Microwave-assisted solvent- and ligand-free copper-catalysed cross-coupling between halopyridines and nitrogen nucleophiles†
Zhen-Jiang
Liu
a,
Jean-Pierre
Vors
b,
Ernst R. F.
Gesing
*c and
Carsten
Bolm
*a
aInstitute of Organic Chemistry, RWTH Aachen University, Landoltweg 1, D-52056, Aachen, Germany. E-mail: Carsten.Bolm@oc.rwth-aachen.de
bBayer CropScience S, Centre de Recherche de La Dagoire 14–20, Rue Pierre Baizet, 69009, Lyon, France
cBayer CropScience AG, BCS-R-I-CI, Building 6550, Alfred-Nobel-Strasse 50, D-40789, Monheim am Rhein, Germany. E-mail: ernst-rudolf.gesing@bayer.com
First published on 12th November 2010
Abstract
N-Heteroarylated products are obtained in good yields by microwave-assisted solvent- and ligand-free copper-catalysed amination of halopyridines with nitrogen nucleophiles.
Substituted N-heterocycles are prevalent in biologically active compounds. Pyridine derivatives such as N-pyridinylpyrazoles, -imidazoles, -pyrroles, -indoles and -amides have attracted particular attention, and several of those compounds have found use in the biological, pharmaceutical and material sciences.1 Furthermore, they proved promising as ligands in metal catalysis.2 The required core structures can generally be assembled in a straightforward manner by copper-catalysed Ullmann-type reactions. However, the classical versions of this cross-coupling3 have several drawbacks, which greatly restrict their applications in industry. For example, they often require stoichiometric amounts of a copper reagent, a high reaction temperature and a polar solvent. Consequently, great efforts have been made to improve the efficiency of Ullmann-type reactions.4 Although many copper/ligand combinations are known to perform these transformations under comparatively mild conditions, there is still a need to find even simpler catalyst systems.4a,5 Ideally, they should be ligand-free6 and proceed in “green” solvents or even be solvent-free.7,8 Also microwave irradiation is of great importance in the context of green synthesis and sustainable chemistry,9 and it is also known to accelerate copper-mediated C–N coupling.6i,7b–c,10 In 2004, Gawley and co-workers reported SNAr reactions of halopyridines with pyrrolidine and piperidine derivatives under microwave irradiation, but a high temperature (130 °C) was required, and 3-halopyridines did not react at all.7b More recently, Liu and co-workers described copper-promoted amination reactions using microwave techniques.6i This method, however, needs stoichiometric amounts of copper reagents. In a continuation of our search for simple and environmentally friendly catalyst systems for C–N couplings,6b we herein disclose our findings on microwave-assisted solvent- and ligand-free copper-catalysed amination of halopyridines with various nitrogen nucleophiles leading to pyridinyl-substituted pyrazoles, imidazoles, pyrroles, indoles and amides.11
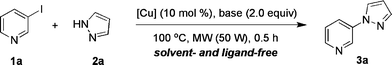
To establish the most efficient solvent- and ligand-free catalyst system under microwave irradiation conditions, various bases and copper sources were screened using the coupling of 3-iodopyridine (1a) with pyrazole (2a) as a model reaction. The results are summarised in Table 1. With reference to our previous report on ligand-free copper-catalysed N-arylation,6b all experiments were initially performed with 10 mol% of Cu2O and 2.0 equiv. of base under microwave irradiation at 100 °C for 0.5 h (Table 1, entries 1–8).12 Several bases proved applicable furnishing the coupling product, N-pyridinylpyrazole (3a), in yields ranging from 10–91%. Use of Cs2CO3 and K3PO4·H2O gave the best results affording the coupling product in 83 and 91% yield, respectively (entries 1 and 7). Using an excess of 3-iodopyridine resulted in a decrease in yield (entry 8). Also other copper sources such as CuI and CuBr catalysed the coupling reaction, but the yields of 3a were slightly lower than with Cu2O (entries 9 and 10). Control experiments confirmed that neither with the base alone (entry 11) nor in the absence of copper and base (entry 12) was the heteroarylated product formed. Thus, the cross-coupling did not proceed by a SNAr mechanism.13
|
|
|||
|---|---|---|---|
| Entry | Cu source | Base | Yield of 3a (%)b |
| a Reaction conditions: 3-iodopyridine (1a, 0.5 mmol), pyrazole (2a, 0.65 mmol), copper source (0.05 mmol), base (1.0 mmol), 100 °C, MW (50 W), 0.5 h. b Referring to the amount of product isolated by chromatography. c Use of 0.65 mmol of 1a and 0.5 mmol of 2a. | |||
| 1 | Cu2O | Cs2CO3 | 83 |
| 2 | Cu2O | K2CO3 | 70 |
| 3 | Cu2O | Na2CO3 | 10 |
| 4 | Cu2O | t-BuOK | 39 |
| 5 | Cu2O | t-BuONa | 69 |
| 6 | Cu2O | K3PO4 | 76 |
| 7 | Cu2O | K3PO4·H2O | 91 |
| 8c | Cu2O | K3PO4·H2O | 61 |
| 9 | CuI | K3PO4·H2O | 66 |
| 10 | CuBr | K3PO4·H2O | 77 |
| 11 | — | K3PO4·H2O | Traces |
| 12 | — | — | 0 |
Encouraged by the results of the reaction between 1a and 2a, the substrate scope of the microwave-assisted solvent- and ligand-free coupling was investigated. As shown in Table 2, the reactions between 3-iodopyridine (1a) and various heterocycles such as pyrazole, imidazole, pyrrole and indole proceeded well. In all cases the corresponding N-3-pyridinyl-substituted heterocycles were obtained, and the yields were moderate to excellent (up to 91%, Table 2, entries 1–4). Furthermore, 2-pyrrolidinone and acetamide coupled well with 1a affording the corresponding products in 90 and 74% yields, respectively (entries 5 and 6). The N-heteroarylation of phenylmethyl sulfoximine14 gave product 3g in 55% yield (entry 7).15Alkyl and aromatic amines did not react with 1a under these conditions (entries 8 and 9). However, when the temperature was raised to 130 °C and Cs2CO3 was used instead of K3PO4·H2O the coupling between 3-iodopyridine (1a) and benzylamine led to 3h albeit only in 12% yield (entry 8).
To compare relative reactivity, coupling between pyrazole (2a) and substituted pyridines having various leaving groups in 2,- 3- or 4-position was examined. The data are shown in Table 3. The best results were achieved in coupling of the iodo-substituted pyridines leading to the corresponding products in up to 91% (entries 1, 6, and 10). Also bromo derivatives coupled, but the yields are lower (up to 74%; entries 2, 7, and 11). 3-Chloro- and 3-fluoropyridine showed a very low reactivity, and the corresponding product was obtained in only 7 and 10% yield, respectively (entries 3 and 4). The coupling of pyrazole with 2-fluoropyridine and the hydrochlorides of 4-chloro- and 4-fluoropyridine afforded the products in moderate yields (entries 8, 9 and 13), and those reactions probably involved a SNAr mechanisms. Low yields were observed in coupling of 2-chloropyridine (entry 12) and 3-substituted chloro- fluoro- and trifluoromethylsulfonyl pyridines (entries 3–5). Overall the following reactivity order could be inferred: I > Br ≫ Cl ≈ F ≫ OTf.13
|
|
||||
|---|---|---|---|---|
| Entry | Halopyridine | Product | Yield (%)a | |
| a Referring to the amount of product isolated by chromatography. b Starting from hydrochloride of the halo pyridine and using 3.0 equiv. of K3PO4·H2O. | ||||
| 1 |

|

|
3a | 91 (X = I) |
| 2 | 60 (X = Br) | |||
| 3 | 7 (X = Cl) | |||
| 4 | 10 (X = F) | |||
| 5 | 0 (X = OTf) | |||
| 6 |

|

|
4 | 86 (X = I) |
| 7b | 42 (X = Br) | |||
| 8b | 58 (X = Cl) | |||
| 9b | 47 (X = F) | |||
| 10 |

|

|
5 | 80 (X = I) |
| 11 | 74 (X = Br) | |||
| 12 | 22 (X = Cl) | |||
| 13 | 50 (X = F) | |||
In summary, copper-catalysed coupling reaction of halo pyridines with various nitrogen nucleophiles can be performed under microwave-assisted solvent- and ligand-free conditions providing the corresponding coupling products in moderate to high yields. Further studies to find alternative and “greener” catalyst systems and to expand the substrate scope are currently in progress in our laboratories.
Experimental section
Procedure for N-heteroarylations of nitrogen nucleophiles (example: synthesis of 3-(1H-pyrazol-1-yl)pyridine (3a)
After cooling of an oven-dried tube to room temperature under argon, it was charged with 3-iodopyridine (1a, 102.5 mg, 0.5 mmol), pyrazole (2a, 45 mg, 0.65 mmol), Cu2O (7.2 mg, 0.05 mmol) and K3PO4·H2O (231 mg, 1.0 mmol). The tube was sealed under argon and placed into a CEM Discover microwave apparatus. Initially, an irradiation power of 50 W was applied. When the temperature reached 100 °C, the instrument automatically adjusted the power to maintain a constant temperature. After a total heating time of 1 h, the reaction mixture was cooled to room temperature and diluted with ethyl acetate (10 mL; use of less solvent can reduce the yield.) The resulting solution was filtered through a pad of silica gel and concentrated to give the crude product. Purification by silica gel chromatography (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 pentane/ethyl acetate) gave 3-(1H-pyrazol-1-yl)pyridine (3a, 66 mg, 91%) as a yellowish oil. The identity and purity of the product was confirmed by 1H and 13C NMR spectroscopic analysis. See the ESI† for full details.
1 pentane/ethyl acetate) gave 3-(1H-pyrazol-1-yl)pyridine (3a, 66 mg, 91%) as a yellowish oil. The identity and purity of the product was confirmed by 1H and 13C NMR spectroscopic analysis. See the ESI† for full details.
Acknowledgements
We are grateful to the Fonds der Chemischen Industrie for financial support. ZJL thanks Bayer CropScience for postdoctoral funding.Notes and references
-
(a) T. T. Denton, X. Zhang and J. R. Cashman, J. Med. Chem., 2005, 48, 224 CrossRef CAS
; (b) H.-C. Zhang, C. K. Derian, D. F. McComsey, K. B. White, H. Ye, L. R. Hecker, J. Li, M. F. Addo, D. Croll, A. J. Eckardt, C. E. Smith, Q. Li, W.-M. Cheung, B. R. Conway, S. Emanuel, K. T. Demarest, P. Andrade-Gorden, B. P. Damiano and B. E. Maryanoff, J. Med. Chem., 2005, 48, 1725 CrossRef CAS
; (c) J. Roppe, N. D. Smith, D. Huang, L. Tehrani, B. Wang, J. Anderson, J. Brodkin, J. Chung, X. Jiang, C. King, B. Munoz, M. A. Varney, P. Prasit and N. D. P. Cosford, J. Med. Chem., 2004, 47, 4645 CrossRef CAS
; (d) Y. Wang, Y. Li and B. Wang, Int. J. Mol. Sci., 2007, 8, 166 CrossRef CAS
; (e) N. P. Buu-Hoi, R. Rips and R. Cavier, J. Med. Chem., 1960, 2, 335 CrossRef CAS
; (f) A. M. Crider, R. Lamey, H. G. Floss and J. M. Cassady, J. Med. Chem., 1980, 23, 848 CrossRef CAS
; (g) L. He, L. Duan, J. Qiao, R. Wang, P. Wei, L. Wang and Y. Qiu, Adv. Funct. Mater., 2008, 18, 2123 CrossRef CAS
.
-
(a) K. H. Sugiyarto and H. A. Goodwin, Aust. J. Chem., 1988, 41, 1645 CAS
; (b) J. Elguero, A. Fruchier, A. de la Hoz, F. A. Jalón, B. R. Manzano, A. Otero and F. Gómez-de la Torre, Chem. Ber., 1996, 129, 589 CrossRef CAS
; (c) J. A. A. W. Elemans, E. J. A. Bijsterveld, A. E. Rowan and R. J. M. Nolte, Eur. J. Org. Chem., 2007, 751 CrossRef CAS
.
-
(a) F. Ullmann, Ber. Dtsch. Chem. Ges., 1903, 36, 2382 CAS
; (b) F. Ullmann, Ber. Dtsch. Chem. Ges., 1904, 37, 853 CAS
.
- For reviews, see:
(a) F. Monnier and M. Taillefer, Angew. Chem. Int. Ed., 2009, 48, 6954 CrossRef CAS
; (b) D. Ma and Q. Cai, Acc. Chem. Res., 2008, 41, 1450 CrossRef CAS
; (c) S. V. Ley and A. W. Thomas, Angew. Chem. Int. Ed., 2003, 42, 5400 CrossRef CAS
; (d) K. Kunz, U. Scholz and D. Ganzer, Synlett, 2003, 2428 CrossRef CAS
; (e) I. P. Beletskaya and A. V. Cheprakov, Coord. Chem. Rev., 2004, 248, 2337 CrossRef CAS
; (f) J.-P. Corbet and G. Mignani, Chem. Rev., 2006, 106, 2651 CrossRef CAS
; (g) M. Carril, R. SanMartin and E. Domínguez, Chem. Soc. Rev., 2008, 37, 639 RSC
.
- F. Monnier and M. Taillefer, Angew. Chem. Int. Ed., 2008, 47, 3096 CrossRef CAS
.
- For examples of ligand-free catalyst systems, see:
(a) M. Taillefer, N. Xia and A. Oualli, Angew. Chem. Int. Ed., 2007, 46, 934 CrossRef CAS
; (b) A. Correa and C. Bolm, Adv. Synth. Catal., 2007, 349, 2673 CrossRef CAS
; (c) E. Sperotto, J. G. de Vries, G. P. M. van Klink and G. van Koten, Tetrahedron Lett., 2007, 48, 7366 CrossRef CAS
; (d) T. Kubo, C. Katoh, K. Yamada, K. Okano, H. Tokuyama and T. Fukuyama, Tetrahedron, 2008, 64, 11230 CrossRef CAS
; (e) J. L. Bolliger and C. M. Frech, Tetrahedron, 2009, 65, 1180 CrossRef CAS
; (f) X.-M. Wu and Y. Wang, J. Chem. Res., 2009, 555 Search PubMed
; (g) L. Zhu, P. Guo, G. Li, J. Lan, R. Xie and J. You, J. Org. Chem., 2007, 72, 8535 CrossRef CAS
; (h) J. W. W. Chang, X. Xu and P. W. H. Chan, Tetrahedron Lett., 2007, 48, 245 CrossRef CAS
; (i) H. Huang, X. Yan, W. Zhu, H. Liu, H. Jiang and K. Chen, J. Comb. Chem., 2008, 10, 617 CrossRef CAS
.
- For examples of solvent-free catalyst systems, see:
(a) F. Y. Kwong and S. L. Buchwald, Org. Lett., 2003, 5, 793 CrossRef CAS
; (b) S. Narayan, T. Seelhammer and R. E. Gawley, Tetrahedron Lett., 2004, 45, 757 CrossRef CAS
; (c) W. S. Chow and T. H. Chan, Tetrahedron Lett., 2009, 50, 1286 CrossRef CAS
; (d) general review: P. J. Walsh, H. Li and C. Anaya de Parrodi, Chem. Rev., 2007, 107, 2503 Search PubMed
.
- It should be, however, noted that solvent-free exothermic reactions can be problematic because a thermal buffer is missing. For a related study, see: L. Balland, N. Mouhab, J.-M. Cosmao and L. Estel, Chem. Eng. Proc., 2002, 41, 395 Search PubMed
.
-
(a)
Microwave Methods in Organic Synthesis, ed. M. Larhed and K. Olofsson, Springer-Verlag, Berlin, 2006 Search PubMed
; (b) Microwaves in Organic Synthesis, 2nd edition, ed. A. Loupy, Wiley-VCH, Weinheim, 2006 Search PubMed
; (c) C. O. Kappe and A. Stadler, Microwaves in Organic and Medicinal Chemistry, Wiley-VCH, Weinheim, 2005 Search PubMed
.
-
(a) L. Shi, M. Wang, C.-A. Fan, F.-M. Zhang and Y.-Q. Tu, Org. Lett., 2003, 5, 3515 CrossRef CAS
; (b) M. Meciarová, J. Podlesná and S. Toma, Monatsh. Chem., 2004, 135, 419 CrossRef CAS
; (c) S.-Y. Chen, H. Huang, X.-J. Liu, J.-K. Shen, H.-L. Jiang and H. Liu, J. Comb. Chem., 2008, 10, 358 CrossRef CAS
; (d) M. Poirier, S. Goudreau, J. Poulin, J. Savoie and P. L. Beauliu, Org. Lett., 2010, 12, 2334 CrossRef CAS
.
- For other copper-catalysed N-heteroarylations of nitrogen nucleophiles with heteroaryl halides, see:
(a) D. Cheng, F. Gan, W. Qian and W. Bao, Green Chem., 2008, 10, 171 RSC
; (b) F. Li and T. S. A. Hor, Chem. Eur. J., 2009, 15, 10585 CrossRef CAS
; (c) Z. Zhang, J. Mao, D. Zhu, F. Wu, H. Chen and B. Wan, Tetrahedron, 2006, 62, 4435 CrossRef CAS
; (d) D. Zhu, R. Wang, J. Mao, L. Xu, F. Wu and B. Wan, J. Mol. Catal. A: Chem., 2006, 256, 256 CrossRef CAS
; (e) Z. Xi, F. Liu, Y. Zhou and W. Chen, Tetrahedron, 2008, 64, 4254 CrossRef CAS
; (f) P. Vera-Luque, R. Alajarín, J. Alvarez-Builla and J. J. Vaquero, Org. Lett., 2006, 8, 415 CrossRef CAS
; (g) M. A. Khan and J. B. Polya, J. Chem. Soc. (C), 1970, 85 RSC
; (h) J. Baffoe, M. Y. Hoe and B. B. Touré, Org. Lett., 2010, 12, 1532 CrossRef CAS
; (i) F. Lang, D. Zewge, I. N. Houpis and R. P. Volante, Tetrahedron Lett., 2001, 42, 3251 CrossRef CAS
; (j) X. Han, Tetrahedron Lett., 2010, 51, 360 CrossRef CAS
; (k) V. S. C. Yeh and P. E. Wiedeman, Tetrahedron Lett., 2006, 47, 6011 CrossRef CAS
; (l) H. Zhang, Q. Cai and D. Ma, J. Org. Chem., 2005, 70, 5164 CrossRef CAS
; (m) J. B. Arterburn, M. Pannala and A. M. Gonzalez, Tetrahedron Lett., 2001, 42, 1475 CrossRef
; (n) J. C. Antilla, A. Klapars and S. L. Buchwald, J. Am. Chem. Soc., 2002, 124, 11684 CrossRef CAS
; (o) H.-J. Cristau, P. P. Cellier, J.-F. Spindler and M. Taillefer, Eur. J. Org. Chem., 2004, 695 CrossRef CAS
; (p) A. Shafir and S. L. Buchwald, J. Am. Chem. Soc., 2006, 128, 8742 CrossRef CAS
; (q) X. Han, Tetrahedron Lett., 2010, 51, 360 CrossRef CAS
.
- In the MW experiments, the temperature was measured by an internal thermocouple. The setting power of the MW instrument was 50 W during the entire reaction time. When the temperature reached 100 °C, the instrument automatically adjusted the power to maintain this temperature.
- For recent mechanistic studies on copper-catalysed cross-couplings, see:
(a) E. R. Strieter, B. Bhayana and S. L. Buchwald, J. Am. Chem. Soc., 2009, 131, 78 CrossRef CAS
; (b) G. O. Jones, P. Liu, K. N. Houk and S. L. Buchwald, J. Am. Chem. Soc., 2010, 132, 6205 CrossRef CAS
; (c) P.-F. Larsson, C. Bolm and P.-O. Norrby, Chem. Eur. J. Search PubMed
accepted for publication.
- Sulfoximines have been found to be effective chiral ligands in several catalytic asymmetric reactions by our group. For recent examples, see:
(a) M. Frings, I. Atodiresei, J. Runsink, G. Raabe and C. Bolm, Chem. Eur. J., 2009, 15, 1566 CrossRef CAS
; (b) M. Frings and C. Bolm, Eur. J. Org. Chem., 2009, 4085 CrossRef CAS
; (c) S.-M. Lu and C. Bolm, Chem. Eur. J., 2008, 14, 7513 CrossRef CAS
; (d) S.-M. Lu and C. Bolm, Adv. Synth. Catal., 2008, 350, 1101 CrossRef CAS
; (e) M. Langner and C. Bolm, Angew. Chem., Int. Ed., 2004, 43, 5984 CrossRef CAS
; (f) M. Langner, P. Rémy and C. Bolm, Chem. Eur. J., 2005, 11, 6254 CrossRef CAS
; (g) P. Rémy, M. Langner and C. Bolm, Org. Lett., 2006, 6, 1209
; (h) C. Moessner and C. Bolm, Angew. Chem. Int. Ed., 2005, 44, 7564 CrossRef CAS
.
- The optimised solvent- and ligand-free conditions [10 mol% of Cu2O, 2.0 equiv. of Cs2CO3, 100 °C, MW (50 W), 1 h] can also be applied in the coupling between phenyl iodide and S-methyl-S-phenylsulfoximine to give the corresponding N-arylated product in 72% yield.
Footnote |
| † Electronic supplementary information (ESI) available: Further experimental details. See DOI: 10.1039/c0gc00296h |
| This journal is © The Royal Society of Chemistry 2011 |