Glycerol eutectics as sustainable solvent systems†
Andrew P.
Abbott
*a,
Robert C.
Harris
a,
Karl S.
Ryder
a,
Carmine
D'Agostino
b,
Lynn F.
Gladden
b and
Mick D.
Mantle
b
aChemistry Department, University of Leicester, Leicester, UK LE1 7RH
bDepartment of Chemical Engineering and Biotechnology, University of Cambridge, Cambridge, UK CB2 3RA. E-mail: Andrew.abbott@le.ac.uk; Fax: +44 116 252 3789
First published on 27th October 2010
Abstract
In this work the use of glycerol as a hydrogen bond donor in Deep Eutectic Solvents is studied. The physical properties of choline chloride mixtures with glycerol are quantified and it is shown that eutectic mixtures can circumvent some of the difficulties of using glycerol as a solvent viz. high viscosity and high melting point. The solvent properties are characterised using polarity parameters and the values are similar to other ionic liquids although it is shown that this procedure is a poor method of characterising Lewis basicity. The application of these liquids to the esterification of glycerol is used as a demonstration of the ability to tune a reaction with the quaternary ammonium halide acting as a quasi-protecting group. The liquids represent a sustainable way of preparing non-toxic, tuneable solvent systems.
Introduction
Glycerol is a by-product of a range of hydrolysis and trans-esterification processes of oils and fats most notably in the soap and biodiesel manufacturing industries. Glycerol has some uses principally as viscosity modifiers and freezing point suppressants however with annual production in excess of 1 million tonnes p.a. the market is saturated. The fact that glycerol is poorly combustible means that it cannot even be used as a fuel and it is regarded by many to be a waste product. One of the limiting factors with its use as a bulk solvent is its high viscosity and high boiling point making it difficult to separate species by filtration or distillation. A recent review1 has assessed the use of glycerol as a green solvent.We have recently shown that the addition of a stoichiometric equivalent of a quaternary ammonium salt to glycerol forms a deep eutectic solvent (DES) and decreases the viscosity of the liquid. This mixture was shown to be useful for the purification of biodiesel.2 DESs are mixtures of hydrogen bond donors with simple halide salts which produce liquids which have physical and solvent properties that are comparable with ionic liquids.3,4 DESs have been used for a variety of applications including metal deposition, metal oxide processing, metal dissolution and a variety of synthetic processes. The majority of systems studied to date have been based on choline chloride HOC2H4N+(CH3)3Cl− (ChCl) because it is non-toxic and readily available as a bulk commodity chemical. Its common use stems in part from its simple manufacture; an efficient gas phase reaction between trimethylamine, ethylene oxide and HCl. This means that the Sheldon E factor5 for this salt is close to zero because almost no waste products are formed during this reaction. The combination of the salt with glycerol produces a liquid which is non-flammable and non-toxic; the mixture can even be prepared from food-grade components.
In the current work we characterise the physical properties of these liquids and use them to propose an improved model for the mobility of species in ionic liquids. We also demonstrate that the chemical properties of these liquids are different from bulk glycerol by characterising the solvatochromic parameters and demonstrate that esterification reactions of glycerol produce significantly different product distributions.
Experimental
Synthesis of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) 2 ChCl: glycerol system
2 ChCl: glycerol system
ChCl (Aldrich 99%) was recrystallised from absolute ethanol, filtered and dried under vacuum. Glycerol (Aldrich 99%) was used as received. The eutectic mixtures were formed by stirring the two components at ∼80 °C until a homogeneous colourless liquid was formed.
Physical property measurements
The viscosity of the various ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycerol mixtures were measured using a Brookfield DV-E Viscometer fitted with a thermostated jacket and the conductivity of these systems was measured using a Jenway 4071 Conductivity Meter conductivity probe. Surface tension and density measurements were carried out using a Krüss Tensiometer K9 with a platinum plate which was connected to a thermostated water bath. All readings were taken after 25–30 min at each temperature to ensure the system was at equilibrium. For clarity error bars have been omitted from most plots but these are typically less than ±3% of the given value.
glycerol mixtures were measured using a Brookfield DV-E Viscometer fitted with a thermostated jacket and the conductivity of these systems was measured using a Jenway 4071 Conductivity Meter conductivity probe. Surface tension and density measurements were carried out using a Krüss Tensiometer K9 with a platinum plate which was connected to a thermostated water bath. All readings were taken after 25–30 min at each temperature to ensure the system was at equilibrium. For clarity error bars have been omitted from most plots but these are typically less than ±3% of the given value.
Solvatochromism experiments
All UV-Vis measurements were carried out using a Shimadzu model UV-1601 spectrophotometer to measure the solvatochromic shift of the different indicator dyes in the visible absorbance spectrum. The program UV PROBE was used to obtain the maximum absorbance of the UV-Vis spectra of the solvatochromic dyes in each system. Sample concentration was kept in the range of 10−5–10−6 mol dm−3 in order to reduce any possible solute–solute interactions being observed.Esterification experiments
The progression of the esterification reactions was measured by gel permeation chromatography (GPC). The GPC used was a Polymer Laboratories PL-GPC 220 with two PLgel 5 μm 50 Å columns. The analysis was carried out at 40 °C at a flow rate of 1 cm3 min−1. The solvent used was dry THF. Each sample vial contained 0.02 g of product and was dissolved in 1.25 mL of dry THF. Under this setup the elution times of the reagents and products are shown in Table 1. As would be expected the product yields were less reproducible than the physical measurements but values of the equilibrium constant are ±10% of the values quoted.Diffusion coefficient studies
The Pulsed Field Gradient NMR experiments were carried out with a Bruker DMX 300 equipped with a diffusion probe capable of producing magnetic field gradient pulses up to 11.6 T/m. The measurements were performed over the temperature range from 298 K to 333 K, with increments of 5 K using a Bruker Variable Temperature unit, BVT 300. Samples were prepared in 5 mm NMR tubes filled with liquid to a height of approximately 20 mm. For each temperature, the sample was left for 15 min before starting the measurement, to achieve thermal equilibrium. Diffusion measurements were carried out using the PGSTE pulse sequence (Fig. 1), holding the gradient pulse duration, δ, constant and varying the magnetic field gradient strength, g, for 16 points. A homospoil gradient between the second and the third 90° radiofrequency pulse (RF) was applied in order to destroy any unwanted residual magnetization on the transverse plane. The observation time, Δ, was set to 50 ms. Typical values of δ were 3 and 4 ms. The numerical values of the diffusion coefficient, D, were obtained by fitting the NMR signal decay to the echo attenuation equation, given by: | (1) |
 | ||
| Fig. 1 PGSTE pulse sequence showing gradient pulse duration, δ, echo time, τ, storage interval, T, homospoil gradient (crosshatched pattern) and observation time, Δ. | ||
Results
Physical properties of glycerol with ChCl
The high viscosity of glycerol (1200 cP at room temperature) makes its use as a solvent very difficult. However, due to its low toxicity and ready availability, it could make an ideal solvent. The addition of a salt has a significant effect upon the fluid properties. Fig. 2 shows the effect of ChCl on the viscosity,η, of glycerol as a function of composition and temperature. It can be seen that the viscosity decreases as the salt concentration increases which is in direct contrast to diol based systems where the viscosity increases as salts are added.6 It is worth noting that the addition of 33 mol% ChCl decreases the viscosity of glycerol by a factor of 3. Whilst this is higher than most molecular solvents it is similar to many ionic liquids and DESs. | ||
| Fig. 2 Plot of temperature versus viscosity as a function of ChCl composition for glycerol (a) and a plot of all viscosities at 298 K (b). | ||
The addition of 33 mol% ChCl significantly decreases the freezing point of glycerol from 17.8 °C down to −40 °C circumventing one of the problems with the use of the pure liquid. Fig. 3 shows the corresponding conductivity of the systems shown in Fig. 2. DESs have been studied in depth previously and many have shown a correlation between molar conductivity and fluidity.6
 | ||
| Fig. 3 Plot of temperature versus conductivity as a function of ChCl composition for glycerol systems. | ||
This trend is more obvious when the viscosity is taken at one temperature and compared as a function of molar percentage composition of ChCl (Fig. 2 (b)). Like most ionic liquids the viscosity-temperature profiles follow an Arrhenius-like behaviour. The change in viscosity, η with temperature can be described by the expression;7
 | (2) |
 | (3) |
The effect of the salt upon the structure of the liquid can be more clearly seen by measuring the liquid density. The amount of free volume can be determined using a hard sphere model. Using the density data shown in Fig. 4 the molar volume Vm can be calculated using
| Vm = Mr/ρ | (4) |
| Mr = xChClMChCl + xHBDMHBD | (5) |
| Vcomp = (xChClVChCl + xHBDVHBD)NA | (6) |
| Vfree = (Vm− Vcomp)/Vm | (7) |
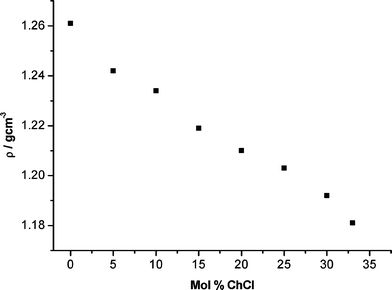 | ||
| Fig. 4 Density of the glycerol systems as a function of molar composition of the system in terms of ChCl. | ||
Fig. 4 shows the raw density data for the ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycerol systems. As well as a decrease in viscosity, ChCl also decreases the density which causes an increase in free volume. The change in Vfree with mole fraction ChCl can be observed in Fig. 5 (a). Glycerol is a viscous liquid because of the lack of free volume caused by the extent of hydrogen bonding. The fluidity (1/η) of the liquid is related to the free volume of the liquid (Fig. 5 (b)).
glycerol systems. As well as a decrease in viscosity, ChCl also decreases the density which causes an increase in free volume. The change in Vfree with mole fraction ChCl can be observed in Fig. 5 (a). Glycerol is a viscous liquid because of the lack of free volume caused by the extent of hydrogen bonding. The fluidity (1/η) of the liquid is related to the free volume of the liquid (Fig. 5 (b)).
We have previously proposed a new model that explains the mechanism of motion in an ionic liquid which fits the behaviour of DESs. It proposed that the mechanism of charge transfer is limited by the migration of holes through the liquid. Since the fraction of suitably sized holes in ambient temperature ionic liquids is very low the holes can be assumed to be at infinite dilution. The molar conductivity, Λ, can therefore be described by combining the Stokes–Einstein and Nernst–Einstein equations (eqn (8))11
| Λ = z2F e/6 π η (R++ R−) | (8) |
An alternative analysis of mobility in ionic liquids has used NMR measurements to calculate self diffusion coefficients, D. These differ from those calculated using the Stokes–Einstein equation (eqn (9)).
| D = kT/6πηR | (9) |
This difference between the calculated radius and the hard sphere radius was ascribed to changes in ionic character, termed “ionicity”, resulting from ionic association.13–16 In the current work we aim to show that the disparity between the values is due to the identity of the mobile species.
The diffusion coefficient for Ch+ and glycerol was determined as a function of temperature using Pulsed Field Gradient NMR and these data are shown in Table 2. From eqn (9), if the fluid behaves like a Newtonian fluid then the radius of the diffusing species can be calculated and these are shown in columns 4 and 5. The values are considerably below the hard sphere radius of Ch+ (3.29 Å) and glycerol (3.00 Å).
| Dmeas ×1011/m2s−1 | Ra/Å | r H /Å | Dc ×1011/m2s−1 | Dd ×1011/m2s−1 | ||||
|---|---|---|---|---|---|---|---|---|
| T/K | Ch+ | Glycerol | Ch+ | Glycerol | Hole | Ch+ | Glycerol | Hole |
| a Calculated using eqn (9) with the D values in columns 2 and 3. b Calculated using eqn (10). c Calculated using hard sphere radii (Ch+ = 3.26 Å and glycerol = 3.00 Å) from molecular simulation.4 d Calculated using rH#. | ||||||||
| 298 | 0.38 | 0.52 | 2.33 | 1.69 | 1.54 | 0.25 | 0.28 | 0.55 |
| 303 | 0.51 | 0.70 | 2.43 | 1.75 | 1.56 | 0.36 | 0.39 | 0.75 |
| 308 | 0.66 | 0.92 | 2.57 | 1.88 | 1.58 | 0.49 | 0.54 | 1.02 |
| 313 | 0.86 | 1.17 | 2.65 | 1.89 | 1.60 | 0.67 | 0.73 | 1.37 |
| 318 | 1.07 | 1.50 | 2.64 | 1.88 | 1.62 | 0.86 | 0.95 | 1.75 |
| 323 | 1.40 | 1.97 | 2.58 | 1.88 | 1.64 | 1.12 | 1.23 | 2.25 |
It has previously been suggested that the movement of holes in an ionic liquid aids in mass transport. The average size of the void, rH, in the liquid can be determined from the surface tension of the liquids as described in eqn (10).
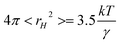 | (10) |
 | ||
| Fig. 5 Plot of (a) free volume vs. mole composition of ChCl and (b) free volume vs. 1/ŋ. | ||
 | ||
| Fig. 6 Plot of temperature versus surface tension for the glycerol systems. | ||
Chemical properties of glycerol with ChCl
By using Reichardt's Dye 30, a value for ET(30) can be calculated using eqn (11).
| ET(30) (kcal mol−1) = hCUmaxNA = (2.8591 × 10−3) Umax(cm−1) = 28591/λmax | (11) |
In addition to the ET(30) scale, there is a normalised scale, the ENT scale. The normalised value for a solvent is calculated using eqn (12);
 | (12) |
To calculate π* the eqn (13) was used with the data obtained from the indicator molecule 4-nitroaniline
| π* = (vsolvent - vcyclohexane)/(vdmso - vcyclohexane) | (13) |
| α = (ET(30) - 14.6 (π* - 0.23) - 30.31)/16.5 | (14) |
The β values were calculated from the data obtained for 4-nitroaniline and N,N-dimethyl-4-nitroaniline
| β = 0.9 (Δvcyclohexane - Δvsolvent)/(Δvcyclohexane - Δvpropan-1-ol) | (15) |
The data in Table 3 shows that the choline chloride based DES's exhibit a polarity that is similar to RNH3+X− and R2NH2+X− ionic liquids with discrete anions.28 It can also be seen that the addition of choline chloride increases the ET(30), π* and α parameters of the system. The changes in these parameters as a function of choline chloride concentration can be seen in Fig. 7.
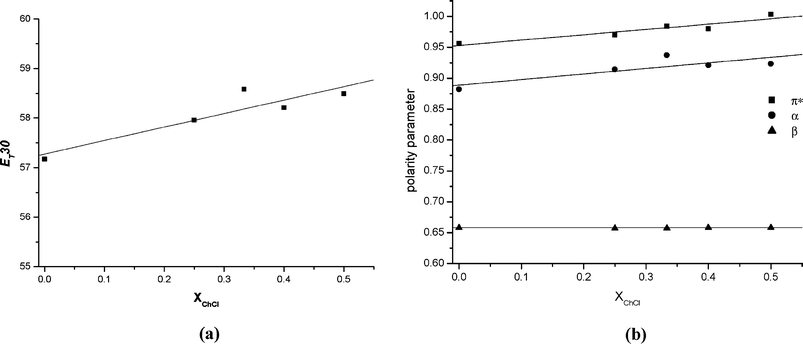 | ||
| Fig. 7 Variation of ET(30) (a), π*, α and β (b) with mole fraction of ChCl in glycerol. | ||
Fig. 7 shows a roughly linear increase in ET(30) with ChCl concentration. Extrapolating this trend to 100% ChCl gives a value of approximately 59 kcal mol−1 which is comparable to that for ethylammonium nitrate28 which is reasonable. Similar trends are observed for π* and α, although the parameters are relatively insensitive to changes in polarity. The β parameter in particular does not appear to show significant changes with increasing chloride concentration. To some extent this is not surprising since the number glycerol OH groups present are in excess to the indicator solute and will act as a better hydrogen bond donor. This suggests that in high polarity solvents, solvatochromic indicators are insensitive to compositional changes because solvent–solvent interactions dominate over solvent–solute interactions.
Esterification of glycerol
To demonstrate the different chemical properties of ChCl–glycerol mixtures the esterification of glycerol with lauric acid was chosen as a model experiment. The aim of this work was to determine the effect of hydrogen bonding of Cl− to the OH moieties on glycerol. It was envisaged that the strength of the H-bonding would affect the reactivity of glycerol and this could change the product distribution of the reaction.The esterification of the triol can be broken down into three elementary equilibria. Analysis of such a complex process is clearly complicated and here only a simple qualitative analysis is carried out. Attempts to fit all of the data were unsuccessful. The scheme for this reaction is as follows:
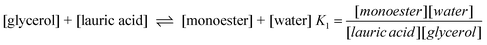 | (16) |
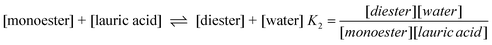 | (17) |
 | (18) |
The plots shown in Fig. 8 show the progression of the esterification of lauric acid in various ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycerol mixtures and also pure glycerol. In each system, 60 cm3, (0.82 mol) of glycerol was used and the corresponding molar amount of ChCl needed to make up the desired ratio was added. It should be noted that the addition of ChCl changes the volume of the system and so the data have not been analysed in terms of the concentration, but rather the number of moles of each reagent. In each case the acid catalyst added was trifluoroacetic acid (6 cm3, 0.078 mol). In these systems 0.1 moles of lauric acid was added. The reactions were all carried out at 150 °C, in a sealed vessel. As lauric acid and the respective esters are poorly soluble in glycerol and the glycerol based DESs, aliquots were taken from the top organic layer after being allowed to phase separate, which at this temperature was liquid. The isomeric form of the mono- and di-esters were not investigated in the analysis. Only the formation of the mono- and di-esters were investigated.
glycerol mixtures and also pure glycerol. In each system, 60 cm3, (0.82 mol) of glycerol was used and the corresponding molar amount of ChCl needed to make up the desired ratio was added. It should be noted that the addition of ChCl changes the volume of the system and so the data have not been analysed in terms of the concentration, but rather the number of moles of each reagent. In each case the acid catalyst added was trifluoroacetic acid (6 cm3, 0.078 mol). In these systems 0.1 moles of lauric acid was added. The reactions were all carried out at 150 °C, in a sealed vessel. As lauric acid and the respective esters are poorly soluble in glycerol and the glycerol based DESs, aliquots were taken from the top organic layer after being allowed to phase separate, which at this temperature was liquid. The isomeric form of the mono- and di-esters were not investigated in the analysis. Only the formation of the mono- and di-esters were investigated.
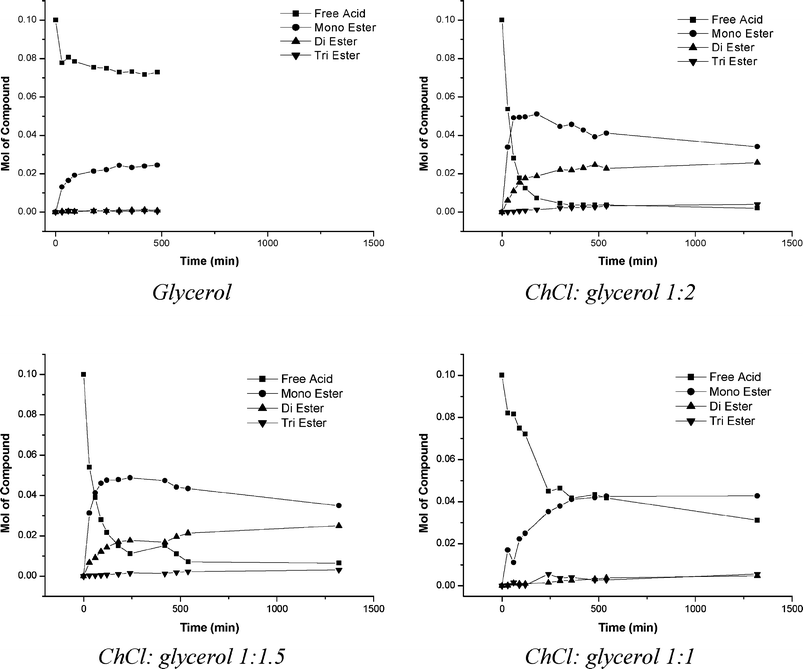 | ||
Fig. 8 Esterification data of 0.1 moles of lauric acid in various ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycerol DES mixtures. glycerol DES mixtures. | ||
The data in Fig. 8 show that the addition of ChCl does have a marked effect on the product distribution of the reaction. These systems are operating in large excess of glycerol, with a greater than 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of glycerol
1 ratio of glycerol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lauric acid. The mono-ester would be the most abundant product predicted due to the large excess of glycerol. This is indeed the case with pure glycerol where negligible amounts of di- and tri-esters formed. It also shows that after 8 h there is still approximately 70% of the free acid remaining. This is a surprising result since there is such a large amount of glycerol available for the lauric acid to react with but this could be due to the high viscosity of the system.
lauric acid. The mono-ester would be the most abundant product predicted due to the large excess of glycerol. This is indeed the case with pure glycerol where negligible amounts of di- and tri-esters formed. It also shows that after 8 h there is still approximately 70% of the free acid remaining. This is a surprising result since there is such a large amount of glycerol available for the lauric acid to react with but this could be due to the high viscosity of the system.
The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ChCl
1 ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycerol mixture shows a faster consumption of the lauric acid than the pure glycerol system, resulting in a much greater formation of the mono-ester. It should be noted that approximately twice as many moles of products are made when ChCl is added which clearly negates any effect of dilution. The 1
glycerol mixture shows a faster consumption of the lauric acid than the pure glycerol system, resulting in a much greater formation of the mono-ester. It should be noted that approximately twice as many moles of products are made when ChCl is added which clearly negates any effect of dilution. The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 and 1
1.5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 systems show similar overall conversion to the monoester but noticeably the monoester concentration goes through a maximum due to its use in eqn (17). The concentration of the diester is significantly increased with respect to both the pure glycerol and the 1
2 systems show similar overall conversion to the monoester but noticeably the monoester concentration goes through a maximum due to its use in eqn (17). The concentration of the diester is significantly increased with respect to both the pure glycerol and the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ChCl systems. The rate of consumption of the lauric acid follows 1
1 ChCl systems. The rate of consumption of the lauric acid follows 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ≈ 1
2 ≈ 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 > 1
1.5 > 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 > glycerol. This could be due to the pseudo site inhibition, where the interaction between the glycerol OH moieties interact with the Cl− from ChCl, but a more logical explanation is that the process is mass transport controlled.
1 > glycerol. This could be due to the pseudo site inhibition, where the interaction between the glycerol OH moieties interact with the Cl− from ChCl, but a more logical explanation is that the process is mass transport controlled.
The results shown in Fig. 8 indicate that the addition of choline chloride alters the product distribution of the esterifcation of glycerol and lauric acid. The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mixture shows that it is possible to form the di-ester product in relatively large amounts considering that the initial ratio of glycerol
2 mixture shows that it is possible to form the di-ester product in relatively large amounts considering that the initial ratio of glycerol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lauric acid is very high. Deviating to a ratio of 1
lauric acid is very high. Deviating to a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 does not appreciably alter this product distribution, however changing the ratio to 1
1.5 does not appreciably alter this product distribution, however changing the ratio to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the product distribution more closely resembles that of the of the pure glycerol system. There are likely to be many factors governing how these reactions progress; the addition of ChCl to glycerol lowers the viscosity, which would increase the mass transport in the system however it also acts as a diluent of the glycerol.
1, the product distribution more closely resembles that of the of the pure glycerol system. There are likely to be many factors governing how these reactions progress; the addition of ChCl to glycerol lowers the viscosity, which would increase the mass transport in the system however it also acts as a diluent of the glycerol.
The experiments shown in Fig. 8 were repeated at four different initial lauric acid concentrations; 0.2, 0.3, 0.4 and 0.6 mol L−1. The results are given summarised in Table 4 and full details are provided in the ESI†. The K values in Table 4 are apparent equilibrium constants based on the number of moles of species in solution. Although there is a relatively complex relationship between the concentrations of ChCl and lauric acid upon the product distribution, it can be concluded that the ChCl leads to a considerably higher proportion of product in the form of the diester whereas pure glycerol results predominantly in the monoester. It can also be seen that almost no tri-ester forms. Most importantly in the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 mixture the di-ester was effectively the only product formed. The supplementary information shows that most systems have reached equilibrium within 8 h however all systems were left for 24 h to obtain an equilibrium value. Where the data point taken after 24 h differs significantly from that after 8 h the last data point was ignored. However in most cases the tri-ester was still being formed at a slow rate due to the back reactions of eqn (16) and (17) yielding more acid. While there is some fluctuation in the K3 values the trend compared to K1 and K2 are constant.
1.5 mixture the di-ester was effectively the only product formed. The supplementary information shows that most systems have reached equilibrium within 8 h however all systems were left for 24 h to obtain an equilibrium value. Where the data point taken after 24 h differs significantly from that after 8 h the last data point was ignored. However in most cases the tri-ester was still being formed at a slow rate due to the back reactions of eqn (16) and (17) yielding more acid. While there is some fluctuation in the K3 values the trend compared to K1 and K2 are constant.
Ratio of ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycerol glycerol |
Lauric acid/mol L−1 | K1 | K2 | K3 |
|---|---|---|---|---|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
0.2 | 1.038 | 1.26 | 0.053 |
| 0.3 | 1.053 | 1.080 | 0.13 | |
| 0.4 | 1.28 | 0.97 | 0.14 | |
| 0.6 | 0.64 | 2.58 | 1.41 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0.1 | 1.43 | 0.79 | 0.11 |
| 0.2 | 0.24 | 0.66 | 0.026 | |
| 0.3 | 0.40 | 1.36 | 0.174 | |
| 0.4 | 1.01 | 1.83 | 0.22 | |
| 0.6 | 0.44 | 1.91 | 0.78 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
0.1 | 0.48 | 0.21 | 0.021 |
| 0.2 | 0.006 | 0.16 | 0.005 | |
| 0.3 | 0.31 | 0.70 | 0.070 | |
| 0.4 | 0.97 | 1.40 | 0.17 | |
| 0.6 | 0.90 | 0.77 | 0.290 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.1 | 0.077 | 0.007 | 0.005 |
| 0.2 | 0.58 | 0.34 | 0.040 | |
| 0.3 | 0.44 | 0.50 | 0.061 | |
| 0.4 | 0.52 | 0.61 | 0.082 | |
| 0.6 | 0.41 | 1.11 | 0.74 | |
| No ChCl | 0.2 | 1.58 | 0.41 | 0.02 |
| 0.3 | 2.43 | 0.79 | 0.04 | |
| 0.4 | 2.24 | 0.62 | 0.04 | |
| 0.6 | 2.14 | 1.11 | 0.15 |
In the equimolar ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycerol mixture the rate of conversion decreases and the monoester is the favoured product. The same trend was observed using 0.1 moles of free acid. This suggests there is a pseudo site inhibition effect from the Cl− interacting with the OH moieties that occurs at high ChCl concentrations.
glycerol mixture the rate of conversion decreases and the monoester is the favoured product. The same trend was observed using 0.1 moles of free acid. This suggests there is a pseudo site inhibition effect from the Cl− interacting with the OH moieties that occurs at high ChCl concentrations.
The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 system shows a faster rate of formation of the mono-ester than in the 0.2 M system, as would be expected since the amount of lauric acid is greater. In all of the ChCl containing systems the amount of di-ester formed is approximately constant (0.01 mol). This is significantly higher that the pure glycerol system. All of the systems containing ChCl are slow to reach equilibrium, again because the tri-ester appears to form slowly and removes di-ester from the system.
1 system shows a faster rate of formation of the mono-ester than in the 0.2 M system, as would be expected since the amount of lauric acid is greater. In all of the ChCl containing systems the amount of di-ester formed is approximately constant (0.01 mol). This is significantly higher that the pure glycerol system. All of the systems containing ChCl are slow to reach equilibrium, again because the tri-ester appears to form slowly and removes di-ester from the system.
Increasing the lauric acid concentration to 0.4 moles results in a similar product distribution to that found with 0.3 moles and as Table 4 shows the equilibrium constants for pure glycerol remain relatively similar. As the lauric acid concentration increases the data for the ChCl containing systems approach equilibrium. The equilibrium constants are shown in Table 4. In all of the pure glycerol systems the equilibrium constants are in the order K1 > K2 > K3 whereas for the ChCl containing mixtures the order is K2 > K1 > K3. The only way in which the equilibrium can be driven to the right is if one of the components is removed or more accurately if it decreases its activity in the system. One possible explanation for the data observed in Table 4 is that the presence of ChCl causes a decrease in the activity of water due to hydrogen bonding between the chloride anion and the water molecules.
One complication is that the reaction is carried out at 150 °C which would normally be enough to drive the water from the reaction vessel however in these experiments the reaction vessel was closed and so the ChCl would also affect the concentration of water vapour in the headspace. Nevertheless, inspection of the data in Table 4 suggests that both K2 and K3 increase as the ChCl concentration increases which supports this idea. Furthermore the central premise of this work was that ChCl could act as a pseudo-site inhibitor. This could either prevent esterification, which is clearly not the case, or it could reduce the activity of glycerol in eqn (16). If this were the case, then di- and tri-esterification would be preferred, which is indeed what appears to occur.
The results for the system containing 0.6 mol L−1 of ChCl superficially support all of the discussions above. The concentration of the mono-ester is up to ten times larger than that observed in some of the ChCl containing systems. Given that glycerol is still present in a large excess it is important to notice that the overall lauric acid concentration is approximately the same at the end of each experiment. While this could be viewed in terms of decreased glycerol activity in the ChCl an alternative view could be that the ChCl breaks up the structure of the glycerol and therefore actually enables further reaction on the OH moieties that could normally be inter-molecularly hydrogen bonded. It is probably a mixture of both effects that lead to the observed product distribution.
Equilibrium constants have been calculated for the ChCl based systems, and are shown in Table 4. An additional point worth discussing at this juncture is that the choline cation contains an OH moiety which in principle could be esterified. Despite numerous experiments no such esters were observed. Bell et al. also studied esterification reactions in choline based ionic liquids and deep eutectic solvents. The choline cation was found to be very unreactive and it was only when strong Lewis acids such as ZnCl2 were present with acetic anhydride that any significant acylation took place.29
Conclusion
The work here has shown that by adding ChCl to glycerol there is a marked reduction in the viscosity, whereas in the case of several previously reported ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) diol mixtures the viscosity increases.5 This is due to the 3D intermolecular H-bond interactions in glycerol that are broken up on addition of ChCl resulting in a less ordered system. This is also backed up by the density data which decreases as ChCl is added, where this is not true for the diol systems. As would be expected the conductivity increases as a function of ChCl addition due to more charge carrying species being made available in an increasingly more fluid solvent.
diol mixtures the viscosity increases.5 This is due to the 3D intermolecular H-bond interactions in glycerol that are broken up on addition of ChCl resulting in a less ordered system. This is also backed up by the density data which decreases as ChCl is added, where this is not true for the diol systems. As would be expected the conductivity increases as a function of ChCl addition due to more charge carrying species being made available in an increasingly more fluid solvent.
Several polarity parameters; ENT, α, β and π* have been obtained for the first time for several alcohol based DESs. The data has provided information on the relative polarity of these systems, which suggests that they are similar to RNH3+X−, R2NH2+X− and imidazolium ionic liquids, which is not surprising given the similarity in the structure of ChCl. There is a linear increase in the ENT, π* and α values of the glycerol based DESs as the ChCl concentration is increased.
By performing esterification of glycerol it has been shown that at low fatty acid concentrations and high ChCl concentrations the monoester can be selectively produced at a significantly higher rate than in pure glycerol. Solventless esterification of glycerol and lauric acid was carried out using ChCl with the intention of using its hydrogen bonding interaction with glycerol to behave as a potential site inhibitor to selectively form mono- or di-ester products. The work has shown that the addition of ChCl to the system actually encourages the reaction to progress further towards the di-ester product, as opposed to the pure glycerol system which encouraged only mono-ester formation. It is thought that because ChCl breaks up the 3-D structure of glycerol, this actually works in favour of the esterification reaction as it can further react with glycerol molecules already esterified. It is also possible that ChCl is interacting with water molecules produced in the reaction, effectively removing them from the equilibrium, which in turn helps drive the reaction forward. The ChCl also decreases the viscosity of the liquid and at low acid concentrations it is suggested that the process is mass transport controlled when the system has not reached equilibrium.
Acknowledgements
The authors would like to acknowledge the EPSRC (for a studentship for RCH), the Technology and Strategy Board (under the Glycerol Challenge) and Scionix Ltd. for funding this work.References
- Y. Gu and F. Jerome, Green Chem., 2010, 12, 1127 RSC.
- A. P. Abbott, P. M. Cullis, M. J. Gibson, R. C. Harris and E. Raven, E., Green Chem., 2007, 9, 868 RSC.
- A. P. Abbott, G. Capper, D. L. Davies, R. K. Rasheed and V. Tambyrajah, Chem. Commun., 2003, 70 RSC.
- A. P. Abbott, D. Boothby, G. Capper, D. L. Davies, R. K. Rasheed and V. Tambyrajah, J. Am. Chem. Soc., 2004, 126, 9142 CrossRef CAS.
- R. A. Sheldon, Chem. Ind. (London), 1992, 903 CAS.
- A. P. Abbott, R. C. Harris and K. S. Ryder, J. Phys. Chem. B, 2007, 111, 4910 CrossRef CAS.
- R. Fürth, Math. Proc. Cambridge Philos. Soc., 1941, 37, 252 Search PubMed.
- J. O'M. Bockris and G. W. Hooper, Discuss. Faraday Soc., 1961, 32, 218 RSC.
- A. P. Abbott, ChemPhysChem, 2004, 5, 1242 CrossRef CAS.
- A. P. Abbott, G. Capper and S. Gray, ChemPhysChem, 2006, 7, 803 CrossRef CAS.
- H. Jin, B. O'Hare, J. Dong, S. Arzhantsev, G. A. Baker, J. F. Wishart, A. J. Benesi and M. Maroncelli, J. Phys. Chem. B, 2008, 112, 81 CrossRef CAS.
- H. Zhao, Z.-C. Liang and F. Li, J. Mol. Liq., 2009, 149, 55 CrossRef CAS.
- A. Noda, K. Hayamizu and M. Watanabe, J. Phys. Chem. B, 2001, 105, 4603 CrossRef CAS.
- H. Tokuda, K. Hayamizu, K. Ishii, M. A. B. H. Susan and M. Watanabe, J. Phys. Chem. B, 2004, 108, 16593 CrossRef CAS.
- H. Tokuda, K. Hayamizu, K. Ishii, M. A. B. H. Susan and M. Watanabe, J. Phys. Chem. B, 2005, 109, 6103 CrossRef CAS.
- H. Tokuda, K. Ishii, M. A. B. H. Susan, S. Tsuzuki, K. Hayamizu and M. Watanabe, J. Phys. Chem. B, 2006, 110, 2833 CrossRef CAS.
- M. Tariq, A. P. Serro, J. L. Mata, B. Saramago, J. M. S. S. Esperanc, J. N. Canongia Lopes and L. P. N. Rebelo, Fluid Phase Equilib., 2010, 291, 131 CrossRef.
- M. H. Ghatee, M. Zare, A. R. Zolghadr and F. Moosavi, Fluid Phase Equilib., 2010, 291, 188 CrossRef CAS.
- A. Taylor, P. License and A. P. Abbott, manuscript in preparation.
- A. P. Abbott and C. A. Eardley, J. Phys. Chem. B, 1998, 102, 8574 CrossRef CAS.
- A. P. Abbott and C. A. Eardley, J. Phys. Chem. B, 1999, 103, 2504 CrossRef CAS.
- A. P. Abbott, C. A. Eardley and J. E. Scheirer, Phys. Chem. Chem. Phys., 2001, 3, 3722 RSC.
- K. Dimroth, C. Reichardt, T. Siepmann and F. Bohlmann, Justus Liebigs Ann. Chem., 1963, 661, 1 CrossRef CAS.
- C. Reichardt, Chem. Rev., 1994, 94, 2319 CrossRef CAS.
- M. J. Kamlet, J.-L. M. Abboud and R. W. Taft, J. Am. Chem. Soc., 1976, 98, 377 CrossRef CAS.
- M. J. Kamlet, J.-L. M. Abboud and R. W. Taft, J. Am. Chem. Soc., 1977, 99, 6027 CrossRef CAS.
- C. Reichardt, Green Chem., 2005, 7, 339 RSC.
- M. G. Del Po'polo, J. Kohanoff and R. M. Lynden-Bell, J. Phys. Chem. B, 2006, 110, 8798 CrossRef CAS.
- A. P. Abbott, T. J. Bell, S. Handa and B. Stoddart, Green Chem., 2006, 8, 784 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Tables of data used in Figures 2, 3 and 6 together with the full data used to create Table 4 in the form shown in Figure 8.See DOI: 10.1039/c0gc00395f |
| This journal is © The Royal Society of Chemistry 2011 |
