me | 24.0 | 3r | 29 |
| 11 | 1k | 6-Me | 1.0 | 3k | 75 |
| 12 | 1l | 7-Me | 2.0 | 3l | 73 |
Catalytic enantioselective Friedel–Crafts alkylation of indoles
Our success in the catalyst-free F–C reactions of indoles in Solkane® 365mfc prompted us to extend to catalytic asymmetric reactions (Table 3). Török, Prakash and co-workers have reported that natural cinchona alkaloids catalyze the highly enantioselective F–C alkylation of indoles with ethyl trifluoropyruvate.6b The reaction proceeded in ether at −8 °C. We thus examined whether their asymmetric catalytic system was applicable to Solkane® 365mfc. Regrettably, the natural cinchona alkaloids, i.e., cinchonidine (CD), cinchonine (CN), quinine (QN) and quinidine (QD), gave F–C adducts with much lower ee values in Solkane® 365mfc compared to ether, despite excellent yields being achieved (Table 3, entries 1–8). This is probably due to the poor solubility of the natural cinchona alkaloids in Solkane® 365mfc at −8 °C. In fact, when the temperature of the QD-catalyzed F–C reaction of 1a was increased to rt, the selectivity increased from 22 to 48% ee (Table 3, entries 8 and 9). To further improve the enantioselectivity, three effective catalysts 4a, 4b and 4c, reported by the Deng group,6d were attempted to catalyze the reaction in Solkane® 365mfc. However, the ee values were moderate, 46–58% (Table 3, entries 10–12). Considering that fluorinated compounds should dissolve well in fluorinated solvents, we designed novel fluorinated cinchona alkaloids 4d and 4e (see the ESI† for details of their preparation). The ee values improved slightly to 60–62% (Table 3, entries 13 and 14). Since the non-catalytic F–C reaction of indoles with trifluoropyruvate was very fast in Solkane® 365mfc, as revealed in the first part of this article, ethyl trifluoropyruvate was slowly added to the reaction mixture over 1 h; ee values were improved to 70–72% (Table 3, entries 15 and 16).|
|
||||||
|---|---|---|---|---|---|---|
| Entry | Catalyst | Solvent | Temperature | Yield (%)a | ee (%)b | Configurationc |
| a Isolated yield by silica gel column chromatography. b Determined by chiral HPLC analysis. c The absolute configuration was assigned by comparison with the reported optical rotation.11b d Trifluoropyruvate was added slowly to the reaction mixture for 1 h. | ||||||
| 1 | CD | Et2O | −8 °C | 91 | 76 | S |
| 2 | CD | Solkane® | −8 °C | 98 | 10 | S |
| 3 | CN | Et2O | −8 °C | 97 | 62 | R |
| 4 | CN | Solkane® | −8 °C | 97 | 14 | R |
| 5 | QN | Et2O | −8 °C | 98 | 77 | S |
| 6 | QN | Solkane® | −8 °C | 96 | 46 | S |
| 7 | QD | Et2O | −8 °C | 94 | 64 | R |
| 8 | QD | Solkane® | −8 °C | 98 | 22 | R |
| 9 | QD | Solkane® | rt | 98 | 48 | R |
| 10 | 4a | Solkane® | rt | 96 | 58 | S |
| 11 | 4b | Solkane® | rt | 89 | 50 | S |
| 12 | 4c | Solkane® | rt | 78 | 46 | S |
| 13 | 4d | Solkane® | rt | 98 | 62 | S |
| 14 | 4e | Solkane® | rt | 91 | 60 | S |
| 15d | 4d | Solkane® | rt | 91 | 70 | S |
| 16d | 4e | Solkane® | rt | 93 | 72 | S |
The asymmetric F–C alkylation of various indole substrates in Solkane® 365mfc was next carried out to examine the generality and scope of the reaction using catalyst 4e and its pseudo-enantiomer 4e′ prepared from quinine (see the ESI† for details of the preparation) (Table 4). As can be seen in Table 4, catalysts 4e and 4e′ proved to be effective for the asymmetric F–C reaction of indoles in Solkane® 365mfc to furnish the products in high to excellent yields with moderate to high enantioselectivities (Table 4, entries 1–18). It should be mentioned that both enantiomers of the products were selectively obtained by the choice of catalyst, 4e or 4e′. Substituents at the 4, 5 and 6-positions gave lower ee values compared to reactant 1a (Table 4, entries 2–4 and 11–13), while the substituents at position 7 gave higher ee values (Table 4, entries 5–9 and 14–18). The best results were obtained when 7-methylindole reacted with catalyst 4e to give the S-isomeric product with an 85% ee (Table 4, entry 5) and when 7-ethylindole reacted with catalyst 4e′ to give the R-isomeric product with an 82% ee (Table 4, entry 15).
|
|
||||||
|---|---|---|---|---|---|---|
| Entrya | 1 | R | 4 | 2 b | Yield (%)c | ee (%)d |
| a Trifluoropyruvate was added slowly to the reaction mixture over 1 h. b The absolute configuration was assigned by comparison with the reported optical rotation.11g c Isolated yield by silica gel column chromatography. d Determined by chiral HPLC analysis. | ||||||
| 1 | 1a | H | 4e | (S)-2a | 93 | 72 |
| 2 | 1d | 4-Me | 4e | (S)-2d | 84 | 48 |
| 3 | 1g | 5-F | 4e | (S)-2g | 98 | 70 |
| 4 | 1k | 6-Me | 4e | (S)-2k | 88 | 55 |
| 5 | 1l | 7-Me | 4e | (S)-2l | 92 | 85 |
| 6 | 1m | 7-Et | 4e | (S)-2m | 97 | 81 |
| 7 | 1n | 7-But | 4e | (S)-2n | 81 | 79 |
| 8 | 1o | 7-Br | 4e | (S)-2o | 80 | 79 |
| 9 | 1p | 7-Ph | 4e | (S)-2p | 95 | 75 |
| 10 | 1a | H | 4e′ | (R)-2a | 98 | 71 |
| 11 | 1d | 4-Me | 4e′ | (R)-2d | 78 | 43 |
| 12 | 1g | 5-F | 4e′ | (R)-2g | 99 | 56 |
| 13 | 1k | 6-Me | 4e′ | (R)-2k | 91 | 63 |
| 14 | 1l | 7-Me | 4e′ | (R)-2l | 99 | 77 |
| 15 | 1m | 7-Et | 4e′ | (R)-2m | 92 | 82 |
| 16 | 1n | 7-But | 4e′ | (R)-2n | 86 | 80 |
| 17 | 1o | 7-Br | 4e′ | (R)-2o | 79 | 58 |
| 18 | 1p | 7-Ph | 4e′ | (R)-2p | 93 | 74 |
Importantly, this asymmetric catalytic system was effectively used in asymmetric F–C alkylations with ethyl glyoxylate (Table 5). In most cases, good to excellent yields (except for 1r; Table 5, entry 7) and good to excellent ee values were obtained (Table 5, entries 1–9). Again, both enantiomers of the products were selectively obtained by the choice of catalyst, 4e or 4e′. The reactions with 7-methylindole gave the best results for both isomeric products with 94% and 96% ee, respectively (entry 9).
|
|
||||||
|---|---|---|---|---|---|---|
| Entrya | 1 | R | Time/h | 3 | Yield (%)b | ee (%)c |
| a Commercially available ethyl glyoxylate (∼50 wt% in toluene) was used. b Isolated yield by silica gel column chromatography. Values in parentheses indicate the yield catalyzed by 4e′. c Determined by chiral HPLC analysis. Values in parentheses indicate the ee catalyzed by 4e′. | ||||||
| 1 | 1a | H | 1.0 | 3a | 80 (92) | 90 (92) |
| 2 | 1b | 2-Me | 1.0 | 3b | 99 (99) | 85 (85) |
| 3 | 1d | 4-Me | 1.0 | 3d | 77 (84) | 89 (92) |
| 4 | 1e | 5-Me | 1.0 | 3e | 99 (91) | 87 (91) |
| 5 | 1f | 5-OMe | 1.0 | 3f | 96 (92) | 83 (88) |
| 6 | 1g | 5-F | 1.0 | 3g | 74 (90) | 89 (92) |
| 7 | 1r | 5-CO2Me | 3.0 | 3r | 8 (25) | 81 (90) |
| 8 | 1k | 6-Me | 1.0 | 3k | 79 (99) | 89 (92) |
| 9 | 1l | 7-Me | 1.0 | 3l | 99 (99) | 94 (96) |
Catalyst-free and Catalytic enantioselective Friedel–Crafts alkylation of indoles in Solkane® 365/227 blend solvent
Finally, we examined the F–C reactions in commercially available Solkane® 365/227 blend solvent (365/227 = 93/7).12 It is disclosed that Solkane® 365mfc has the flash point ≤ −27 °C; however, its blend form (93/7 mixture of Solkane® 365mfc and Solkane® 227, 1,1,1,2,3,3,3-heptafluoropropane) as Solkane® 365/227 is known to have no flash point (non-flammable) and could also provide the benefits of Solkane® 365mfc.9b Therefore, we compared the reactivity pattern of the present F–C alkylations of indoles with trifluoropyruvate and glyoxylate in both solvents-Solkane® 365mfc and Solkane® 365/227. The results are shown in Table 6 and Table 7. As might be expected, reactivity behaviour such as reaction rates, chemical yields and enantioselectivity were almost identical in Solkane® 365mfc and Solkane® 365/227.|
|
||||
|---|---|---|---|---|
| Entrya | Solvent | Catalyst | Yield (%)b | ee (%)c |
| a Trifluoropyruvate was added slowly to the reaction mixture over 1 h (entries 3–6). b Isolated yield by silica gel column chromatography. c Determined by chiral HPLC analysis. | ||||
| 1 | Solkane®365mfc | — | 92 | — |
| 2 | Solkane®365/227 (93/7) | — | 97 | — |
| 3 | Solkane®365mfc | 4e | 93 | 72 |
| 4 | Solkane®365/227 (93/7) | 4e | 91 | 73 |
| 5 | Solkane®365mfc | 4e′ | 98 | 71 |
| 6 | Solkane®365/227 (93/7) | 4e′ | 89 | 72 |
|
|
||||
|---|---|---|---|---|
| Entrya | Solvent | Catalyst | Yield (%)b | ee (%)c |
| a Commercially available ethyl glyoxylate (∼50 wt% in toluene) was used. b Isolated yield by silica gel column chromatography. c Determined by chiral HPLC analysis. | ||||
| 1 | Solkane®365mfc | — | 73 | — |
| 2 | Solkane®365/227 (93/7) | — | 71 | — |
| 3 | Solkane®365mfc | 4e | 80 | 90 |
| 4 | Solkane®365/227 (93/7) | 4e | 92 | 90 |
| 5 | Solkane®365mfc | 4e′ | 92 | 92 |
| 6 | Solkane®365/227 (93/7) | 4e′ | 96 | 91 |
Conclusions
The environmentally benign solvent Solkane® 365mfc is introduced as a medium for the first time for the F–C reaction of indoles with trifluoropyruvate and glyoxylate. The reaction proceeds nicely in Solkane® 365mfc at rt without any help from a promoter. Enantioselective variants of this reaction have also been achieved by virtue of the high-affinity of fluorous cinchona alkaloid catalysts to Solkane® 365mfc to afford the desired products in excellent yields with good to excellent ee values. Solkane® 365mfc is also useful for both catalyst-free and catalytic F–C reactions, even in the presence of 7 vol% of Solkane® 227, which provides additional benefits from a green chemistry point of view, since the Solkane® 365/227 blend (365/227 = 93/7) is completely non-flammable. The F–C reaction is ubiquitous and one of the key transformations, not only in laboratory-scale chemistry, but also in industrial process chemistry. We hope that Solkane® 365mfc will become a green organic solvent of choice alongside water and ionic liquids in industrial process chemistry. Extensions to other ubiquitous organic reactions with Solkane® 365mfc as a medium is currently under investigation.Experimental
Typical procedure for the catalyst-free F–C alkylation of indole 1a with ethyl trifluoropyruvate in Solkane® 365mfc
To a stirred solution of 1a (23.4 mg, 0.20 mmol) in Solkane® 365mfc (0.5 mL) was added ethyl trifluoropyruvate (29.2 μL, 0.22 mmol) at room temperature under a nitrogen atmosphere. The mixture was stirred at room temperature for 1 h. Then, the solvent was removed in vacuo, and the residue was purified by column chromatography (n-hexane–ethyl acetate = 80/20) to give 2a as a white solid (52.9 mg, 92%).Typical procedure for the catalytic enantioselective F–C alkylation of indole 1a with ethyl trifluoropyruvate in Solkane® 365mfc
To a stirred solution of 1a (11.7 mg, 0.10 mmol) and catalyst 4e (8.2 mg, 0.01 mmol) in Solkane® 365mfc (0.3 mL) was added slowly ethyl trifluoropyruvate (14.6 μL, 0.11 mmol) in Solkane® 365mfc (0.2 mL) over 1 h at room temperature under a nitrogen atmosphere. The mixture was stirred at room temperature for 1 h. Then the solvent was removed in vacuo, and the residue was purified by column chromatography (n-hexane–ethyl acetate = 80/20) to give (S)-2a as a white solid (26.6 mg, 93%, 72% ee).Acknowledgements
This study was financially supported in part by Grants-in-Aid for Scientific Research (B21390030, 22106515) and the Chubu Bureau of Economy Trade and Industry. We thank Dr Max Braun and Mr Toyofuku Yoshitaka, Solvay Fluor GmbH, for the generous gift of Solkane® 365mfc and Solkane® 365/227. We are also grateful to Central Glass Co. Ltd. for a gift of ethyl trifluoropyruvate.Notes and references
- (a) G. A. Olah, in Friedel–Crafts and Related Reactions, Wiley, New York, 1963 Search PubMed; (b) G. A. Olah, in Friedel–Crafts Chemistry, Wiley, New York, 1973 Search PubMed; (c) R. M. Roberts, A. A. Khalaf, in Friedel–Crafts Alkylation Chemistry. A Century of Discovery, Dekker, New York, 1984 Search PubMed; (d) G. A. Olah, R. Krishnamurti, G. K. S. Prakash, Friedel–Crafts Alkylation in Comprehensive Organic Synthesis, ed. B. M. Trost and I. Flemming, Pergamon Press, Oxford, 1991, Vol. 3, pp. 293 Search PubMed; (e) R. C. Larock, in Comprehensive Organic Transformations, VCH, New York, 1999 Search PubMed.
- For reviews, see: (a) M. Bandini, A. Melloni and A. Umani-Ronchi, Angew. Chem., Int. Ed., 2004, 43, 550 CrossRef CAS; (b) T. B. Poulsen and K. A. Jørgensen, Chem. Rev., 2008, 108, 2903 CrossRef CAS; (c) S.-L. You, Q. Cai and M. Zeng, Chem. Soc. Rev., 2009, 38, 2190 RSC.
- For reviews, see: M. Bandini and A. Eichholzer, Angew. Chem., Int. Ed., 2009, 48, 9608 Search PubMed.
- (a) P. T. Anastas and M. M. Kirchhoff, Acc. Chem. Res., 2002, 35, 686 CrossRef CAS; (b) M. Doble, A. K. Kruthiventi, in Green Chemistry and Processes, Academic Press, Burlington, MA, 2007 Search PubMed; (c) V. K. Ahluwalia, in Green Chemistry: Environmentally Benign Reactions, CRC, Taylor & Francis, Boca Raton, FL, 200 8 Search PubMed.
- Solvent-free systems are obviously another solution; however, the solvent-free F–C alkylation of indoles only relies on racemic synthesis and might not be applied for enantioselective F–C reactions. See: (a) J.-L. Zhao, L. Liu, H.-B. Zhang, Y.-C. Wu, D. Wang and Y.-J. Chen, Synlett, 2006, 96 CAS; (b) J.-L. Zhao, L. Liu, H.-B. Zhang, Y.-C. Wu, D. Wang and Y.-J. Chen, Tetrahedron Lett., 2006, 47, 2511 CrossRef CAS; (c) K. Tabatabaeian, M. Mamaghani, N. O. Mahmoodi and A. Khorshidi, Tetrahedron Lett., 2008, 49, 1450 CrossRef CAS; (d) G. Bartoli, G. D. Antonio, S. Giuli, E. Marcantoni, M. Marcolini and M. Paoletti, Synthesis, 2008, 320 CAS.
- For recent examples of the enantioselective F–C alkylation of indoles, see: (a) M. Bandini, P. G. Cozzi, P. Melchiorre and A. Umani-Ronchi, Angew. Chem., Int. Ed., 2004, 43, 84 CrossRef; (b) B. Török, M. Abid, G. London, J. Esquibel, M. Török, S. C. Mhadgut, P. Yan and G. K. S. Prakash, Angew. Chem., Int. Ed., 2005, 44, 3086 CrossRef; (c) S. Shirakawa, R. Berger and J. L. Leighton, J. Am. Chem. Soc., 2005, 127, 2858 CrossRef CAS; (d) H.-M. Li, Y.-Q. Wang and L. Deng, Org. Lett., 2006, 8, 4063 CrossRef CAS; (e) Y.-Q. Wang, J. Song, R. Hong, H.-M. Li and L. Deng, J. Am. Chem. Soc., 2006, 128, 8156 CrossRef CAS; (f) M. Boudou, C. Ogawa and S. Kobayashi, Adv. Synth. Catal., 2006, 348, 2585 CrossRef CAS; (g) M. Terada, S. Yokoyama, K. Sorimachi and D. Uraguchi, Adv. Synth. Catal., 2007, 349, 1863 CrossRef CAS; (h) Q. Kang, Z.-A. Zhao and S.-L. You, J. Am. Chem. Soc., 2007, 129, 1484 CrossRef CAS; (i) M. Terada and K. Sorimachi, J. Am. Chem. Soc., 2007, 129, 292 CrossRef CAS; (j) D. Enders, A. A. Narine, F. Toulgoat and T. Bisschops, Angew. Chem., Int. Ed., 2008, 47, 5661 CrossRef CAS; (k) Y.-H. Hui, Q. Zhang, J. Jiang, L.-L. Lin, X.-H. Liu and X.-M. Feng, J. Org. Chem., 2009, 74, 6878 CrossRef CAS; (l) P. Bachu and T. Akiyama, Chem. Commun., 2010, 46, 4112 RSC; (m) L. Yang, Q.-M. Zhu, S.-M. Guo, B. Qian, C.-G. Xia and H.-M. Huang, Chem.–Eur. J., 2010, 16, 1638 CrossRef CAS; (n) W.-T. Wang, X.-H. Liu, W.-D. Cao, J. Wang, L.-L. Lin and X.-M. Feng, Chem.–Eur. J., 2010, 16, 1664 CrossRef CAS; (o) F.-F. Guo, G.-Y. Liu, S.-S. Xiong, S.-J. Wang and Z. Y. Wang, Chem.–Eur. J., 2010, 16, 6438 CAS; (p) H. Jiang, M. W. Paixão, D. Monge and K. A. Jørgensen, J. Am. Chem. Soc., 2010, 132, 2775 CrossRef CAS; (q) J. Lv, X. Li, L. Zhong, S.-Z. Luo and J.-P. Cheng, Org. Lett., 2010, 12, 1096 CrossRef CAS; (r) Y.-Y. Huang, E. Tokunaga, S. Suzuki, M. Shiro and N. Shibata, Org. Lett., 2010, 12, 1136 CrossRef CAS.
- (a) K. Manabe, N. Aoyama and S. Kobayashi, Adv. Synth. Catal., 2001, 343, 174 CrossRef CAS; (b) W. Zhuang and K. A. Jørgensen, Chem. Commun., 2002, 1336 RSC; (c) S. Shirakawa and S. Kobayashi, Org. Lett., 2006, 8, 4939 CrossRef CAS; (d) N. Azizi, F. Arynasab and M. R. Saidi, Org. Biomol. Chem., 2006, 4, 4275 RSC; (e) H. Lin and X.-W. Sun, Tetrahedron Lett., 2008, 49, 5343 CrossRef CAS; (f) V. P. Kumar, R. Sridhar, B. Srinivas, M. Narender and K. R. Rao, Can. J. Chem., 2008, 86, 907 CrossRef CAS; (g) M. Bandini and R. Sinisi, Org. Lett., 2009, 11, 2093 CrossRef CAS; (h) A. J. Boersma, B. L. Feringa and G. Roelfes, Angew. Chem., Int. Ed., 2009, 48, 3346 CrossRef CAS; (i) M. Ghandi and A. Taheri, Molecules, 2009, 14, 1056 Search PubMed; (j) M. D. Rosa and A. Soriente, Eur. J. Org. Chem., 2010, 1029; (k) M. L. Kantam, R Chakravarti, B. Rajashree and S. Bhargava, Synlett, 2008, 1449 CrossRef CAS; (l) J.-H. Lin, C.-P. Zhang, Z.-Q. Zhu, Q.-Y. Chen and J.-C. Xiao, J. Fluorine Chem., 2009, 130, 394 CrossRef CAS.
- (a) A. Kusuda, H. Kawai, S. Nakamura and N. Shibata, Green Chem., 2009, 11, 1733 RSC; (b) N. Shibata, A. Matsnev and D. Cahard, Beilstein J. Org. Chem., 2010, 6 Search PubMed; (c) N. Shibata, T. Furukawa and D. S. Reddy, Chem. Today, 2009, 27, 38 Search PubMed; (d) N. Shibata, S. Mizuta and H. Kawai, Tetrahedron: Asymmetry, 2008, 19, 2633 CrossRef CAS; (e) N. Shibata, T. Ishimaru, S. Nakamura and T. Toru, J. Fluorine Chem., 2007, 128, 469 CrossRef CAS; (f) N. Shibata, S. Mizuta and T. Toru, J. Synth. Org. Chem. Jpn., 2006, 65, 14.
- (a) See Solvay Solkane® 365 mfc brochure: http://www.solvay-fluor.com/product/lib/0,0,-_EN-1000798,00.html; (b) See Solvay Solkane® 365/227 brochure: http://www.solvay-fluor.com/product/lib/0,0,-_EN-1000292,00.html.
- (a) I. Ojima, J. R. McCarthy, J. T. Welch, in Biomedical Frontiers of Fluorine Chemistry, American Chemical Society, Washington, DC, 1996 Search PubMed; (b) T. Himaya, Organofluorine Compounds, Springer-Verlag, Berlin, Feidelberg, 2001 Search PubMed; (c) K. Uneyama, in Organofluorine Chemistry, Blackwell, Oxford, 2006 Search PubMed; (d) L. Paquette, in Fluorine-Containing Reagents, Wiley-VCH, Weinheim, 2007 Search PubMed; (e) J.-A. Ma and D. Cahard, Chem. Rev., 2008, 108, PR1 CrossRef CAS; (f) P. Kirsch, in Modern Fluoroorganic Chemistry, Wiley-VCH, Weinheim, 2004 Search PubMed.
- (a) N. Gathergood, W. Zhuang and K. A. Jørgensen, J. Am. Chem. Soc., 2000, 122, 12517 CrossRef CAS; (b) W. Zhuang, N. Gathergood, R. G. Hazell and K. A. Jørgensen, J. Org. Chem., 2001, 66, 1009 CrossRef CAS; (c) G. K. S. Prakash, P. Yan, B. Török and G. A. Olah, Synlett, 2003, 527 CrossRef CAS; (d) M. P. A. Lyle, N. D. Draper and P. D. Wilson, Org. Lett., 2005, 7, 901 CrossRef CAS; (e) M. Abid and B. Török, Adv. Synth. Catal., 2005, 347, 1797 CrossRef CAS; (f) M. Soueidan, J. Collin and R. Gil, Tetrahedron Lett., 2006, 47, 5467 CrossRef CAS; (g) S. Nakamura, K. Hyodo, Y. Nakamura, N. Shibata and T. Toru, Adv. Synth. Catal., 2008, 350, 1443 CrossRef CAS; (h) J. Nie, G.-W. Zhang, L. Wang, A.-P. Fu, Y. Zheng and J.-A. Ma, Chem. Commun., 2009, 2356 RSC; (i) J. Nie, G.-W. Zhang, L. Wang, D.-H. Zheng, Y. Zheng and J.-A. Ma, Eur. J. Org. Chem., 2009, 3145 CrossRef CAS; (j) H.-M. Dong, H.-H. Lu, L.-Q. Lu, C.-B. Chen and W.-J. Xiao, Adv. Synth. Catal., 2007, 349, 1597 CrossRef CAS.
- The idea of testing the F–C reaction in Solkane® 365/227 blend solvent in order to provide additional benefits of Solkane® 365mfc was kindly suggested by a reviewer of this manuscript.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details. See DOI: 10.1039/c0gc00619j |
| This journal is © The Royal Society of Chemistry 2011 |