β-Cyclodextrin catalysed synthesis of tryptanthrin in water†
Atul
Kumar
*,
Vishwa Deepak
Tripathi
and
Promod
Kumar
Medicinal and Process Chemistry Division, Central Drug Research Institute, CSIR, Lucknow, India. E-mail: dratulsax@gmail.com; Fax: +91-522-26234051; Tel: +91-522-2612411
First published on 2nd December 2010
Abstract
An efficient and green method has been developed for the synthesis of tryptanthrin employing β-cyclodextrin as a catalyst in aqueous media at room temperature from isatoic anhydride and isatin. The reactions were performed under mild conditions to afford biologically active natural product tryptanthrin in excellent yields.
In recent years growing awareness about environmental safety and global warming has attracted worldwide concern towards use of renewable sources and reduction of waste. This has shifted paradigm towards the use of ecofriendly and green protocols in organic synthesis. A catalyst derived from biomass and green solvent increases the greenness of a process. Tryptanthrin (indolo[2,1-b]quinazoline-6,12-dione) is weak basic alkaloid found in a number of plant species.1Tryptanthrin and its analogues have emerged as potential biologically active molecules because of their broad spectrum of activity against various pathogens. Tryptanthrin possesses antibacterial activity against Bacillus subtilis, Mycobacterium tuberculosis and resistant strains of tuberculosis,2 antifungal activity against Leishmania donovani, Trypanosoma brucei and Plasmodium falciperum. Tryptanthrin and derivatives are also well known to be potential anticancer agents against MCF-7, NCI-H460 and SF-268 human cancer cell lines.3–6Tryptanthrin consists of quinazoline and indole core structures. The quinazoline core itself forms the building block for approximately 150 naturally occurring alkaloids isolated from a number of plant families, animals and microorganisms, such as batracylin, (−)-vascicine and lutonin (Fig. 1). Hence indolo[2,1-b]quinazoline-6,12-dione is a very important class of heterocyclic compound for its structural diversity as well as pharmacological value.7–8
 | ||
| Fig. 1 Representative biologically active molecules that posses 3-arylquinazoline structural motif. | ||
Tryptanthrin can be produced by Candida lipolytica when grown in media containing an excess of tryptophan, hence name tryptanthrin.9 In view of the importance of these heterocycles various synthetic methods have been developed for synthesis of tryptanthrin. The current methods available for synthesis of quinazoline nucleus involve use of anthranilic acid, primary amines, thionyl chloride and pyridine.9a Electrosynthesis of tryptanthrin also has been reported by Barba et al. starting from isatin.9b Weaver et al. have reported oxidative radical cyclization for synthesis of quinazolines from quinazolin-4(3H)-one.10–11 Mitscher et al. have described intramolecular aza-Wittig reaction using triethylamine.12 Jao synthesized tryptanthrin from isatin and POCl3.13 Though different approaches have been reported, there are many limitations such as use of strong basic conditions, elevated temperatures, long reaction time, hazardous organic solvents, reagents, catalyst for activation and low yield. Synthesis of compounds via a green, mild and simpler procedure, eliminating the use and generation of hazardous substances is the foremost goal of green chemistry today. Hence, in a quest for a new easy and ecofriendly procedure for the synthesis of tryptanthrin, we planned our strategy to exploit cyclodextrins as catalysts in aqueous medium. Cyclodextrin-mediated organic reactions in aqueous medium are very useful both from economical and environmental point of view. Cyclodextrins apart from being nontoxic are considered to be metabolically safe.
In this communication we have reported the synthesis of tryptanthrin (indolo[2,1-b]quinazoline-6,12-dione) derivatives with β-cyclodextrin as catalyst in aqueous medium from isatoic anhydrides and isatins at room temperature (Scheme 1). Cyclodextrins are cyclic glucose oligomers with cylindrical shape having primary hydroxyl groups at the more restricted rim of the cylinder. They catalyze reactions by supramolecular catalysis involving the formation of a host–guest complex by noncovalent bonding interactions.14 Supramolecular catalysis is the discipline of chemistry which involves all intermolecular interactions where covalent bonds are not established between the interacting species i.e., molecules, ions, or radicals.15 The majority of these interactions are of the host–guest type. The internal cavity of the cyclodextrin (CD) molecule is strongly hydrophobic in nature and this particular characteristic of CD molecules enables them to bind a wide range of guest molecules.16Cyclodextrins bind substrates in its hydrophobic cavity and catalyze reactions in a selective manner. Cyclodextrin used in the reaction can be recovered after completion.
 | ||
| Scheme 1 Synthesis of tryptanthrin derivatives. | ||
The three most common cyclodextrins are α, β and γ-species having 6, 7 and 8 sugar molecules respectively in the ring system. For optimization of the catalyst, the reaction of isatoic anhydride and isatin (without any substitution) was taken as the model reaction and all three forms of cyclodextrin were explored as catalysts. Very good results were obtained with β-CD as catalystTable 1. Product yield was low with α and γ-cyclodextrins. No product formation was detected in absence of cyclodextrin, which showed that cyclodextrin plays an essential role to catalyze the reaction. Hence β-CD was chosen as catalyst for the reaction.
We also screened different solvents such as CH3OH, CHCl3, DMF, DMSO, THF, acetonitrile and water with cyclodextrin as catalyst. After some optimization we found promising results with water as a solvent due to better solubility of cyclodextrin in water. Subsequently to verify the general procedure of reaction, various substituted isatins and substituted isatoic anhydride were tested under optimised reaction conditions, the results have been summarised in Table 2.
| Entry | Compound | R1 | R2 | R3 | Timea | Yield(%)b |
|---|---|---|---|---|---|---|
| a Time in hours. b Isolated yields of purified fractions. | ||||||
| 1 | 3a | H | H | H | 6 | 90 |
| 2 | 3b | H | Br | H | 6 | 82 |
| 3 | 3c | H | NO2 | H | 6 | 81 |
| 4 | 3d | OCH3 | NO2 | H | 5 | 86 |
| 5 | 3e | H | NH2 | H | 6 | 86 |
| 6 | 3f | Br | H | H | 5 | 83 |
| 7 | 3g | OCH3 | H | H | 5 | 86 |
| 8 | 3h | CH3 | H | H | 8 | 82 |
| 9 | 3i | Cl | H | H | 6 | 87 |
| 10 | 3j | NO2 | H | H | 9 | 80 |
| 11 | 3k | F | H | H | 5 | 82 |
| 12 | 3l | I | H | H | 5 | 81 |
| 13 | 3m | Br | OCH3 | OCH3 | 6 | 84 |
| 14 | 3n | Br | NO2 | H | 7 | 80 |
| 15 | 3o | Br | NH2 | H | 5 | 85 |
| 16 | 3p | NO2 | Br | H | 9 | 79 |
| 17 | 3q | NO2 | OCH3 | OCH3 | 8 | 81 |
| 18 | 3r | NO2 | Cl | H | 9 | 80 |
| 19 | 3s | Cl | NO2 | H | 8 | 82 |
| 20 | 3t | Cl | OCH3 | OCH3 | 6 | 83 |
| 21 | 3u | F | Cl | H | 7 | 84 |
| 22 | 3v | F | NO2 | H | 7 | 86 |
| 23 | 3w | OCH3 | Cl | H | 6 | 85 |
The reaction was carried out by dissolving cyclodextrin in water followed by addition of substituted isatoic anhydride (1) and isatin (2). The reaction mixture was stirred at room temperature to give the desired compound in excellent yields. Reaction goes smoothly without formation of any side product.
The fact that these reactions do not take place in absence of cyclodextrin indicates the essential role of cyclodextrin, which appears to activate the carbonyl carbon at position 4 of isatoic anhydride leading to cleavage of anhydride ring and formation of intermediate (5). Which then reacts at position 2 of isatin to form the product (6) (Fig. 2).
 | ||
| Fig. 2 Plausible mechanistic pathway for reaction. | ||
Evidence for association of isatoic anhydride and cyclodextrin is provided by 1H NMR spectroscopy. A comparison of 1H NMR spectra (D2O solutions) of β-CD, β-CD-isatoic anhydride complex and freeze-dried reaction mixture after 2 h were undertaken (Fig. 3). It is evident from Fig. 3 that there is an upfield shift of H-3 (0.034 ppm) and H-5 (0.058 ppm) of cyclodextrin in complex as compared to β-CD indicating the formation of an inclusion complex of isatoic anhydride with β-cyclodextrin. It is further observed from spectra of reaction mixture after two hours that the upfield character of protons retains in the reaction mixture showing retention of complex during reaction after 2 h. This indicates the formation of β-CD complex from primary rim of cylinder. Thus the role of cyclodextrin is not only to catalyze the reaction, but also to provide a new mechanistic pathway to the reaction.
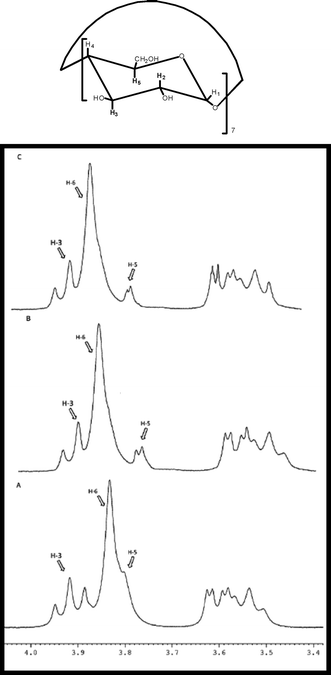 | ||
| Fig. 3 1H NMR spectra of A) β-CD B) β-CD complex C) freeze-dried reaction mixture after 2 h. | ||
The catalyst reusability was studied five times including the use of fresh catalyst for the synthesis of compound (3a) and there was inevitable loss of catalyst during recovery process. Besides this no significant loss in catalytic activity was observed (Fig. 4) and catalyst was reused in next batch without any treatment.
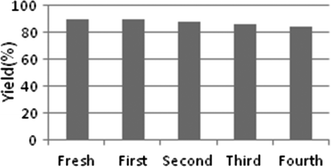 | ||
| Fig. 4 Catalyst (β-cyclodextrin) recyclability data. | ||
Conclusions
In conclusion, an extremely efficient and green process has been developed for the synthesis of biologically active natural product tryptanthrin, using β-cyclodextrin as a catalyst in aqueous medium. This method is bestowed with merits like high yield, cost effectiveness, biomimetic, neutral aqueous phase conditions and environmentally benign nature. These advantages of the catalyst made this process very useful for the synthesis of tryptanthrin derivatives.Experimental
All the reactions were carried out at room temperature that is 28–32 °C. Unless otherwise specified, all the reagents were purchased from Sigma-Aldrich Chemical Co, Lancaster and were used directly without further any purification. NMR spectra were obtained using the Brucker DRX 300 MHz spectrometer. Chemical shifts (δ) are given in ppm relative to TMS, coupling constants (J) in Hz. IR spectra were taken on VARIAN FT-IR spectrometer as KBr pellets. Elemental analysis was preformed using a Perkin Elmer Autosystem XL Analyzer. Melting points were measured using a COMPLAB melting-point apparatus. Reactions were monitored by thin-layer chromatography (TLC) carried out on 0.25 mm silica gel plates visualized with UV light.General procedure for synthesis of compounds 3(a–x)
Isatin (1 mmol.) and isatoic anhydride (1 mmol.) were combined with 10 ml distilled water in a 50 ml roundbottomed flask equipped with a stir bar. β-cyclodextrin (20 mol%) was added. Reaction was allowed to stir at room temperature for the appropriate amount of time. After completion of reaction, the reaction mixture was extracted with ethyl acetate. Organic layer was dried over Na2SO4 and concentrated in vacuo to give crude product which was further purified by column chromatography.Indolo[2,1-b]quinazoline-6,12-dione (3a)
Yellow solid. mp > 250 °C. Found C, 72.52, H, 3.19, N, 11.22 C15H8N2O2 requires C, 72.58, H, 3.25, N, 11.28%. νmax(KBr): 3062, 1721, 1645, 1435, 1256, 804, 747 cm−1. 1H NMR (300 MHZ, CDCl3) δ = 8.61 (d, 1H, J = 3.4 Hz), 8.44 (d, 1H, J = 5.4 Hz), 8.06 (d, 1H, J = 8.1 Hz), 7.94–7.80 (m, 3H), 7.69 (t, 1H, J = 7.08 Hz), 7.28 (t, 1H, J = 7.1 Hz). 13C NMR (75 MHZ, CDCl3) δ = 117.5, 120.6, 125.4, 126.3, 127.1, 129.7, 130.0, 133.2, 134.6, 145.3, 146.6, 160.4, 183.8.˙ ESIMS: m/z 249 (M+H).2-Bromoindolo[2,1-b]quinazoline-6,12-dione(3b)
Light yellow solid; mp > 250 °C. Found C, 54.98, H, 2.13 N, 8.48 C15H7BrN2O2 requires C, 55.07; H, 2.16; N, 8.56%. νmax(KBr):3124, 1724, 1647, 1440, 1255, 812, 746 cm−1. 1H NMR (300 MHZ, CDCl3) δ = 8.24 (s, 1H), 8.10–8.02 (m, 2H), 7.98–7.82 (m, 2H), 7.63 (t, 1H, J = 7.2 Hz), 7.38 (t, 1H, J = 6.3 Hz). 13C NMR (75 MHZ, CDCl3) δ = 117.8, 120.7, 123.0, 123.5, 125.7, 129.4, 129.9, 132.3, 134.8, 136.2, 146.4, 160.8, 182.5. ESIMS: m/z 326 (M+H).References
- (a) C. M. Martıínez-Viturro and D. Domıínguez, Tetrahedron Lett., 2007, 48, 1023–1026 CrossRef; (b) K. Dzierzbicka, P. Trzonkowski, P. L. Sewerynek and A. Mysliwski, J. Med. Chem., 2003, 46, 978–986 CrossRef CAS.
- S.-T. Yu, T.-M. Chen, S.-Y. Teng and Y.-H. Chen, Biochem. Biophys. Res. Commun., 2007, 358, 79–84 CrossRef CAS.
- T. Yasutaka, S. Takao, W. Nobuhisa, A. Hideyuki, S. Shigeru and S. Isao, J. Med. Chem., 1994, 37, 2106–2111 CrossRef.
- L. A. Mitscher and W. Baker, Med. Res. Rev., 1998, 18, 363–374 CrossRef CAS.
- A. K. Bhattacharjee, D. J. Skanchy, B. Jennings, T. H. Hudson, J. J. Brendle and K. A. Werbovetz, Bioorg. Med. Chem., 2002, 10, 1979–1989 CrossRef CAS.
- W. R. Baker and L. A. Mitscher, Indolo{2,1-biquinazoline-6.12-dione Antibacterial Compounds and Methods of Use Thereof, U.S. Patent, 5, 441, 955, 1995.
- S. B. Phadtare and G. S. Shankarling, Green Chem., 2010, 12, 458–462 RSC.
- S. C. Harvey, in Goodman and Gilman's The Therapeutic Basis of Therapeutics; A. G. Gilman, L. S. Goodman, ed. 6th ed.; MacMillan, New York, 1980, p 367 Search PubMed.
- (a) E. S. Lee, J. G. Park and Y. Jahng, Tetrahedron Lett., 2003, 44, 1883–1886 CrossRef CAS; (b) B. Batanero and F. Barba, Tetrahedron Lett., 2006, 47, 8201–8203 CrossRef CAS.
- (a) K. M. Amin, M. M. Kamel, M. M. Anwar, M. Khedr and Y. M. Syamb, Eur. J. Org. Chem., 2010, 45, 2117–2131 CAS; (b) K. C. Jahng, S. I. Kim, D. H. Kim, C. S. Seo, J. K. Son, S. H. Lee and Y. Jahng, Chem. Pharm. Bull., 2008, 56, 607–609 CrossRef CAS.
- W. R. Bowman, M. R. J. Elsegood, T. Stein and G. W. Weaver, Org. Biomol. Chem., 2007, 5, 103–113 RSC.
- (a) W. A. Bergman, Curr. Org. Chem., 2003, 7, 1–19; (b) L. A. Mitscher, W. C. Wong, T. De Meulenere, J. Sulko and S. Drake, Heterocycles, 1981, 15, 1017–1019 CrossRef CAS.
- C. en.-W. Jao, W.-C. Lin and Y.-T. Wu, J. Nat. Prod., 2008, 71, 1275–1279 CrossRef CAS.
- R. Villalonga, R. Cao and A. Fragoso, Chem. Rev., 2007, 107, 3088–3116 CrossRef CAS.
- J. Szejtli, Chem. Rev., 1998, 98, 1743–1753 CrossRef CAS.
- F. Hapiot, S. Tilloy and E. Monflier, Chem. Rev., 2005, 106, 767–781.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c0gc00523a |
| This journal is © The Royal Society of Chemistry 2011 |
