Synergy of boric acid and added salts in the catalytic dehydration of hexoses to 5-hydroxymethylfurfural in water†
Thomas S.
Hansen
,
Jerrik
Mielby
and
Anders
Riisager
*
Centre for Catalysis and Sustainable Chemistry, Department of Chemistry, Technical University of Denmark, DK-2800 Kgs. Lyngby,
Denmark. E-mail: ar@kemi.dtu.dk; thomh@kemi.dtu.dk; Tel: (+45) 45 25 22 33
First published on 30th November 2010
Abstract
In combination with various salts boric acid, B(OH)3, was shown to be an efficient, weak Lewis acid catalyst in the aqueous dehydration of fructose to 5-hydroxymethylfurfural (HMF) due to strong complexation between the boron atom and the hexoses. In the dehydration of a highly concentrated aqueous fructose solution (30 wt%), a HMF yield of 60% was achieved at 92% fructose conversion and a HMF selectivity of 65% with 100 g L−1B(OH)3 and 50 g L−1sodium chloride in the aqueous phase and methyl-isobutylketone (MIBK) as extracting solvent. Furthermore, the dehydration of glucose resulted in a yield of 14% HMF at 41% glucose conversion after 5 h at similar conditions. These results are highly competitive with currently reported aqueous HMF dehydration systems. In combination with the non-corrosive and non-toxic nature of the boric acid, compared to other well known homogeneous catalysts applicable for the dehydration process (e.g., H2SO4 and HCl), clearly, the boric acid-salt mixture is a very attractive catalyst system.
1. Introduction
Development of novel CO2 neutral and sustainable synthetic strategies to supply fuel and chemicals are necessary as a response to the ongoing concern about the climate changing along with depletion of fossil fuel reserves. Such demands can be met by a shift from the petrochemical industry to one based on biomass as the carbon source.1–6An attractive compound that is obtainable directly from the dehydration of carbohydrates, such as fructose and glucose, is 5-hydroxymethylfurfural (HMF). The molecule constitutes a platform compound that can be converted into a wide range of industrially important chemicals.7,8 In this context, the oxidation product of HMF, 2,5-furandicarboxylic acid (FDA), is of specific interest as it can potentially replace terephthalic acid in polyester manufacturing (Scheme 1).9
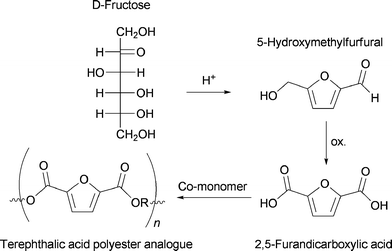 | ||
| Scheme 1 The reaction path from D-fructose to a terephthalic acid polyester analogue of 2,5-furandicarboxylic acid (FDA). | ||
Currently the most successful efforts to produce HMF have been done in ionic liquids,10–12 high-boiling organic solvents (e.g.dimethylsulfoxide, DMSO),13,14 by using strong and highly corrosive mineral acids such as HCl, H2SO4 and H3PO4 with an organic solvent to extract the HMF as it is produced in a two phase system5,15–17 or by employing microwaves as the heating source.18–20
Herein, we report our results from the development of a novel21 method to produce HMF from highly concentrated aqueous fructose solutions employing only a weak, non-toxic and non-corrosive acid, namely boric acid (pKa = 9.27, T = 293.15 K)22 as the dehydration catalyst.
2. Experimental
2.1 Materials
Fructose (99%), glucose (≥99.5%), B(OH)3 (99.8%), levulinic acid (98%), formic acid (99.8%), methyl-isobutylketone (MIBK) (≥99.0%), and tetrahydrofuran (THF) (99.9%) were purchased from Sigma-Aldrich. 5-Hydroxymethylfurfural (>99%) was purchased from SAFC and sucrose (99%) from Alfa Aesar and all used without further purification. Similarly, all salts applied for the salting-out experiments were acquired in p.a. quality and used as received. Distilled water was used for preparation of all aqueous solutions.2.2 Dehydration of sugars to HMF
As a general procedure the dehydration of fructose, glucose or sucrose was performed in a sealed pressure stable glass tube (Ace, pressure limit: 20 bar) charged with an aqueous solution of the sugar (30 wt%, 3.0 mL, 5.7 mmol), a specified mass of solid B(OH)3 and a magnet. In experiments with organic solvent extraction, these were used in the volume ratio 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (org
1 (org![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) aq). The tube with the reaction mixture was placed in a preheated oil bath for a specified time under magnetic stirring (420 rpm) at a fixed temperature. After the reaction, the tube was removed from the oil bath and cooled to room temperature before a sample was taken for analysis.
aq). The tube with the reaction mixture was placed in a preheated oil bath for a specified time under magnetic stirring (420 rpm) at a fixed temperature. After the reaction, the tube was removed from the oil bath and cooled to room temperature before a sample was taken for analysis.
2.3 Product analysis
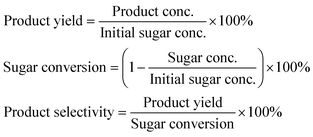
Reaction samples from the fructose, glucose and sucrose experiments were filtered through a syringe filter (VWR, 0.45 μm PTFE), mixed with an internal standard (i-PrOH) for calibration and filtered again, prior to analysis by high pressure liquid chromatography (HPLC, Agilent 1200 series, Bio-Rad Aminex HPX-87H, 300 mm × 7.8 mm pre-packed column). A 0.005 M H2SO4 mobile phase was employed as eluent at 60 °C with a flow rate of 0.6 mL min−1.During sucrose analysis the substrate was hydrolyzed to glucose and fructose on the HPLC column resulting in a poor elution profile on the HPLC chromatogram. Thus, in order to analyze experiments with sucrose, an aqueous 30 wt% solution was hydrolyzed at 50 °C for 6 h in 0.05 M HCl and subsequently analyzed viaHPLC. The resulting amount of glucose and fructose was used to determine the initial amount of sucrose.
Concentrations of products were determined from calibration curves obtained with reference samples and corrected with an internal standard, while product yields (%), product selectivities (%) and fructose conversions (%) were based on the initial concentration of sugars and calculated as:
The mass balances did not add up to 100% due to formation of insoluble polymers (humins), soluble polymers and other unidentified products during the dehydration process.
3. Results and discussion
3.1 Boric acid
Boric acid, B(OH)3 or H3BO3, acts as a Lewis acid in aqueous solutions forming tetrahydroxyborate and a proton (or hydronium ion) upon reaction with water. The nature of the boric acid suggests (based on previous published results by Kuster and Temmink23) that it is too weak an acid to efficiently catalyze the dehydration reaction of fructose to HMF. However, boric acid forms strong chelates with diols and hexoses, e.g.fructose and glucose,24–26 shifting the equilibrium reactions towards a doubly coordinated borate–hexose complex. This complexation results in an overall release of protons leading to acidification of the aqueous media and so facilitates the acid catalyzed dehydration (Scheme 2).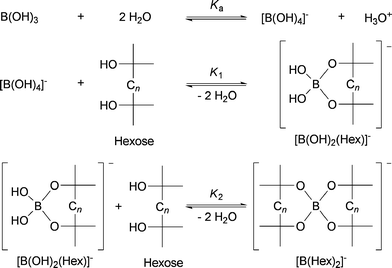 | ||
| Scheme 2 Equilibria between boric acid and hexoses in water. | ||
In principle, the pH will gradually increase during the HMF dehydration reaction and return to a value determined by the boric acid-water equilibrium when all sugars are converted. However, by-product formation of formic acid (FA) and levulinic acid (LA) by competitive rehydration of HMF during the reaction27,28 prevent the pH from increasing. From a downstream HMF processing point of view, this phenomenon could prove very useful, as the isolation of LA and FA, which are also valuable by-products, results in a HMF product solution which is not nearly as acidic compared to using mineral acids. This is likely to make the overall HMF production process more economically attractive by reducing costs related to both neutralization and the use of corrosion resistant materials.
3.2 Titration of fructose and glucose with boric acid
A 1.88 M aqueous solution of fructose and a 1.93 M solution of glucose (both ∼30 wt%) were titrated with solid B(OH)3 to the point of saturation at room temperature to monitor the associated pH change as a function of the B(OH)3 concentration (Fig. 1). As reference, pH was measured in a B(OH)3 saturated (50 g L−1) aqueous solution (pH = 3.65).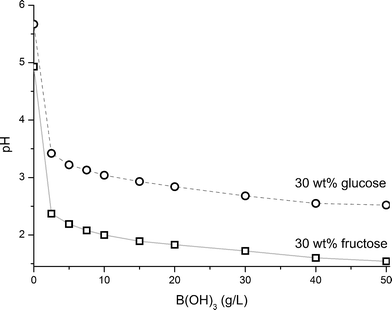 | ||
| Fig. 1 Titration of a 1.88 M fructose solution and a 1.93 M glucose solution (both ∼30 wt%) with solid B(OH)3. Between additions of boric acid, the system was allowed sufficient time to stabilize before pH measurements. | ||
A rapid decrease in pH in the 30 wt% sugar solutions was observed upon the first boric acid addition which then levelled out as more B(OH)3 was added. The change was more pronounced for fructose than glucose suggesting a stronger complexation between B(OH)3 and fructose than with glucose, in accordance with previous findings.25
3.3 Dehydration of fructose with boric acid
The dehydration of fructose to HMF is known to proceed even without the addition of catalyst at elevated temperatures and prolonged reaction times.14 However, when the dehydration of fructose is carried out in an aqueous environment alone high HMF yields and selectivities are difficult to achieve. One way to overcome this problem is by employing an organic phase that extracts HMF as it is produced thereby reducing the rehydration of HMF to FA and LA, as well as humin formation to some extent.15,16 To separate this inherent catalytic effect from any effects of the added catalyst, reaction temperature and time was initially investigated in order to find conditions where the non-catalyzed reaction was negligible. Here it was found, that reaction of a 30 wt% fructose solution at 150 °C for 45 min with MIBK as the extracting solvent with a MIBK![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) aqueous volume ratio of 4
aqueous volume ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 possessed minimal intrinsic activity resulting in only 5% fructose conversion and 2% HMF yield (Fig. 2). Notably, these conditions are, however, still suitable for HMF production if a dehydration catalyst is added (vide infra).
1 possessed minimal intrinsic activity resulting in only 5% fructose conversion and 2% HMF yield (Fig. 2). Notably, these conditions are, however, still suitable for HMF production if a dehydration catalyst is added (vide infra).
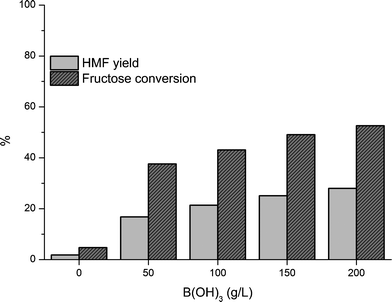 | ||
Fig. 2 Dehydration of 30 wt% fructose solutions with varied B(OH)3 concentrations and MIBK as extracting solvent (150 °C, 45 min, MIBK![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) aqueous volume ratio = 4 aqueous volume ratio = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1). | ||
Using the reaction conditions where the intrinsic conversion to HMF was found to be minimal (i.e. 150 °C, 45 min, MIBK![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) aqueous volume ratio = 4
aqueous volume ratio = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), a set of experiments were conducted varying the catalyst amount in a 30 wt% fructose solution in order to investigate how the B(OH)3 concentration influenced the dehydration of highly concentrated fructose solutions (Fig. 2).
1), a set of experiments were conducted varying the catalyst amount in a 30 wt% fructose solution in order to investigate how the B(OH)3 concentration influenced the dehydration of highly concentrated fructose solutions (Fig. 2).
A clear relation between the B(OH)3 concentration and the fructose conversion was found, showing an increase in fructose conversion with increasing B(OH)3 concentration. The most commonly used catalysts for the dehydration process are strong acids, as previously mentioned, so increasing the amount of the weak Lewis acid B(OH)3 was expected to result in a higher fructose conversion and HMF yield.
3.4 Synergetic catalytic effect between boric acid and salts
When sugars are dehydrated to HMF in acidic, aqueous-organic systems with dissolved salts, the salts usually facilitate only a salting-out effect of the formed HMF to the organic phase and no significant catalytic effect.16 In accordance with this, only a weak catalytic effect of NaCl was found in the dehydration of 30 wt% aqueous fructose solution (Fig. 3).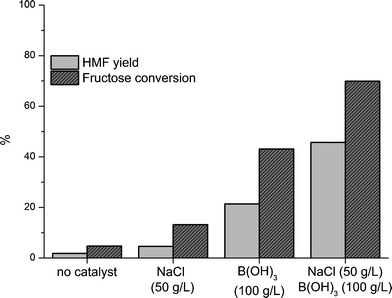 | ||
Fig. 3 Dehydration of 30 wt% fructose solutions with MIBK as extracting solvent and no catalyst or added NaCl and/or B(OH)3 (150 °C, 45 min, MIBK![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) aqueous volume ratio = 4 aqueous volume ratio = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1). | ||
The addition of 50 g L−1NaCl to the reaction mixture resulted in a HMF yield of 5% at 13% fructose conversion compared to 2% HMF at 5% fructose conversion without NaCl present. This weak catalytic effect is believed to arise from the slight acidification observed when salts are added to a concentrated solution of fructose. The dehydration reaction of fructose to HMF is highly dependent on the pH in solution, hence a small decrease in pH is expected to have a positive influence on the rate of fructose conversion and HMF formation.
Surprisingly the addition of NaCl in low concentrations together with B(OH)3 was found not only to increase the HMF yield, due to the salting-out effect as described by Román-Leshkov and Dumesic,16 but also to significantly increase the fructose conversion and HMF yield. Thus, reaction at 150 °C for 45 min with 50 g L−1NaCl, 100 g L−1B(OH)3 and MIBK as extracting solvent (MIBK![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) aqueous volume ratio = 4
aqueous volume ratio = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) resulted in a fructose conversion of 70% and 46% HMF yield (Fig. 3). The synergistic effect of using both B(OH)3 and NaCl is believed to occur as a result of the increased acidity of aqueous B(OH)3 solutions in combination with salts, suggesting that the salts stabilizes one or more of the charged intermediates and products shown in Scheme 2. The acidifying effect of different salts on boric acid has been correlated to the energy of hydration by Shishido29 and shows an increase in acidity with increasing hydration energy. As already mentioned, the addition of salt to a concentrated fructose solution also results in a slight acidification of the solution. Hence, several factors add up in the observed synergy and complex behavior of NaCl and B(OH)3 in the dehydration of aqueous fructose solutions to HMF.
1) resulted in a fructose conversion of 70% and 46% HMF yield (Fig. 3). The synergistic effect of using both B(OH)3 and NaCl is believed to occur as a result of the increased acidity of aqueous B(OH)3 solutions in combination with salts, suggesting that the salts stabilizes one or more of the charged intermediates and products shown in Scheme 2. The acidifying effect of different salts on boric acid has been correlated to the energy of hydration by Shishido29 and shows an increase in acidity with increasing hydration energy. As already mentioned, the addition of salt to a concentrated fructose solution also results in a slight acidification of the solution. Hence, several factors add up in the observed synergy and complex behavior of NaCl and B(OH)3 in the dehydration of aqueous fructose solutions to HMF.
An experiment was conducted in order to find the highest obtainable HMF yield from a 30 wt% aqueous fructose solution near full substrate conversion with the NaCl-containing catalytic system. Increasing the reaction time to 90 min subsequently resulted in a 92% fructose conversion, a 60% HMF yield and a HMF selectivity of 65%.
A range of other salts, primarily containing alkali or alkaline earth metal cations, were further employed in the dehydration of fructose in order to investigate the influence of the salts on the synergistic interplay with B(OH)3 (Table 1).
| Entry | Salt | Fructose conversion (%) | HMF yield (%) | HMF selectivity (%) |
R valueb (MIBK![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) aq) aq) |
|---|---|---|---|---|---|
a Reaction conditions: 100 g L−1B(OH)3, 0.87 M salt with respect to the anion, 45 min, 150 °C, MIBK![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) aqueous volume ratio = 4 aqueous volume ratio = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
b The R value is the HMF distribution obtained between the MIBK phase and the aqueous phase, i.e.[HMF]MIBK/[HMF]aq. 1.
b The R value is the HMF distribution obtained between the MIBK phase and the aqueous phase, i.e.[HMF]MIBK/[HMF]aq.
|
|||||
| 1 | LiCl | 69 | 45 | 66 | 1.1 |
| 2 | LiBr | 61 | 38 | 62 | 1.0 |
| 3 | LiNO3 | 49 | 21 | 42 | 0.9 |
| 4 | NaCl | 70 | 46 | 65 | 1.0 |
| 5 | NaBr | 60 | 38 | 64 | 0.9 |
| 6 | NaNO3 | 49 | 20 | 41 | 0.9 |
| 7 | Na2SO4 | 90 | 41 | 45 | 1.7 |
| 8 | KCl | 67 | 44 | 65 | 1.0 |
| 9 | KBr | 63 | 39 | 62 | 0.9 |
| 10 | KI | 56 | 35 | 63 | 0.7 |
| 11 | KNO3 | 49 | 20 | 40 | 0.8 |
| 12 | K2SO4 | 89 | 40 | 46 | 1.5 |
| 13 | MgCl2 | 81 | 52 | 65 | 1.1 |
| 14 | AlCl3 | 100 | 21 | 21 | 1.1 |
| 15 | FeCl3 | 99 | 36 | 36 | 1.1 |
A clear relationship between the nature of the anion and the resulting fructose conversion and HMF yield was observed. Generally, sulfates (entries 7 and 12) resulted in high fructose conversions and a high HMF distribution in the organic phase but low HMF selectivities and yields. The phase distribution selectivity is commonly expressed as the R value (calculated as [HMF]org/[HMF]aq), which quantify the extracting power of the organic solvent. In contrast, the nitrates (entries 3, 6 and 11) resulted in lower fructose conversions, HMF yields, HMF selectivities and R values compared to the halide salts, which were shown to be superior when comparing HMF yields and selectivities. The size of the halide anions did not seem to affect the selectivity towards HMF, whereas the fructose conversion rate decreased in the order: chloride > bromide > iodide.
The nature of the alkali cation did also not seem to have great importance on the dehydration reaction and only small variations in HMF yields and selectivities within the estimated experiment error were observed. Similar trends were observed in the control experiments conducted without B(OH)3 in order to test the activity of the salts alone (results not presented here).
Interestingly, the high R values, representing the distribution of HMF in the organic phase relative to the aqueous phase, observed in experiments with sulfate salts did not result in correspondingly high relative HMF yields and selectivities, as would be expected when the mean residence time of HMF in the aqueous solution was reduced due to diminished rehydration. The same conclusion was reached by Román-Leshkov and Dumesic16 in their investigation of fructose dehydration reactions employing different salts and a strong mineral acid as the dehydration catalyst, thus suggesting the sulfate anion has some influence on the dehydration reactivity, e.g. shifting the pyranose-furanose equilibrium.
In combination, the strong Lewis acidic salts, AlCl3 or FeCl3, and B(OH)3 (entries 14 and 15) resulted in formation of large amounts of polymers and the rehydration products FA and LA, demonstrating that HMF was no longer the favored product.
3.5 Boric acid-salt catalysis with common extracting solvents
Experiments with different organic extraction solvents were conducted with 30 wt% fructose solutions at 150 °C for 45 min with 100 g L−1 of B(OH)3 catalyst and 50 g L−1NaCl using an organic![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) aqueous volume ratio of 4
aqueous volume ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. (Fig. 4).
1. (Fig. 4).
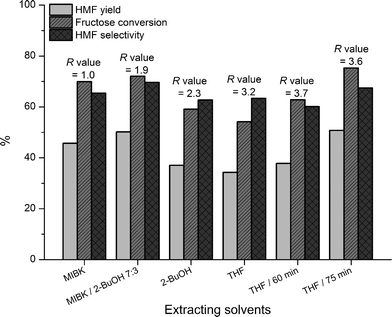 | ||
Fig. 4 Dehydration of 30 wt% fructose solutions with organic extraction solvents, 100 g L−1B(OH)3 and 50 g L−1NaCl (150 °C, 45 min unless otherwise mentioned, organic![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) aqueous volume ratio = 4 aqueous volume ratio = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1). | ||
Reactions with THF as extraction solvent were found to be substantially slower than analogous reactions with MIBK. This could be attributed to an effective decrease in the fructose concentration at elevated temperatures as a result of miscibility of THF and saline aqueous mixtures, or an increased ability of THF to extract the produced organic acids compared to MIBK. However, when increasing the reaction time with THF as extracting solvent comparable HMF yields and selectivities to experiments with MIBK were obtained.
THF is an interesting extraction solvent for the dehydration reaction due to avoidance of humin formation which, if formed, would impose a severe drawback from a process point of view. Although the apparent HMF selectivity with THF was not significantly higher than experiments with visible humin formation, the selectivity was observed to increase with increasing reaction time, most likely due to reversion of isomeric and dimer forms of fructose. The disadvantage and concern of applying THF is obviously its low flashpoint (−14 °C), its tendency to form peroxides over time and the relatively high chemical aggressiveness of THF fumes. Unfortunately, the latter made longer term experiments (>75 min) impossible with our available apparatus.
3.6 Dehydration of glucose with boric acid
Despite the ability to selectively form HMF from fructose, boric acid proved ineffective in the dehydration of glucose to HMF. Thus, dehydration of a 30 wt% glucose solution at 150 °C for 45 min with 100 g L−1B(OH)3 and 50 g L−1NaCl only resulted in 8% glucose conversion and a poor HMF yield of 2% (Table 2, entry 2). Prolonging the reaction time to 3 h increased the HMF yield somewhat to 10% at 36% glucose conversion compared to the un-catalyzed reaction which only resulted in 1% HMF yield at 13% glucose conversion (entries 3 and 4). Further increase of the reaction time to 5 h resulted in a 41% glucose conversion and 14% HMF yield compared to the un-catalyzed experiment which resulted in a 24% glucose conversion and 3% HMF yield (entries 5 and 6). Thus, even though the catalytic system performed poorly on a glucose feedstock, the catalyzed reactions were substantially better than the un-catalyzed ones.| Entry | Catalyst | Time (min) | Glucose conversion (%) | HMF yield (%) | HMF selectivity (%) |
|---|---|---|---|---|---|
a Reaction conditions: 150 °C, MIBK![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) aqueous volume ratio = 4 aqueous volume ratio = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
b Reaction conditions: 100 g L−1B(OH)3, 50 g L−1NaCl, 150 °C, MIBK 1.
b Reaction conditions: 100 g L−1B(OH)3, 50 g L−1NaCl, 150 °C, MIBK![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) aqueous volume ratio = 4 aqueous volume ratio = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1.
|
|||||
| 1a | — | 45 | <1 | 0 | 0 |
| 2b | B(OH)3 + NaCl | 45 | 8 | 2 | 25 |
| 3a | — | 180 | 13 | 1 | 10 |
| 4b | B(OH)3 + NaCl | 180 | 36 | 10 | 27 |
| 5a | — | 300 | 24 | 3 | 13 |
| 6b | B(OH)3 + NaCl | 300 | 41 | 14 | 34 |
The dehydration of glucose is much more difficult than the dehydration of fructose, presumably because the first step in the Lobry de Bruyn-van Ekenstein transformation30 of glucose to fructose proceeds rapidly in basic media, but very slowly in acidic media where HMF production is possible. Furthermore the HMF selectivity increased over time indicating that some intermediate hexoses formed from glucose were able to revert back to fructose and dehydrate to HMF.
3.7 Dehydration of sucrose with boric acid
The disaccharide sucrose consists of a fructose unit and a glucose unit connected via an α-1,4′-glycosidic linkage which can be hydrolyzed relatively easily. Indeed, the fructose part of the disaccharide could selectively be converted into HMF by the catalytic B(OH)3-NaCl system. Interestingly, the glucose part was not converted in any significant amount and could still be detected after the dehydration reaction. At prolonged reaction times of 2 h at 150 °C with a 30 wt% aqueous sucrose solution, 94% of the initial fructose units were converted resulting in a good HMF yield of 33% from sucrose corresponding to a 70% selectivity assuming that all HMF originated from fructose (Table 3).314. Conclusions
In this work, we have shown that the Lewis acid, boric acid B(OH)3, is a very efficient catalyst in the dehydration of highly concentrated aqueous fructose solutions to HMF. This result, combined with desirable properties such as non-toxicity, low corrosiveness, low acid strength and readily availability, makes B(OH)3 a very attractive alternative to known catalysts for the process.32HMF selectivities and yields were further improved by employing a combined catalytic system of B(OH)3 and NaCl, which in combination showed a synergistic effect on the dehydration reaction.Boric acid in combination with NaCl was found to be less efficient for the dehydration of glucose in water, which is also generally considered to be a more challenging task to achieve. Hence, only poor HMF yields were accomplished. However, the catalytic system developed here performed much better than the un-catalyzed reaction and could also be applied for highly concentrated aqueous sucrose solutions.
This study is, to the best of our knowledge, the first to demonstrate the use of boric acid in the dehydration of sugars to HMF and to provide a detailed parameter study of the dehydration process for fructose. A simple modification of the catalytic system by addition of salt was shown to have a synergistic effect of the dehydration of fructose to HMF allowing good yields and selectivities of HMF to be reached.
Since boric acid is a weak Lewis acid, non-toxic, cheap and already widely used in industrial processes it is desirable from an industrial point of view compared to other mineral acids such as HCl and H2SO4, which are highly corrosive. This could clearly make future implementation of B(OH)3 in HMF production attractive compared to other acids.
Acknowledgements
The work was supported by The Danish National Advanced Technology Foundation and Novozymes A/S.Notes and references
- B. Kamm and M. Kamm, Appl. Microbiol. Biotechnol., 2004, 64, 137 CrossRef CAS.
- B. Kamm and M. Kamm, Adv. Biochem. Eng./Biotechnol., 2007, 105, 175 Search PubMed.
- C. H. Christensen, J. Rass-Hansen, C. C. Marsden, E. Taarning and K. Egeblad, ChemSusChem, 2008, 1, 283 CrossRef CAS.
- A. J. Ragauskas, C. K. Williams, B. H. Davison, G. Britovsek, J. Cairney, C. A. Eckert, W. J. Frederick Jr., J. P. Hallett, D. J. Leak, C. L. Liotta, J. R. Mielenz, R. Murphy, R. Templer and T. Tschaplinski, Science, 2006, 311, 484 CrossRef.
- Y. Román-Leshkov, C. J. Barrett, Z. Y. Liu and J. A. Dumesic, Nature, 2007, 447, 982 CrossRef CAS.
- M. Mascal and E. B. Nikitin, Angew. Chem., Int. Ed., 2008, 47, 7924 CrossRef CAS.
- A. Corma, S. Iborra and A. Velty, Chem. Rev., 2007, 107, 2411 CrossRef CAS.
- C. Moreau, M. N. Belgacem and A. Gandini, Top. Catal., 2004, 27, 11 CrossRef CAS.
- T. Werpy and G. Petersen, US DOE, 2004, No. DOE/GO-102004-1992, http://www.nrel.gov/docs/fy04osti/35523.pdf.
- H. Zhao, J. E. Holladay, H. Brown and Z. C. Zhang, Science, 2007, 316, 1597 CrossRef CAS.
- G. Yong, Y. Zhang and J. Y. Ying, Angew. Chem., Int. Ed., 2008, 47, 9345 CrossRef CAS.
- T. Ståhlberg, M. G. Sørensen and A. Riisager, Green Chem., 2010, 12, 321 RSC.
- J. N. Chheda, Y. Román-Leshkov and J. A. Dumesic, Green Chem., 2007, 9, 342 RSC.
- R. M. Musau and R. M. Munavu, Biomass, 1987, 13, 67 CrossRef CAS.
- Y. Román-Leshkov, J. N. Chheda and J. A. Dumesic, Science, 2006, 312, 1933 CrossRef CAS.
- Y. Román-Leshkov and J. A. Dumesic, Top. Catal., 2009, 52, 297 CrossRef CAS.
- B. F. M. Kuster, Starch/Staerke, 1990, 42, 314 Search PubMed.
- X. Qi, M. Watanabe, T. M. Aida and R. L. Smith, Jr., Green Chem., 2008, 10, 799 RSC.
- X. Qi, M. Watanabe, T. M. Aida and R. L. Smith, Jr., Catal. Commun., 2008, 9, 2244 CrossRef CAS.
- T. S. Hansen, J. M. Woodley and A. Riisager, Carbohydr. Res., 2009, 344, 2568 CrossRef CAS.
- Although B(OH)3 is mentioned as a possible catalyst for the dehydration of fructose to HMF in at least three patent applications by Dumesic et al.: WO 2007/146636, WO 2008/151178 A1 and US 2008/0033188 A1, no experimental results are reported.
- Handbook of Chemistry and Physics, CRC, 2008–2009, 89, pp. 8–40 Search PubMed.
- B. F. M. Kuster and H. M. G. Temmink, Carbohydr. Res., 1977, 54, 185 CrossRef CAS.
- J. C. Norrild and H. Eggert, J. Am. Chem. Soc., 1995, 117, 1479 CrossRef CAS.
- M. Makkee, A. P. G. Kieboom and H. van Bekkum, Recl. Trav. Pays-Bas., 1985, 104, 230 Search PubMed.
- M. van Duin, J. A. Peters, A. P. C. Kieboom and H. van Bekkum, Tetrahedron, 1984, 40, 2901 CrossRef CAS.
- L. Cottier and G. Descotes, Trends in Heterocycl. Chem., 1991, 2, 233 Search PubMed.
- J. Horvat, B. Klaić, B. Metelko and V. Šunjić, Tetrahedron Lett., 1985, 26, 2111 CrossRef CAS.
- S. Shishido, Bull. Chem. Soc. Jpn., 1952, 25, 199 CrossRef CAS.
- W. L. Evans, Chem. Rev., 1942, 31, 537 CrossRef.
- To assume that all HMF originates from the fructose moiety of sucrose is not entirely correct, as dehydration experiments with pure glucose indeed indicated that a small amount was converted to HMF. However, we have rationalized that this is the best way of indicating the selectivity of the sucrose dehydration.
- A. Boisen, T. B. Christensen, W. Fu, Y. Y. Gorbanev, T. S. Hansen, J. S. Jensen, S. K. Klitgaard, A. Riisager, T. Ståhlberg and J. M. Woodley, Chem. Eng. Res. Des., 2009, 87, 1318 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Tables with experimental values reported in the figures. See DOI: 10.1039/c0gc00355g |
| This journal is © The Royal Society of Chemistry 2011 |