Investigation of glycerol polymerization in the clinker grinding process†‡
Andrei
Parvulescu
a,
Michele
Rossi
*b,
Cristina Della
Pina
b,
Rosaria
Ciriminna
c and
Mario
Pagliaro
*c
aDebye Institute for Nanomaterials Science, Utrecht University, Sorbonnelaan 16, 3508 TC, Utrecht, The Netherlands. E-mail: michele.rossi@unimi.it; mario.pagliaro@cnr.it
bDipartimento di Chimica Inorganica, Metallorganica e Analitica, Università degli Studi di Milano, via Venezian 21, 20133, Milano, Italy
cIstituto per lo Studio dei Materiali Nanostrutturati, CNR, via U. La Malfa 153, 90146, Palermo, Italy
First published on 17th November 2010
Abstract
Concrete production is a large scale process that involves high energy consumption. In order to increase the sustainability of this process, the reduction of energy input is necessary. Bio-glycerol was demonstrated to be a highly efficient renewable-based additive in the grinding process for concrete production and helped reduce energy costs and improve the quality of the resulting product. In order to understand its excellent aiding properties, the interaction of glycerol with cement clinkers was investigated; both chemical and physical interactions were taken into account. The results of this investigation points to surface tension modification of the clinker particles as one of the main effects of bio-glycerol addition during the grinding process.
Introduction
Despite the recession that started in 2008, the global production of concrete, which is currently over 2 billion tonnes per year,1 is expanding rapidly due to China's,2 Brazil's and India's booming construction industries. By 2050, concrete use is predicted to reach four times the 1990 level. Cement production is also a highly energy intensive process, and particularly, the grinding of the cement clinker accounts for as much as 40% of the total production cost. Therefore, grinding additives are widely used by the cement industry as they can lead to a significant decrease of electrical energy consumption by about 10%.The development of new concrete additives (admixtures) to produce a stronger, handy material is a topic of intense investigation.3 In general, cement additives are primarily composed of strongly polar and hydrophilic organic molecules, usually derived from non-renewable petroleum feedstocks. They include aliphatic alkali carboxylates3 and nitrogen-containing hydroxyl compounds such as triethanolamine,4 which, overall, are used in 60% of the world's cement production. Water-soluble, biomass-derived polyols, mainly glycols and polyglycerols,5 sulfonated lignins,6 and the acetic acid esters of glycerol,7 are also useful cement additives, but are generally more expensive. A combination of more than one component is frequently employed.8 These additives are introduced during the grinding process within the range 150–500 g per ton of cement, and they are considered to be able to adsorb the newly created surface of the particles, changing their electrokinetic potentials and reducing their tendency to re-agglomerate (Table 1).9 This reduces the energy needed to grind the clinker. Admixtures actively interact with hydration processes by controlling them in terms of reaction rate, composition, morphology of the hydration products and by reducing pack set inhibition.10
| Increased cement flowability and reduced pack set | Increased grinding efficiency and mill output | Reduced unit power costs | Reduced handling and pumping costs |
Recently, some of us reported that crude glycerol obtained as a by-product of biodiesel manufacture (bio-glycerol) is an excellent concrete additive that is capable of enhancing cement performance by (i) improving the compression strength, and (ii) aiding the grinding and handling properties.11 Compression strength is the capability of a manufactured concrete material to withstand pressure. When the ultimate compression strength is reached, fractures are generated at the surface that may cause the material to break. Since a high compression strength is so important, a variety of additives have been developed to improve this property.
The use of bio-glycerol in this application has significant environmental, economic and technical benefits. The environmental benefits can be achieved through better energy efficiency in grinding and handling processes by the use of a renewable resource—bio-glycerol—in place of additives of petrochemical origin. Industry benefits are derived from having a single, readily available material that is capable of offering all three major technical advantages required of cement additives (enhanced concrete strength, grinding and handling aids for cement). Furthermore, bio-glycerol is expected to be an important platform molecule of future bio-refinery schemes and therefore available in high quantities from renewable sources.12
The other major advantage of employing crude glycerol as a cement additive is that the use of low levels of raw glycerol gives significant improvements in the morphological and rheological properties of the materials after the milling step of different cement clinkers. These performances are either similar or better compared to the more expensive triethanolamine (TEA) and diethylene glycol (DEG) that are typically used as grinding and handling aids. Moreover, in our original study,11 crude glycerol from a biodiesel plant was found to induce improved mechanical properties with respect to those produced by pure glycerol.
To understand the causes of this particular behaviour, we have tried to investigate whether the beneficial effect of glycerol is just due to physical properties or whether is it due to chemical reactions taking place during the grinding of clinker–bio-glycerol admixtures. Indeed, Ruppert et al. recently discovered that colloidal CaO particles, similar to those that might be present in cement, have a very high activity in promoting glycerol etherification (Scheme 1).13
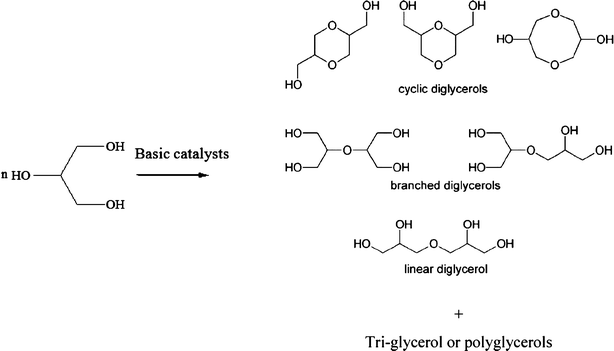 | ||
| Scheme 1 Glycerol polymerization by basic catalysts. | ||
In this paper, we report our investigations on the interaction of bio-glycerol and cement clinkers obtained from industry. Both the catalytic properties of the clinkers in glycerol etherification, as well as the physical interactions, were investigated.
Experimental conditions
Materials
Three clinker samples originating from plants located in three different countries (France—clinker A, China—clinker B and Germany—clinker C, kindly donated by Grace Construction Products), were used throughout the study. To study interactions, we tested the catalytic activity of the clinkers in (bio-)glycerol etherification, followed by characterization of the clinkers before and after the reaction, and finally we examined the stability of the clinkers in the presence of glycerol solutions. Raw glycerol was received from a bio-diesel plant with the following composition: 85 wt% glycerol and 15 wt% water solution containing a mixture of inorganic salts, fatty acid salts, methanol and ashes.14Catalytic reaction
Clinkers powdered before the etherification reaction and Na2CO3 (Acros, anhydrous pure) were used as received. The catalytic tests were carried out at temperatures between 120–220 °C, both in a 40 ml Parr autoclave and a three-necked 250 ml flask equipped with a mechanical stirrer (500 rpm) under a flow of argon connected to a Dean–Stark apparatus, with a reflux condenser for removal of water by-product according to the method of Ruppert et al.13 Typical loadings were 50 g of glycerol substrate and 1 g of clinker catalyst.Analytical methods
The products were analyzed using a GC 2010 system from Shimadzu with a CP-WAX 57CB (25 m × 0.2 mm × 0.2 μm) column using internal calibration, a GC-MS from Shimadzu with a CP-WAX 57CB column (25 m × 0.2 mm × 0.2 μm) and also by HPLC-MS using an HPLC-ESI-MS from Shimadzu with a Pathfinder PS column (RP-18, 3.5 μm, 4.6 × 150 mm). Details of these methods can be found in the work of Ruppert et al.13Catalyst characterization
X-Ray powder diffraction (XRD) was performed using a Bruker-AXS D8 Advance powder X-ray diffractometer equipped with an automatic divergence slit, Våntec-1 detector and cobalt Kα1,2 (λ = 1.79026 Å) source. Infrared measurements were recorded on a Perkin-Elmer 2000 FTIR spectrometer with a data point resolution of 4 cm−1 using a DTGS detector with an accumulation of 16 scans per spectrum. The samples were diluted prior to measurement with KBr in a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 mixture ratio.
95 mixture ratio.
Results and discussion
Calcination of a mixture of calcareous and argillaceous materials comprising limestone, sand, clay and iron ore at a temperature of about 1400–1500 °C produces a sintered nodular material called “clinker”, whose major components are tricalcium silicate (typically about 65% of the total), dicalcium silicate (about 15% of the total), tricalcium aluminate (typically about 7% of the total) and tetracalcium aluminoferrite (typically about 8% of the total).15 Overall, these phases constitute about 90% of the material composition, whereas the mineralogical structure differs of course from clinker to clinker. No calcium sulfate was present in the clinker samples analyzed in this work as CaSO4 is used as a cement constituent added during the grinding process and is not considered an additive.Due to the above mentioned complex chemical composition, it is very difficult to distinguish between the clinker components by FT-IR or Raman spectroscopy. Fig. 1a, for example, shows the FT-IR spectra of the three clinker samples. All three samples present a strong band at 927 cm−1, with additional peaks at 1100 cm−1, which most probably correspond to the stretching vibration of the T–O–T groups (where T is either Si or Al) and the Si–O–Ca bond.16 Additional bands are observed in the region 400–600 cm−1 that can be attributed to the tetrahedral alumina stretching band at 532 cm−1 and to silica at 453 cm−1. A small broad band with a maxima at 3400 cm−1 is also observed that corresponds to water (–OH) stretching vibrations. Furthermore, as the spectra are very similar, the FT-IR measurements indicate that all three clinker samples contain the same major structural components.
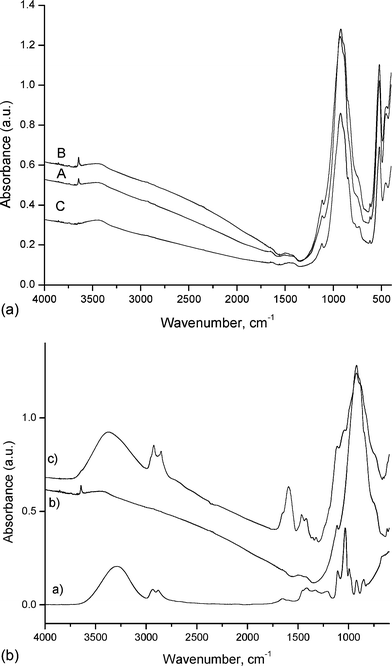 | ||
| Fig. 1 (a) FT-IR spectra of the three clinker samples: (A) is clinker A, (B) is clinker B and (C) is clinker C; (b) FT-IR spectra of a: bio-glycerol, b: clinker B and c: clinker B + 10 wt% bio-glycerol. The compounds were physically mixed. | ||
In order to investigate the influence of bio-glycerol mixing with the clinker, samples were prepared and analyzed by FT-IR spectroscopy. The simple physical mixing of the clinkers with bio-glycerol does not induce any important chemical transformations in the structure of the cement precursor, as indicated for clinker B (Fig. 1b). Similar results were observed for the other two clinkers (spectra not shown).
The FT-IR spectra of the fresh clinker sample (B), mixed with 10 wt% bio-glycerol and of bio-glycerol alone are presented and compared in Fig. 1b. The main characteristic bands of the clinker material in the region 400–1000 cm−1 are present in the mixed material (Fig. 1b, B and C). The new bands that appear in the IR spectrum are due to bio-glycerol (Fig. 1b, A and C). The strong broad band with a maxima at 3400 cm−1 is due to water and glycerol O–H stretching vibrations, where the band at 1600 cm−1 corresponds to adsorbed water molecules.
The bands at 2932 and 2861 cm−1 correspond to the antisymmetric stretching vibration of –CH3 and the symmetric stretching vibration of –CH2– groups of the glycerol molecule, as well of the other organic impurities (fatty acids or fatty esters are normally present in bio-glycerol mixtures). The presence of alkyl groups is further evidenced by the bands in the 1409–1463 cm−1 region, which correspond to the asymmetric bending of –CH3.16
In the spectrum of bio-glycerol alone (Fig. 1b, C), the bands at 1000–1200 cm−1 correspond to the C–O symmetric stretching vibration, whereas the presence of some small fatty acid residues is suggested by a small shoulder at 1705 cm−1, which corresponds to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations of carboxyl groups.
O stretching vibrations of carboxyl groups.
XRD measurements confirm that the clinker–bio-glycerol admixtures have no effect on the chemical structure of the clinkers, even after heating the mixtures at 800 °C for 1 h. Fig. 2a, comprising the XRD patterns of the three clinkers, shows that their composition is very similar; the only differences being in the ratio of the different crystalline phases. The positions of the main diffraction lines of the three main components of the clinker mixtures (dicalcium silicate, tricalcium silicate and calcium aluminate) are indicated in Fig. 2a.
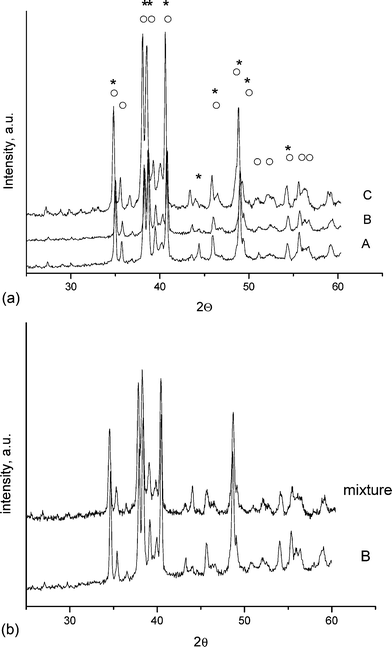 | ||
| Fig. 2 (a) XRD patterns of the clinkers: A—clinker A, B—clinker B and C—clinker C; * = Ca2SiO4, ○ = Ca3SiO5 (according to the Powder Diffraction file from the International Centre for Diffraction Data, 2000). (b) Samples a—clinker A and b—clinker A mixed with 10 wt% of bio-glycerol and heated to 800 °C for 1 h. | ||
Clearly, the thermal treatment at 800 °C of the physical mixture of clinker and bio-glycerol has no effect on the XRD pattern of clinker B (Fig. 2b), and the same result was obtained for the other two clinker–bio-glycerol admixtures.
In conclusion, as both the FT-IR and XRD analyses of the clinker–bio-glycerol admixtures indicate that no chemical transformation occurs in the structure of the clinker in the presence of the polyol, we decided to investigate if indeed the positive effect of bio-glycerol addition during clinker grinding originates from its catalytic activity.
Clinkers’ catalytic activity in glycerol etherification
Temperatures in ball mills or grinders are generally up to 120 °C, and normally range from 80 to 120 °C. Under these conditions, water tends to evaporate; but in the real system, both water and glycerol will be constantly adsorbed and desorbed from the surface of the solid material. In the production process, crude glycerol addition is continuous, namely the additive is constantly added to the fresh feed of the mill and exits the mill adsorbed onto the surface of the finished cement. Depending on the specific mill system, the time spent inside the mill by the clinker along with the added glycerol varies from 15 to 60 min. In this sense, the conditions employed by us during the catalytic tests can be considered extreme, since temperatures of up to 220 °C were used, as well as an excess of glycerol.Table 2 presents the main results of the catalytic etherification of glycerol conducted in the presence of the three clinker samples, as well as of sodium carbonate (a standard catalyst for glycerol etherification). A reaction temperature of 220 °C was previously found to be optimum for a high glycerol etherification yield.13
| Entry | Catalyst | Substrate | Time (h) | T (°C) | Conversion (%) | Selectivity for di-glycerol (%) | Others (%) |
|---|---|---|---|---|---|---|---|
| a Reactions performed in a round-bottomed flask under an Ar flow: 1 g clinker, 50 g glycerol. Other reactions were performed in an autoclave: 6 g catalyst, 18 g glycerol. b Tri-glycerol is the main byproduct (35%). c Conversion < 1%, tri-glycerol was formed. | |||||||
| 1. | Na2CO3a | Glycerol | 20 | 220 | 75 | 60 | 35b |
| 2. | Clinker Ba | Glycerol | 23 | 220 | 15 | 69 | 31 |
| 3. | Clinker Aa | Glycerol | 17 | 220 | 20 | 68 | 32 |
| 4. | Clinker Ca | Glycerol | 20 | 220 | 13 | 68 | 32 |
| 5. | Na2CO3 | Glycerol | 3 | 120 | none | none | — |
| 6. | Clinker B | Glycerol | 3 | 120 | none | none | — |
| 7. | Clinker B | Bio-glycerol | 2 | 120 | 2 | 100 | 0 |
| 8. | Clinker B | Di-glycerol | 2 | 120 | n.d.c | — | — |
Indeed, in agreement with previous results,13aglycerol conversion after 20 h of reaction with Na2CO3 was 75%, mainly yielding di-glycerol (60% selectivity) and tri-glycerol (35% selectivity) (Table 2, entry 1). At 220 °C, all three clinkers showed catalytic activity, but the conversions of glycerol were in each case lower compared to Na2CO3 alone (Table 2, entries 2–4). Hence, clinker B after 23 h caused a glycerol conversion in 15% yield, whereas clinker A gave 20% yield after 17 h. Clinker C gave only 13% conversion after 20 h. For all three clinkers, di-glycerol was obtained with selectivities close to 70% in a mixture of both linear and cyclic isomers. No tri-glycerol formation was observed, and the by-products were mainly composed of a mixture of dehydrated products and their corresponding oligomers.
Acrolein formation is known to take place in glycerol etherification, particularly at high temperature. Here, the formation of acrolein was evidenced by the formation of cyclic products with glycerol and the formation of coke on the clinker catalysts (the formation of a black deposit was observed at the end of the reaction). Another, important dehydration product found was hydroxyacetone. The formation of this product, as well as of the oligomeric products that lead to coke formation, is particularly interesting since it indicates that some Lewis acidity is present in the clinker samples, along with the basicity of the Ca components. Comparing the catalytic activity of the clinkers with that reported by Ruppert et al.13 for CaO catalysts under similar conditions, the one obtained here is considerably lower, as shown by the 20% conversion value compared to 80% by Ruppert et al.
Yet, as mentioned above, 220 °C is a very high temperature compared to the conditions present in a ball mill. At 120 °C (a more realistic temperature), no reaction with pure glycerol was observed either with Na2CO3 or with the clinkers, and this was independent of the reaction setup used (Parr reactor or round-bottomed flask) (Table 2, entries 5–6). At this low temperature, traces of di-glycerol were observed only when bio-glycerol was used as a substrate (Table 2 entry 7). Yet, when 50 wt% water solutions of glycerol or bio-glycerol were used (as in a ball-mill process), no di-glycerol was found. Etherification of di-glycerol was attempted, and some higher glycerol ethers were observed in trace amounts (Table 2, entry 8).
It is important to mention that in these reactions, a glycerol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) clinker ratio of between 5 and 50 was used. Although, we observed di-glycerol formation with clinkers at high temperatures above 200 °C and reaction times of 24 h, at 120 °C (which is theoretically the maximum temperature obtained in a ball mill), only traces of di-glycerol were observed; this after relatively long reaction times. However, we cannot totally exclude the formation of di-glycerol in the grinding process since very small amounts of glycerol are used in the real production process (which makes the ratio between the catalytic species and the substrate very high) and localised overheating may occur in the ball mill.
clinker ratio of between 5 and 50 was used. Although, we observed di-glycerol formation with clinkers at high temperatures above 200 °C and reaction times of 24 h, at 120 °C (which is theoretically the maximum temperature obtained in a ball mill), only traces of di-glycerol were observed; this after relatively long reaction times. However, we cannot totally exclude the formation of di-glycerol in the grinding process since very small amounts of glycerol are used in the real production process (which makes the ratio between the catalytic species and the substrate very high) and localised overheating may occur in the ball mill.
The spent clinker catalysts recovered from reactions at 220 °C were covered by an insoluble black deposit, indicating coke formation. The FT-IR spectrum of the spent clinker catalyst (Fig. 3a) presents a band at 1483 cm−1 corresponding to the –CH3 asymmetric bending vibration, and some small bands at 2932 and 2861 cm −1 corresponding to the antisymmetric stretching vibration of –CH3 and the symmetric stretching vibration of –CH2–. This coke is decomposed at temperatures up to 500 °C, as indicated by the TG analysis (not shown), which may suggest that it is a rather “soft” type of coke.
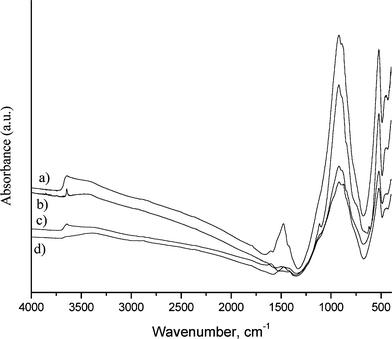 | ||
| Fig. 3 FT-IR spectra before and after the glycerol etherification reaction: (a) etherification of glycerol with clinker B at 220 °C, (b) clinker B, (c) etherification of bio-glycerol with clinker B at 120 °C and (d) etherification of a 85 wt% water solution of glycerol with clinker B at 120 °C. After the reaction, the solid was washed with water and dried at 120 °C overnight. | ||
On the other hand, the FT-IR spectra of the spent clinker samples obtained after reactions at 120 °C are unvaried, no whether or not bio-glycerol or pure glycerol was used.
The XRD analyses of the spent clinkers (not shown) indicate that the composition of the materials is preserved during the reaction.
Finally, the stability of the clinkers during (bio)-glycerol etherification was investigated. All of the clinkers partially dissolved in the glycerol solutions. In Fig. 4, the FT-IR spectra of the recovered materials from the reaction of bio-glycerol and glycerol are presented and compared with the FT-IR spectra of pure clinker B. Unmodified clinker solids were obtained by evaporating the reaction solution, from which the catalyst was previously filtered and centrifuged, at temperatures up to 800 °C (Fig. 4B).
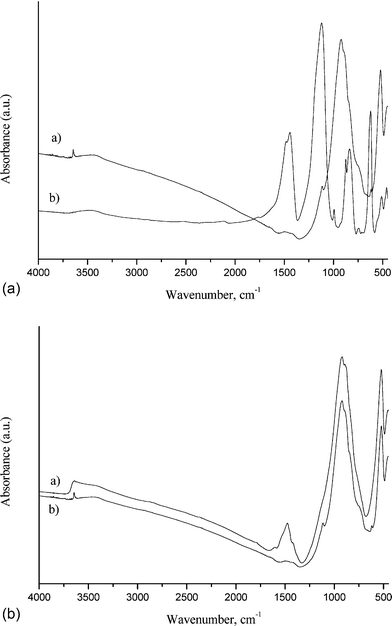 | ||
| Fig. 4 The FT-IR spectrum of the solid recovered from the solution after reactions of glycerol and bio-glycerol with clinker B; the liquid was centrifuged, filtered (0.45 micron) and heated at 800 °C for 6 h: (A-a) clinker B before the reaction, (A-b) recovered material from the reaction of clinker B with bio-glycerol, (B-a) clinker B before the reaction and (B-b) recovered material from the reaction of clinker B with glycerol. | ||
The solid material recovered from the reaction with bio-glycerol presents bands similar to the ones of the pure clinker, plus additional bands most probably due to the inorganic compounds found in the bio-glycerol mixture (Fig. 4A). This experiment is a proof that glycerol helps break down the clinker particle agglomerates during the ball milling process by modification of their surface tension. Similarly, in the etherification of glycerol in the presence of CaO,13 it is clearly observed that glycerol can extract the calcium from the catalyst, helping to dissolve the calcium ions.
Conclusions
In order to understand the excellent aiding properties of biodiesel-derived glycerol (bio-glycerol) as an additive in the grinding process for cement production, we studied the interaction of glycerol with cement clinkers; both chemical and physical interactions were taken into account. The results of these investigations point to surface tension modification of the clinker particles as the main effect of bio-glycerol during the grinding process. No significant change in the composition of the clinker in the presence of the additive was observed, even if high loadings of additive were used, as suggested by FT-IR and XRD analyses. On the other hand, glycerol also has a physical effect on the solids as it helps fragment the colloidal solid particles and therefore improves the mixing of compounds in the grinding process. The formation of polyglycerols or dehydrated/oligomeric species, which in principle can take place due to local overheating in ball mills, is not significant and therefore is not responsible for the significant enhancement of the milling process due to bio-glycerol.Acknowledgements
Thanks go to Grace Construction Products for providing the clinker samples. We also thank Dr Paolo Forni for valuable collaboration, Fouad Soulimani (UU) for help with the spectroscopy measurements and Prof. Bert M. Weckhuysen (UU) for fruitful discussions.References
- J. M. Crow, The concrete conundrum, Chem. World, 2008, 5, 3 Search PubMed : Most efficient cement kilns (cement is concrete's key ingredient) produce about 800 kg of CO2 per tonne, with around 530 kg of carbon dioxide being released by limestone (CaCO3) decomposition into CaO and CO2, and the rest coming from the fossil fuel consumption required to reach reaction temperatures of up to 1500 °C.
- J. S. Damtoft, J. Lukasik, D. Herfort, D. Sorrentino and E. M. Gartner, Cem. Concr. Res., 2008, 38, 115 CrossRef CAS.
- K. L. Scrivener and R. J. Kirkpatrick, Cem. Concr. Res., 2008, 38, 128 CrossRef CAS.
- G. R. Tucker, C. W. Tucker, H. L. Kennedy, M. S. Renner, Concrete and hydraulic cement, US Pat. 2031621 ( 1936) Search PubMed.
- H. H. Moorer, C. M. Anderegg, Cement grinding aid and set retarder, US Pat. 4204877 ( 1980) Search PubMed.
- J. G. Mark, Concrete and hydraulic cement, US Pat. 2141570 ( 1938) Search PubMed.
- I. C. Bechtold, Portland cement and its manufacture, US Pat. 2225146 ( 1940) Search PubMed.
- H. H. Moorer, C. M. Anderegg, Cement grinding aid and pack set inhibitor, US Pat. 3615785 ( 1971) Search PubMed.
- S. Sohoni, R. Sridhar and G. Mandal, Powder Technol., 1991, 67, 277 CrossRef CAS.
- L. A. Jardine, J. H. Cheung, W. M. Freitas, High early strength cement and additives and methods for making the same, US Pat. 6641661 ( 2003) Search PubMed.
- M. Rossi, C. Della Pina, M. Pagliaro, R. Ciriminna and P. Forni, ChemSusChem, 2008, 1, 809 CrossRef CAS.
- M. Pagliaro and M. Rossi, The Future of Glycerol, RSC Publishing, Cambridge, UK, 2nd edn, 2010 Search PubMed; see also: J. J. Bozell and G. R. Petersen, Green Chem., 2010, 12, 539 Search PubMed.
- (a) A. M. Ruppert, J. D. Meeldijk, B. W. M. Kuipers, B. H. Erné and B. M. Weckhuysen, Chem.–Eur. J., 2008, 14, 2016 CrossRef CAS; (b) M. Calatayud, A. Ruppert and B. M. Weckhuysen, Chem.–Eur. J., 2009, 15, 10864 CrossRef CAS.
- R. Palkovits, I. Nieddu, R. J. M. Klein Gebbink and B. M. Weckhuysen, ChemSusChem, 2008, 1, 103 CrossRef CAS.
- http://www.understanding-cement.com/clinker.html .
- G. Socrates, Infrared and Raman Characteristic Group Frequencies Tables and Charts, John Wiley & Sons, Chichester, UK, 3rd edn, 2001 Search PubMed.
Footnotes |
| † This paper is dedicated to Giuseppe and Mario Micciché, friends of M. P. during a splendid youth. |
| ‡ Electronic supplementary information (ESI) available: Further experimental data. See DOI: 10.1039/c0gc00107d |
| This journal is © The Royal Society of Chemistry 2011 |
