Lowbush blueberries inhibit scavenger receptors CD36 and SR-A expression and attenuate foam cell formation in ApoE-deficient mice†
Chenghui
Xie
ab,
Jie
Kang
ab,
Jin-Ran
Chen
ac,
Oxana P.
Lazarenko
ac,
Matthew E.
Ferguson
a,
Thomas M.
Badger
ab,
Shanmugam
Nagarajan
ad and
Xianli
Wu
*ab
aUSDA Arkansas Children's Nutrition Center, Little Rock, AR 72202, USA. E-mail: wuxianli@uams.edu; Fax: +1-501-364-3161; Tel: +1-501-364-2813
bDepartment of Physiology and Biophysics, University of Arkansas for Medical Sciences, Little Rock, AR 72202, USA
cDepartment of Pediatrics, University of Arkansas for Medical Sciences, Little Rock, AR 72202, USA
dDepartment of Microbiology and Immunology, University of Arkansas for Medical Sciences, Little Rock, AR 72202, USA
First published on 28th September 2011
Abstract
Blueberries have recently been reported to reduce atherosclerotic lesion progression in apoE deficient (apoE−/−) mice. However, the underlying mechanisms are not fully understood. The objective of this study was to determine whether lowbush blueberries altered scavenger receptor expression and foam cell formation in apoE−/− mice. ApoE−/− mice were fed AIN-93 diet (CD) or CD formulated to contain 1% freeze-dried lowbush blueberries (BB) for 20 weeks. Gene expression and protein levels of scavenger receptor CD36 and SR-A in aorta and thioglycollate-elicited peritoneal macrophages (PM) were lower in mice fed BB (P < 0.05). In the second experiment, apoE−/− mice were fed CD or BB for 5 weeks. PM were collected and cultured. Gene expression and protein levels of CD36 and SR-A were found to be lower in PM of BB fed mice (P < 0.05). In PM from BB fed mice, fewer oxLDL-induced foam cells were formed compared to those from mice fed CD. Gene expression and protein levels of PPARγ were lower in the PM of BB fed mice (P < 0.05). Detectable isomers of hydroxyoctadecadienoic acids (HODEs) and hydroxyeicosatetraenoic acid (HETEs) were also lower in the PM of BB fed mice (P < 0.05 or P < 0.01). In conclusion, BB inhibited expression of the two major scavenger receptors CD36 and SR-A in PM of apoE−/− mice, at least in part through down-regulating PPARγ and reducing its endogenous ligands HODEs and HETEs. We proposed that BB mediated reduction of scavenger receptor expression and attenuation of oxLDL-induced foam cell formation in PM of apoE−/− mice are important mechanisms of the athero-protective effects of BB.
1. Introduction
It is widely accepted that a diet rich in fruits and vegetables has protective effects against cardiovascular diseases.1–3 However, in vivo experimental evidence that consumption of specific fruits and vegetables reduces the risk of cardiovascular disease and the plausible underlying mechanisms remained to be determined. In a recent publication by our group, apoE deficient mice (apoE−/−) fed 1% freeze-dried lowbush blueberries in the diet developed significantly fewer atherosclerotic lesions in the aorta.4 While our data suggested that certain antioxidant enzymes were up-regulated by lowbush blueberries, these findings did not rule out the possibilities that other mechanisms may also be involved in the athero-protective effects of lowbush blueberries.Atherosclerotic vascular disease arises as a consequence of the deposition and retention of serum lipoproteins in the artery wall. Because cholesterol-laden macrophage foam cells are the primary component of the fatty streak – the earliest atherosclerotic lesion, lipid uptake by these pathways has long been considered a requisite and initiating event in the pathogenesis of atherosclerosis.5 Data from murine models of atherosclerosis have demonstrated a significant role of the two major scavenger receptors: the type A scavenger receptor (SR-A) and type B scavenger receptor CD36, in atherosclerotic foam cell development and vascular lesion initiation.6–8CD36 and SR-A are the principal receptors responsible for the binding and uptake of modified LDL in macrophage. Together the aforesaid account for 75–90% of the uptake and degradation of acetylated and oxidized LDL.9 In addition to modified lipoprotein uptake, these proteins are also known to regulate apoptotic cell clearance, initiate signal transduction, and serve as pattern recognition receptors for pathogens, activities that may contribute both to pro-inflammatory and anti-inflammatory forces regulating atherogenesis.5
Recently, a new role for CD36 as regulator of cellular mobility was revealed.10 Park et al. found that CD36 signaling by oxidized LDL (but not LDL) promotes cellular actin polymerization and “cements” adhesions, thus trapping foam-cell macrophages within lesions.11CD36 signaling triggers this cellular immobilization through the generation of reactive oxygen species (ROS), which indirectly activate a focal adhesion kinase that in turn leads to an increase in actin polymerization. Macrophage migration in the presence of oxLDL was restored by dietary antioxidants, such as resveratrol and NADPH oxidase inhibitors.
Our initial screen of possible mechanisms of the athero-protective effects of lowbush blueberries showed that the expression of CD36 was down-regulated in the aorta of apoE−/− mice fed lowbush blueberries. In the present study, we hypothesized that lowbush blueberries reduce expression of the two major scavenger receptors CD36 and SR-A in macrophages of apoE−/− mice, and further attenuate foam cell formation. The possible mechanisms for scavenger receptor down-regulation, especially viaperoxisome proliferator-activated receptor (PPARγ) pathway, were also investigated.
2. Results
Results from the first animal experiment
2.1 CD36 expression was reduced by PCR-array in aorta of apoE−/− mice fed the BB
After apoE−/− mice were fed AIN-93 diet (CD) or CD formulated to contain 1% freeze-dried lowbush blueberries (BB) for 20 weeks, pooled aorta samples (4 samples/pool, 3 pools) from mice of both diet groups were used for real-time quantitative RT-PCR array analysis of antioxidant/inflammation related gene pathways, including CD36. A 40% reduction in CD36 expression was observed in BB-fed mice compared with CD-fed groups (SR-A was not included in the array).2.2 CD36 and SR-A mRNA expression and protein levels in aorta of apoE−/− mice fed BB
To confirm the RT-PCR array data, CD36 mRNA expression and protein levels were determined using RT-PCR and Western blot analysis with individual aorta samples. Compared with CD-fed mice, both CD36 mRNA expression and protein were significantly lower in BB-fed mice (P < 0.05) (Fig. 1A, 1C). Similarly, mRNA expression and protein levels of a class A scavenger receptor, SR-A, were also lower in aorta from mice fed BB (P < 0.05) (Fig. 1B, 1D).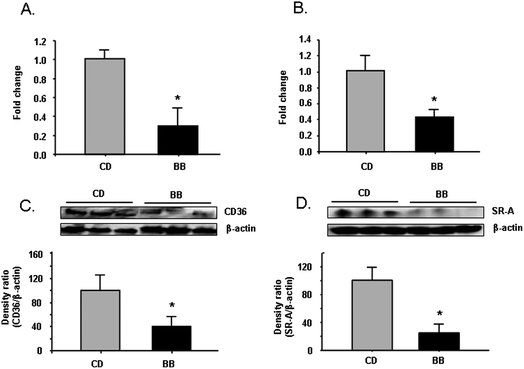 | ||
| Fig. 1 BB decreased mRNA and protein levels of scavenger receptor CD36 and SR-A in aorta samples of apoE−/− mice. After apoE−/− mice were fed CD or BB for 20 wks, BB suppressed mRNA expression of CD36 (A) and SR-A (B) in aorta samples from apoE−/− mice, as measured by RT-PCR. BB decreased the protein levels of CD36 (C) and SR-A (D) in aorta samples of apoE−/− mice. The proteins were separated by SDS-PAGE and then Western blot analysis with specific antibodies against CD36 and SR-A. β-Actin was blotted as an internal control. Data were expressed as mean ± SEM (n = 3). Comparisons were made between CD and BB groups, * P < 0.05, n = 3. | ||
2.3 CD36 and SR-A mRNA expression and protein levels in PM of apoE−/− mice fed BB
Next, CD36 and SR-A mRNA expression and protein levels were compared in thioglycollate-elicited peritoneal macrophages (PM) of apoE−/− mice fed BB or CD for 20 weeks. Compared with CD-fed mice, both mRNA expression and protein levels of CD36 and SR-A were significantly lower in PM of BB-fed mice (P < 0.05) (Fig. 2A–2D).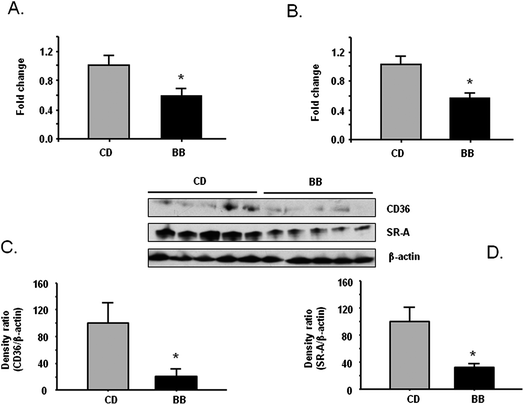 | ||
| Fig. 2 BB decreased mRNA and protein levels of scavenger receptor CD36 and SR-A in peritoneal macrophage from apoE−/− mice. After apoE−/− mice were fed CD or BB for 20 wks, BB suppressed mRNA expression of CD36 (A) and SR-A (B) in peritoneal macrophage from apoE−/− mice measured by RT-PCR. BB decreased the protein levels of CD36 (C) and SR-A (D) in peritoneal macrophage from apoE−/− mice. The proteins were separated by SDS-PAGE and then Western blot analysis with specific antibodies against CD36 and SR-A. β-Actin was blotted as an internal control. Data were expressed as mean ± SEM (n = 5). Comparisons were made between CD and BB groups, * P < 0.05, n = 5. | ||
Results from the second animal experiment
2.4 CD36 and SR-A mRNA expression and protein levels in PM of apoE−/− mice fed BB
After apoE−/− mice were fed CD or BB for 5 weeks, PM were collected and cultured. Similarly to what was observed in the 20-week animal experiment, mRNA and protein levels of both CD36 and SR-A were significantly lower in PM of BB-fed mice compared with CD-fed mice (P < 0.05) (Fig. 3A–3D).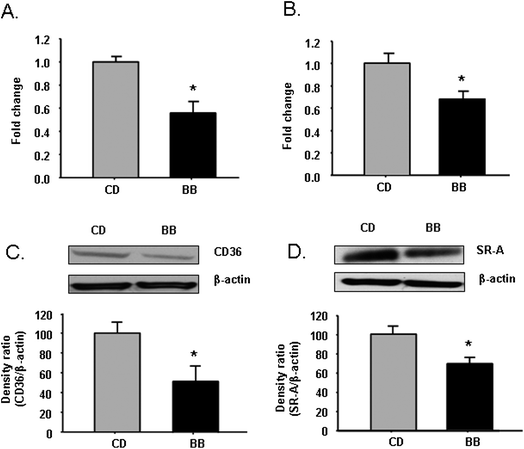 | ||
| Fig. 3 BB decreased mRNA and protein levels of scavenger receptor CD36 and SR-A in peritoneal macrophages of apoE−/− mice. After apoE−/− mice were fed CD or BB for 5 wks, BB suppressed mRNA expressions of CD36 (A) and SR-A (B) in peritoneal macrophages of apoE−/− mice measured by RT-PCR. BB decreased the protein levels of CD36 (C) and SR-A (D) in peritoneal macrophages of apoE−/− mice. The proteins were separated by SDS-PAGE and then Western blot analysis with specific antibodies against CD36 and SR-A. β-Actin was blotted as an internal control. Gel images of CD36 and SR-A represented three individual samples. Data were expressed as mean ± SEM (n = 3). Comparisons were made between CD and BB groups, * P < 0.05, n = 3. | ||
2.5 BB feeding attenuates foam cell formation
To induce foam cell formation, oxLDL was added to the culture media of PM from apoE−/− mice fed BB or CD. After 24-hour incubation with oxLDL, PM from mice fed BB developed significantly fewer foam cells, as indicated by the reduced intensity of red spots after Oil-red O staining, compared with those from CD fed mice (Fig. 4)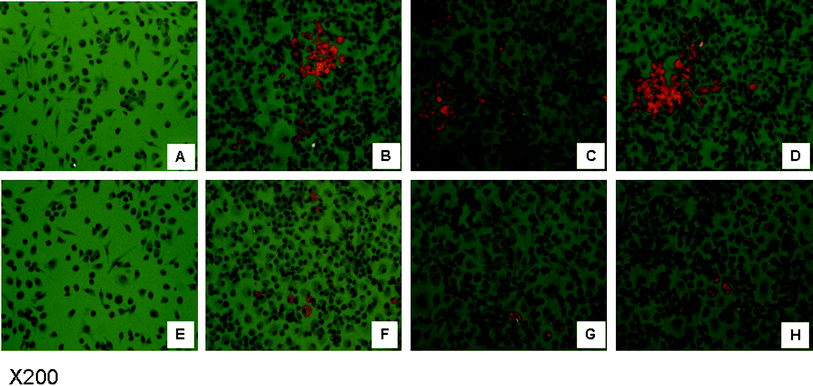 | ||
| Fig. 4 BB attenuated oxLDL induced foam cell formation. OxLDL was added to the culture media of peritoneal macrophages from apoE−/− mice fed BB or CD to induce foam cell formation. After 24 h incubation, foam cells were illustrated by the intensity of red spots after Oil-red O staining. Image A and E represent peritoneal macrophages from apoE−/− mice fed CD or BB before oxLDL incubation. Images B to D are peritoneal macrophages from apoE−/− mice fed CD after oxLDL incubation. Images F to H are peritoneal macrophages from apoE−/− mice fed BB after oxLDL incubation. | ||
2.6 PPARγ mRNA expression and protein levels in PM of apoE−/− mice fed BB
PPARγ is a transcription factor that regulates CD36 expression. PPARγ mRNA expression and protein levels were measured in PM of apoE−/− mice fed CD or BB, without or with oxLDL induction, using RT-PCR and Western blot analysis. Compared with CD fed group, mRNA expression of PPARγ was reduced in PM from mice fed BB without oxLDL incubation (P < 0.05) (Fig. 5A). Protein levels of PPARγ appeared lower in PM from mice fed BB without oxLDL incubation, but this difference was not statistically significant (data not shown). However, with oxLDL incubation, both mRNA expression and protein levels of PPARγ were significantly lower in BB-fed mice (P < 0.05) (Fig. 5B–5C).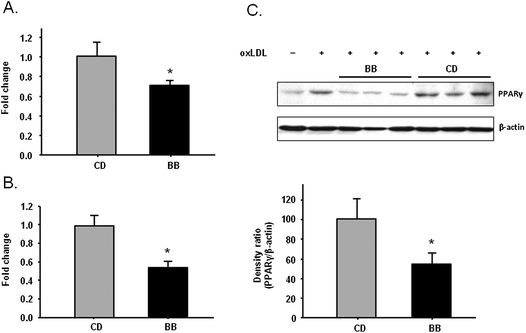 | ||
| Fig. 5 BB decreased PPARγ mRNA expression measured by RT-PCR in peritoneal macrophages from apoE−/− mice fed CD or BB for 5 wks without (A) or with (B) oxLDL incubation. After oxLDL incubation, BB decreased the protein levels of PPARγ in peritoneal macrophages of apoE−/− mice. The proteins were separated by SDS-PAGE and then Western blot analysis with specific antibodies against PPARγ. β-Actin was blotted as an internal control. Data were expressed as mean ± SEM (n = 3). Comparisons were made between CD and BB groups, * P < 0.05, n = 3. | ||
2.7 Reduction of HODEs and HETEs in PM of apoE−/− mice fed BB
Different isomers of HETEs and HODEs were analyzed in supernatants of cultured PM from CD or BB fed mice by means of LC-MS/MS. Of all the isomers, 11-HETE, 9-HODE and 13-HODE, were detected and quantified. All three compounds were significantly lower in PM of BB fed mice (P < 0.05 or P < 0.01) (Table 1).| CD | BB | |
|---|---|---|
| ng mL−1 | ||
| a Results are expressed as mean ± SEM, n = 5 (* P < 0.05, ** P < 0.01). | ||
| 11-HETE | 0.27 ± 0.04 | 0.15 ± 0.02* |
| 9-HODE | 0.42 ± 0.03 | 0.19 ± 0.04** |
| 13-HODE | 1.16 ± 0.16 | 0.58 ± 0.03** |
3. Discussion
Blueberries contain a high level of polyphenols and exhibit strong antioxidant and potential anti-inflammatory effects.12,13 We have recently shown that apoE−/− mice fed 1% freeze-dried lowbush blueberries in the diet developed significantly fewer aortic lesions.4 We provided the evidence that certain antioxidant enzymes were up-regulated by lowbush blueberries. Nevertheless, these findings did not rule out the possibilities that other mechanisms may also be involved in the athero-protective effects of lowbush blueberries. We initially screened for potential mechanisms using a real-time quantitative RT-PCR array with multiple antioxidant/inflammation related genes, and found that the expression of class B scavenger receptor CD36 was reduced by 40% in aorta pooled from apoE−/− mice fed BB for 20 weeks. These data suggested that BB may inhibit scavenger receptors expression.To confirm the findings obtained from RT-PCR array findings, we next performed RT-PCR in individual aorta samples from mice fed BB or CD for 20 weeks. Compared with CD-fed mice, both CD36 mRNA expression and protein were significantly lower in the aortas from mice fed BB. In addition, SR-A mRNA expression and protein level were also lower in individual aorta samples with BB feeding (Fig. 1). Since macrophage is the major cell type in the vascular system that expresses CD36 and SR-A, mRNA expression and protein levels of CD36 and SR-A were further measured in PM. We observed that both mRNA expression and protein levels of CD36 and SR-A were decreased in PM from mice fed BB (Fig. 2). Taken together, our data indicated that BB inhibited scavenger receptor CD36 and SR-A expression in macrophages of apoE−/− mice. CD36 and SR-A are the principal receptors responsible for the binding and uptake of modified LDL in macrophages. Both of them have been implicated to play key roles in macrophage foam cell formation during atherogenesis and in the regulation of inflammatory signaling pathways, including those leading to lesional macrophage apoptosis and plaque necrosis.8
Due to experimental design and limited sample size/volume, we were unable to perform further investigations in the first animal experiment. A second experiment was thus designed where apoE−/− mice were fed CD or BB for 5 weeks. PM were collected and cultured. Similarly to the 20-week study, we observed that mRNA expression and protein levels of CD36 and SR-A were both significantly lower in peritoneal macrophages from mice fed BB for 5 weeks (Fig. 3). These data also indicated that blueberries can indeed inhibit the early stages of the development of atherosclerosis.
Foam cell formation from macrophages with subsequent fatty streak formation is a hallmark of early atherogenesis. OxLDL is the major driving agent to induce foam cell formation via scavenger receptors.14 The scavenger receptors CD36 and SR-A have generally been viewed as essential for foam cell formation according to their roles in mediating the uptake and internalization of modified lipoproteins, like oxidized LDL. Studies have shown that SR-A and CD36 account for greater than 90% of the lipid accumulation in macrophages exposed to oxidized LDL.8 And the other scavenger receptors do not compensate for the absence of SR-A and CD36.9 Since we observed that both SR-A and CD36 were down-regulated in PM with BB feeding, we expected to see less foam cell formation in PM of mice fed BB when the foam cells were induced with oxLDL. The PM derived from BB-fed mice developed significantly fewer foam cells compared to CD-fed mice (Fig. 4), suggesting that the BB diet attenuated oxLDL-induced foam cell formation, possibly through down-regulating CD36 and SR-A expression.
With regard to the regulation of expression of these two scavenger receptors, SR-A offers a more complex scenario than that of CD36 and has not been fully understood.15 The mechanism of induction of CD36 by oxLDL was shown to be due to its ability to activate the transcription factor PPARγ.16PPARγ is essential for basal regulation of CD36; in the absence of PPARγ, CD36 expression is barely detectable and PPARγ agonists do not induce CD36 expression.17 In our study, we found that PPARγ mRNA was significantly down-regulated in PM from BB-fed mice, with or without oxLDL induction (Fig. 5A–5B). However, without oxLDL induction, the protein levels of PPARγ did not differ between CD or BB fed groups (data not shown), possibly due to low basal protein contents in PM. OxLDL significantly increased the protein levels of PPARγ while it was decreased in PM from mice fed BB comparing with PM from mice fed CD (Fig. 5C). Therefore, we suggest that the BB diet reduces CD36 expression through down-regulation of PPARγ.
Oxidized metabolites of linoleic acid – 9-hydroxyoctadecadienoic acid (9-HODE) and 13-HODE, as well as certain isomers of hydroxyeicosatetraenoic acid (HETE) – the oxidized metabolites from arachidonic acid, were potent transcriptional activators of PPARγ activity.18 In this study, different isomers of HETEs and HODEs were measured in the supernates of PM culture media. Only 11-HETE, 9-HODE and 13-HODE reached detectable levels and were quantified, and the levels of these HETEs and HODEs were significantly lower in PM of BB fed mice (Table 1). These data suggest that BB inhibit lipid peroxidation, which might be achieved by boosting antioxidant enzymes as we demonstrated in our previous paper4 or through other mechanisms. 9-HODE and 13-HODE have been known as the endogenous ligands of PPARγ.18 Surprisingly, only one minor HETE isomer 11-HETE was detected, and 12-HETE, the major isomer of HETEs in serum or tissues of apoE−/− mice,19 as well as other isomers, were not detected in supernates of culture media. Thus, the exact role of 11-HETE in related to foam cell formation remains to be determined.
4. Experimental
4.1 Experimental materials and animal diets
Freeze-dried whole lowbush blueberries (BB) (Vaccinium angustifolium) powder (Hi-Actives Wild Blueberry) was provided by VDF/FutureCeuticals (Momence, IL). AIN-93G (CD) or AIN-93G incorporated with 1% freeze-dried BB powder (BB) were made by Harlan Teklad (Madison, WI). To eliminate the caloric density as a confounding variable, all diets were formulated to be isocaloric and isonitrogenous. The diet formulation is presented in our previous paper.44.2 Animals
The animal protocol was approved by the Animal Care and Use Committee of the Arkansas Children's Hospital Research Institute. Mice were handled in compliance with the relevant laws and the guidelines of University of Arkansas for Medical Science Institutional Animal Care and Use Committee. ApoE−/− mice (female, 4 weeks old) in the C57BL/6 genetic background were obtained from Jackson Laboratory (Bar Harbor, ME). Animals were maintained in sterile micro-isolator cages and fed autoclaved-pelleted diet ad libitum and water.In the first animal experiment, apoE−/− mice were fed either CD or BB for 20 weeks. Food intake and body weight were recorded weekly. Three days prior to sacrifice, 3% thioglycollate was injected ip into the mice for peritoneal macrophage collection. At the end of 24 weeks, animals were euthanized using CO2 and serum and PM were collected and stored at −70 °C until analysis.
In the second animal experiment, apoE−/− mice were fed either CD or BB for 5 weeks. Similar to the first animal experiment, at the end of 5 weeks, animals were euthanized using CO2. The PM from mice were collected and cultured.
4.3 Cell culture
PM were plated in RPMI-1640 supplemented with 10% (v/v) FBS, 2 mM L-glutamine, penicillin, streptomycin, and sodium pyruvate. Non-adherent cells were removed after 2 h and macrophages were used after 48 h.For measurements of PPARγ mRNA expression and protein levels induced by oxLDL, PM from CD or BB fed mice were incubated with 5 μg mL−1 of oxLDL (Academy Bio-Medical, Houston, TX) or vehicle for 24 h.
4.4 PCR array of aorta samples
Pooled aorta samples (4/group, 3 pools) were used for RT-PCR array. The sample preparation and the PCR-array using custom real-time PCR-array (SA Biosciences, Frederick, MD) were performed according to the method described previously.44.5 Quantitative RT-PCR analysis
The aorta was perfused with nuclease-free PBS and total RNA was isolated from a proximal portion of the descending aorta using the Trizol reagent (Invitrogen, Carlsbad, CA). Procedures for real time RT-PCR were described previously.20 Real-time PCR primers (from Integrated DNA Technologies, Coralville, IA) were as follows: β-actin sense (GGCTATGCTCTCCCTCACG), β-actin antisense (CGCTCGGTCAGGATCTTCAT), SR-A sense (ATGAACGAGAGGATGCTGACTG), SR-A antisense (GTGCTGTGAGGAAGGGATGC), PPARγ sense (GACATCCAAGACAACCTGCTG), PPARγ antisense (TCTGTGACGATCTGCCTGAG). Primers of CD36 were obtained from SABioscience (Catalog No. PPM03796B, Frederick, MD).4.6 Western-blot analysis
PM were lysed in RIPA buffer (Cell Signaling, Danvers, MA). Cell lyses were centrifuged at 5500 × g for 15 min to remove cell debris. Protein concentrations were determined by Bio-Rad protein assay reagent (Bio-Rad, Richmond, CA). The lysate (10 μg protein/lane) were separated by SDS-PAGE and transferred to a nitrocellulose membrane. Membranes were blocked with 5% BSA/PBS containing 0.05% Tween-20 and probed with antibodies against CD36 (Abcam, Cambridge, MA), SR-A (Abnova, Walnut, CA), PPARγ (Abcam, Cambridge, MA) and β-actin (Sigma, St Louis, MO). Bands were detected using ECL reagents (GE Healthcare, Piscataway, NJ) according to the manufacturer's instructions.4.7 oxLDL-induced foam cell formation
Foam cell assay was conducted based on a previously described method.20 Briefly, PM from CD or BB fed mice were incubated with 10 μg mL−1 of oxLDL (Academy Bio-Medical, Houston, TX) for 24 h. The cells were fixed and stained with Oil Red O and DAPI mount. Lipid droplet accumulation in the foam cells was evaluated by fluorescence microscopy (Olympus, Tokyo, Japan).4.8 Analysis of HETEs and HODEs by HPLC-ESI-MS
Analyses of isomers of hydroxyeicosatetranoic acids (HETEs) and hydroxyoctadecadienoic acids (HODEs) were carried out using an HPLC-MS/MS system based on the method published by our group.19Sample preparation of culture media supernates was based on the method described by our lab.4 Prior to extraction, 12(S)-HETE-d8 and 13(S)-HODE-d6 (Cayman Chemical, Ann Arbor, MI) were added to 100 μL supernates as internal standards.The analysis was carried out using the Agilent 1100 HPLC system (Agilent Technologies, Palo Alto, CA) coupled with a 4000 Q TRAP™ mass spectrometer (Applied Biosystems, Forest City, CA). Separation was performed on a Phenomenex Synergi Max-RP column (150 × 3 mm, 4 μm) using a flow rate of 0.4 mL min−1. The solvent consisted of (A) 0.2% (v/v) of formic acid in water and (B) methanol. The 19 min gradient was as follows: 22–20% A (1–2 min), 20–20% A (2–18 min), 20–22% A (18–19 min). Multi-reaction monitoring (MRM) mode scan was used for quantitation. The transitions monitored were mass to charge ratio (m/z): m/z 295 → 171 for 9-HODE; m/z 295 → 195 for 13-HODE; m/z 319 → 163 for 8-HETE; m/z 319 → 167 for 11-HETE; m/z 319 → 179 for 12-HETE; m/z 327 → 184 for 12(S)-HETE-d8; m/z 299 → 198 for 13(S)-HODE-d4.
4.9 Statistical analysis
Data were expressed as mean value ± SEM. Student's t test was used to analyze differences between two groups. A value of P < 0.05 was considered significant unless otherwise mentioned. Statistical analyses were performed by using SigmaStat (SigmaStat 3.5).5. Conclusion
Lowbush blueberries, when incorporated into the diet at 1%, were capable of inhibiting expression of scavenger receptors CD36 and SR-A in peritoneal macrophages of apoE−/− mice. These effects were achieved, at least in part, through down-regulating PPARγ and reducing its endogenous ligands. Lowbush blueberries likely attenuated foam cell formation by down-regulating the scavenger receptors, which we proposed as an important mechanism of the athero-protective effects of lowbush blueberries. However, accumulation of lipids in the macrophages is a dynamic process involving not only lipids uptake, but also lipid trafficking in the cells and lipid efflux.21,22 Whether lowbush blueberries affect these other processes of lipid accumulation and transferring in macrophages is yet to be determined. Moreover, the bioactive compounds in lowbush blueberries that contributed to these effects have not been characterized. Further research is needed to learn whether the more common commercially available highbush blueberries (Vaccinium corymbosum) and other species of this genus show the same bioactivity as reported for lowbush blueberries.Abbreviations
| apoE−/− | apolipoprotein E deficient |
| BB | blueberries; CD, AIN-93G diet |
| CD36 | cluster of differentiation 36 |
| HETEs | hydroxyeicosatetraenoic acid |
| HODEs | hydroxyoctadecadienoic acids |
| PM | thioglycollate-elicited peritoneal macrophages |
| PPARγ | peroxisome proliferator-activated receptor |
| SR-A | scavenger receptor class A |
Acknowledgements
This study was supported by the USDA Agriculture Research Service CRIS 6251-51000-007-04sReferences
- P. Galassetti and A. Pontello, Curr. Atheroscler. Rep., 2006, 8, 523–529 Search PubMed.
- L. J. Ignarro, M. L. Balestrieri and C. Napoli, Cardiovasc. Res., 2007, 73, 326–340 CrossRef CAS.
- P. Parikh, M. C. McDaniel, M. D. Ashen, J. I. Miller, M. Sorrentino, V. Chan, R. S. Blumenthal and L. S. Sperling, J. Am. Coll. Cardiol., 2005, 45, 1379–1387 CrossRef CAS.
- X. Wu, J. Kang, C. Xie, R. Burris, M. E. Ferguson, T. M. Badger and S. Nagarajan, J. Nutr., 2010, 140, 1628–1632 CrossRef CAS.
- K. J. Moore and M. W. Freeman, Arterioscler., Thromb., Vasc. Biol., 2006, 26, 1702–1711 CrossRef CAS.
- M. Febbraio, E. A. Podrez, J. D. Smith, D. P. Hajjar, S. L. Hazen, H. F. Hoff, K. Sharma and R. L. Silverstein, J. Clin. Invest., 2000, 105, 1049–1056 CrossRef CAS.
- P. I. Makinen, J. P. Lappalainen, S. E. Heinonen, P. Leppanen, M. T. Lahteenvuo, J. V. Aarnio, J. Heikkila, M. P. Turunen and S. Yla-Herttuala, Cardiovasc. Res., 2010, 88, 530–538 CrossRef.
- J. J. Manning-Tobin, K. J. Moore, T. A. Seimon, S. A. Bell, M. Sharuk, J. I. Alvarez-Leite, M. P. de Winther, I. Tabas and M. W. Freeman, Arterioscler., Thromb., Vasc. Biol., 2009, 29, 19–26 CrossRef CAS.
- V. V. Kunjathoor, M. Febbraio, E. A. Podrez, K. J. Moore, L. Andersson, S. Koehn, J. S. Rhee, R. Silverstein, H. F. Hoff and M. W. Freeman, J. Biol. Chem., 2002, 277, 49982–49988 CrossRef CAS.
- L. K. Curtiss, N. Engl. J. Med., 2009, 360, 1144–1146 CrossRef CAS.
- Y. M. Park, M. Febbraio and R. L. Silverstein, J. Clin. Invest., 2009, 119, 136–145 CAS.
- F. C. Lau, D. F. Bielinski and J. A. Joseph, J. Neurosci. Res., 2007, 85, 1010–1017 CrossRef CAS.
- X. Wu, G. R. Beecher, J. M. Holden, D. B. Haytowitz, S. E. Gebhardt and R. L. Prior, J. Agric. Food Chem., 2004, 52, 4026–4037 CrossRef CAS.
- P. Shashkin, B. Dragulev and K. Ley, Curr. Pharm. Des., 2005, 11, 3061–3072 CrossRef CAS.
- M. F. Linton and S. Fazio, Curr. Opin. Lipidol., 2001, 12, 489–495 CrossRef CAS.
- P. Tontonoz and L. Nagy, Curr. Opin. Lipidol., 1999, 10, 485–490 CrossRef CAS.
- A. C. Nicholson, Trends Cardiovasc. Med., 2004, 14, 8–12 CrossRef CAS.
- A. Castrillo and P. Tontonoz, Annu. Rev. Cell Dev. Biol., 2004, 20, 455–480 CrossRef CAS.
- C. Xie, J. Kang, R. Burris, M. E. Ferguson, A. G. Schauss, S. Nagarajan and X. Wu, Atherosclerosis, 2011, 216, 327–333 CrossRef CAS.
- C. Xie, H. Ng and S. Nagarajan, Immunol. Lett., 2011, 137, 15–27 CrossRef CAS.
- R. Ohashi, H. Mu, X. Wang, Q. Yao and C. Chen, QJM: Mon. J. Assoc. Physicians, 2005, 98, 845–856 Search PubMed.
- Y. Zhao, T. J. Van Berkel and M. Van Eck, Curr. Opin. Lipidol., 2010, 21, 441–453 CrossRef CAS.
Footnote |
| † Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. |
| This journal is © The Royal Society of Chemistry 2011 |
