Effect of a cocoa polyphenol extract in spontaneously hypertensive rats
M
Quiñones
a,
M
Miguel
*b,
B
Muguerza†
c and
A
Aleixandre
a
aDpto. Farmacología, Fac. Medicina, U. Complutense, Avda. Complutense s/n, 28040, Madrid, Spain
bInstituto de Investigación en Ciencias de Alimentación (CIAL, CSIC-UAM), C/Nicolás Cabrera, 9 28049, Madrid, Spain. E-mail: marta.miguel@csic.es; Fax: +34-91-0017905; Tel: +34-91-0017931
cNatraceutical Group, Quart de Poblet, Autovía A-3, Salida 343, Camino de Torrent s/n46930, Valencia, Spain
First published on 24th October 2011
Abstract
In this study, we evaluated the short-term effect of a cocoa polyphenol extract (CPE), in spontaneously hypertensive rats (SHR). Male 17–22-week-old SHR were administered by intragastric gavage water, 50 mg kg−1Captopril or CPE at different doses (13, 26, 80 and 160 mg kg−1). The systolic blood pressure (SBP) and diastolic blood pressure (DBP) were recorded by the tail cuff method before the administration and also 2, 4, 6, 8, 24, 48 and 72 h post-administration. Highly significant decreases in the SBP and in the DBP were observed when captopril or CPE was administered to SHR. The cocoa extract produced a dose dependent effect in the SBP of the SHR up to the dose of 80 mg kg−1. Nevertheless this dose of CPE did not decrease the arterial blood pressure in the normotensive Wistar Kyoto rats. The decrease in the SBP caused by 80 mg kg−1 of CPE in the SHR (−39.1 ± 3.7 mm Hg) was maximum 6 h post-administration, and the initial values of SBP were recovered 72 h post-administration of this extract. Paradoxically, 160 mg kg−1 of the cocoa extract caused a decreased antihypertensive effect than lower doses of CPE. In addition, the decrease in DBP was always more accentuated when the dose of CPE administered was lower. Our results suggest that CPE may be used as a functional food ingredient with beneficial effects for controlling arterial blood pressure.
Introduction
Cardiovascular disease is the most common cause of death in industrial societies.1–3 The intake of polyphenols has been inversely related with the reduced risk of this disease.4–6 In fact, many epidemiological studies associate an increased consumption of foods and beverages rich in flavonoids, with a reduced risk of cardiovascular death.7–9 Moreover, the use of products with a natural origin that may cause scarce side-effects, is an attractive possibility to be considered in treating several pathologies.10,11Cocoa is one of the foods that possess a major content of flavanols,12,13 and the cardiovascular benefits of cocoa and cocoa derivatives has been extensively studied.14–16 It was reported that cocoa consumption reduced cardiovascular mortality in the indigenous populations of Kuna islands,17 and more recently cocoa consumption has also been associated with lower cardiovascular mortality.18–20 Cocoa is rich in flavan-3-ols and procyanidins, (−)-epicatechin being the main component.21 Other examples of sources rich in these kind of flavonoids are wine and tea. However, cocoa has been shown to have the highest content of flavanols.22,23
The presence of these polyphenols is important for the healthy effects associated with cocoa consumption. Moreover, it has recently been published that these kind of flavonoids are able to induce a progressive, and sustained reduction in blood pressure when administered chronically to humans or to different rat models of hypertension, including spontaneously hypertensive rats (SHR) and L-NAME-treated rats.24–26 Nevertheless, it should be kept in mind that the cocoa flavanol ingestion will depend on the initial flavanol content of the cocoa beans and the manufacturing process.27
In previous works we have demonstrated the antihypertensive properties of a polyphenol rich cocoa powder, after short28 and long-term treatment,29 and we have also studied the possible mechanisms implicated in their antihypertensive effect.30 However, extracts are easier to handle in the food industry than other traditional functional ingredients, due to some of their physicochemical properties, such as their high solubility. Moreover, the organoleptic profile of the final application is usually not modified when extracts are used as food ingredients, and they could be added to many applications. Therefore, the aim of this study is to evaluate the possible short-term antihypertensive effect of a cocoa polyphenol extract (CPE) derived from a polyphenol rich cocoa powder, after single oral administration, in SHR.
Material and methods
Cocoa polyphenol extract
The extract CPE used for this study was obtained from a polyphenol-rich cocoa powder produced from unfermented, blanch-treated, non-roasted cocoa beans which preserves polyphenol degradation.31 The theobromine content of the extract, determined by High-Performance Liquid Chromatography (HPLC), was 90.6 mg g−1. Total polyphenols, measured by Folin-Ciocalteu's method and flavan-3-ols content (monomers and procyanidins B1 and B2), measured by HPLC-DAD according to Cienfuegos-Jovellanos et al.,28 of CPE were 509.8 and 232.1 mg g−1 respectively. Table 1 shows these data and also the antioxidant capacity of this extract, expressed as μmol of Trolox equivalents (TE) per gram of CPE, that was determined by the hydrophilic ORAC (H-ORAC) assay according to Ramos et al.32 All the analyses have been performed in triplicate and the results are reported on wet basis.| Compounds | mg g−1 wet mattera |
|---|---|
| a The values are expressed as the mean ± SD (n = 3). b Measured by Folin-Ciocalteu's method. c Hydrophilic ORAC (H-ORAC) assay, expressed as μmol of Trolox equivalents (TE) per gram of CPE. | |
| Theobromine | 90.6 ± 0.2 |
| Total polyphenol content b | 509.8 ± 4.0 |
| Epicatechin | 133.5 ± 1.1 |
| Catechin | 10.8 ± 1.0 |
| Procyanidin B1 | 9.1 ± 0.2 |
| Procyanidin B2 | 78.7 ± 1.2 |
| Antioxidant capacity c | 12134 ± 379 |
Experimental procedure in rats
In this study we have used twenty 17–20-week-old male SHR, weighing 314 ± 3 g, and ten 17–20-week-old male normotensive Wistar-Kyoto (WKY) rats, weighing 337 ± 6 g. All these animals were obtained from Charles River Laboratories Spain. The animals were maintained at a temperature of 23 °C with 12 h light/dark cycles, and consumed tap water and a standard diet (A04 Panlab, Barcelona, Spain) ad libitum during the experiments. CPE was dissolved in water and orally administered by gastric intubation, between 9 and 10 am. Distilled water was used as negative control, and Captopril (Sigma, USA) (50 mg kg−1), a known antihypertensive drug, was given as positive control. Different doses of CPE (13, 26, 80 and 160 mg kg−1) were administered. The volume orally administered to the rats was always 1 mL/rat either of water, or of the appropriate solution of CPE or Captopril. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were recorded in the rats by the tail cuff method,33 before administration and 2, 4, 6, 8, 24, 48 and 72 h post-administration. Before the measurement, the rats were kept at 38 °C for 10 min in order to detect the pulsations of the tail artery. To establish the value of SBP and DBP, five measurements were taken, and the average of all of them was obtained. To minimize stress-induced variations in blood pressure all measurements were taken by the same person in the same peaceful environment. Moreover, to guarantee the reliability of the measurements we established a training period of two weeks before the actual trial time, and during this period the rats were accustomed to the procedure.All the above-mentioned experiments were performed as authorized for scientific research (European Directive 86/609/CEE and Royal Decree 223/1988 of the Spanish Ministry of Agriculture, Fisheries and Food).
Statistical analysis
The results are expressed as mean values ± standard error of the mean (SEM) for a minimum of 8 rats, and were analyzed by a two-way analysis of variance (ANOVA), using the GraphPad Prism software. In addition, in order to compare the different treatments and to assess the effect of time within each treatment, some data were also analyzed by a one-way ANOVA, and differences between the groups were assessed by the Bonferroni test. Differences between the means was considered to be significant when P < 0.05.Results
Fig. 1 shows the changes in SBP and DBP obtained in SHR after the administration of the different assayed compounds. Before administration of the different products, the SHR showed SBP values of 216.9 ± 3.3 mm Hg (n = 20) and DBP values of 158.8 ± 3.8 mm Hg (n = 20). The values of SBP and DBP obtained after oral administration of bidistilled water were very similar to those obtained before its administration. Captopril caused a clear decrease in SBP and DBP in SHR. The maximum decrease in SBP and DBP caused by 50 mg Kg−1 of this drug were observed 4 h post-administration. These variables returned to baseline 48 h after the administration of Captopril. The oral administration of CPE also resulted in a significant decrease of the SBP and the DBP in the SHR. The decrease in SBP caused by the extract was dose-dependent only up to the dose of 80 mg kg−1. The change in the SBP caused by this dose of CPE was −39.1 ± 3.7 mm Hg. This decrease in SBP was the maximum decrease in this variable obtained with this extract, and it was observed 6 h post-administration. Nevertheless, 72 h post-administration of 80 mg kg−1CPE, the values of SBP were very similar to those observed before the administration of this extract. The maximum decrease of SBP caused by 13 mg kg−1CPE (−25.9 ± 1.9 mm Hg) and the maximum decrease of SBP caused by 26 mg kg−1 of CPE (−28.6 ± 4.5 mm Hg) were reached 4 h post-administration. Paradoxically, the dose of 160 mg kg−1 of CPE had the lowest antihypertensive effect (−9.3 ± 2.7 mm Hg). In addition, the decrease in DBP was always more accentuated when the dose of CPE administered was lower. Therefore, 13 mg kg−1CPE caused the maximum decrease in the DBP in the rats (−30.6 ± 7.7 mm Hg), and this decrease in this variable was observed 4–6 h post-administration of this dose of CPE.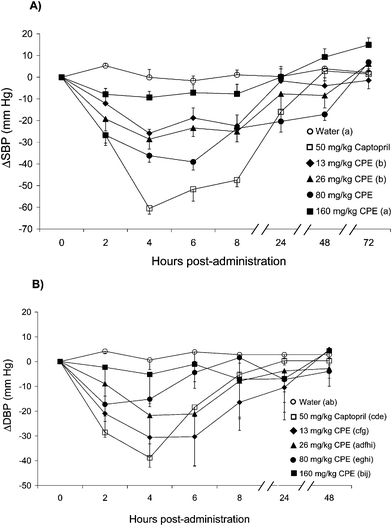 | ||
| Fig. 1 Decrease in systolic blood pressure (SBP) (A) and diastolic blood pressure (DBP) (B) caused in spontaneously hypertensive rats after the administration of different products. Water (○), Captopril (50 mg kg−1) (□) or different doses of CPE: 13 mg kg−1 (♦), 26 mg kg−1 (▲), 80 mg kg−1 (●) and 160 mg kg−1 (■). Data are expressed as mean ± SEM. The experimental groups always have a minimum of 8 animals. Same letters indicate no statistical differences (P > 0.05). P estimated by two-way ANOVA. | ||
The administration of CPE did not modify the arterial blood pressure in the normotensive WKY rats (Fig. 2). This variable was similar in the WKY rats treated with this product and in the WKY rats administered water.
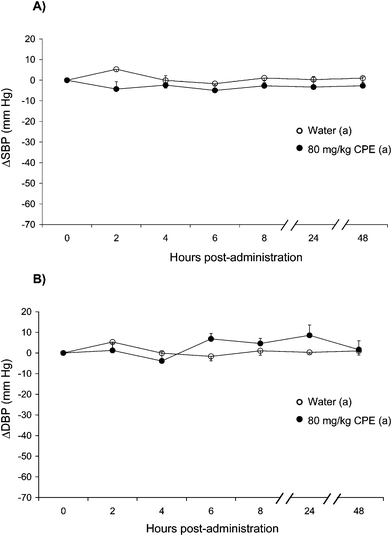 | ||
| Fig. 2 Decrease in systolic blood pressure (SBP) (A) and diastolic blood pressure (DBP) (B) caused in Wistar-Kyoto rats after the administration of water (○), or 80 mg kg−1CPE (●). Data are expressed as mean ± SEM. The experimental groups always have a minimum of 8 animals. No statistical differences were observed. | ||
Discussion
The health benefits of cocoa reported in recent studies have increased the interest to obtain products with high cocoa polyphenol content.34,35 A human study demonstrated that only 30 mg day−1 of flavan-3-ols (up to 5-mers) reduced blood pressure in humans.24 According to previous results of our research group an important content of polyphenols was founded in CPE, in particular of low molecular weight procyanidins.31 In fact, the concentration of total polyphenols and flavan-3-ols of CPE was very high when compared to other cocoa derivatives such as different cocoa powders or chocolates.36–38 Moreover, the total flavan-3-ols content (monomers and procyanidins B1 and B2) and the (−)-epicatechin content of CPE were 2 and 7 times higher than the respective values previously reported for the original polyphenol rich cocoa powder.28 As (−)-epicatechin is the most abundant flavanol in cocoa, the potential of this compound to be responsible for the cocoa blood pressure lowering effect is of singular relevance, as explained by Fraga et al.20 In this context, the high content of flavan-3-ols, and in particular the high content in (−)-epicatechin, measured in CPE, point out that it should be possible to use a small amount of this extract to decrease arterial blood pressure. It should be also noted that the doses of CPE selected for this study had the same (−)-epicatechin content as the doses of a polyphenol rich cocoa powder that had exhibited an antihypertensive effect.28 In addition, low molecular weight procyanidins are probably an important component of CPE, because Cooper et al., in 2008, postulated that the healthy properties attributed to cocoa would be related to the high amount of monomeric and dimeric compounds.39 In fact, the bioavailability of cocoa polyphenols is strongly related with their molecular size, and, in general, the smaller polyphenols (monomers and dimers) are found in higher concentration in the blood than other polyphenols. Polyphenol monomers and dimers have therefore more possibilities to reach their target organs in the body. A high amount of these compounds of low molecular weight in cocoa derivatives, especially for the monomer (−)-epicatechin, could also be accompanied by a dose-dependent increment in plasma antioxidant capacity40,41 and by a dose-dependent decrease in plasma lipid oxidation.40 This monomer is also considered responsible, at least in part, of many of the vascular beneficial effects associated with cocoa consumption,18,20,29,42–44 and their antihypertensive effects have also been demonstrated in rats subjected to Nω-nitro-L-arginine methyl ester pre-treatment20 and in SHR rats (unpublished data).It is well known that theobromine was commonly used to treat hypertension because of its ability to relax smooth muscle tissue and dilate blood vessels. In fact, this methylxanthine could be responsible for the decrease in blood pressure reported after the short-administration of dark chocolate.45 In the present study, an antihypertensive effect of CPE was demonstrated, but theobromine is present in this extract only in a low concentration (90.6 mg g−1) and could therefore hardly justify its antihypertensive properties. A recent study shows that a theobromine-enriched flavanol-rich cocoa with 979 mg of theobromine could decrease central systolic blood pressure in healthy individuals. Nevertheless, the flavanol-rich cocoa with a natural dose of theobromine consisting in 106 mg of this methylxanthine did not significantly change it.46 As in the study carried out with the original polyphenol rich cocoa powder,28 in the present study, a dose dependent antihypertensive effect of CPE could not be demonstrated. On the contrary, we could observe that the highest dose of this polyphenolic compound (160 mg kg−1) was not the most effective one to decrease arterial blood pressure. In this context, different studies have demonstrated that a high quantity of polyphenols could exhibit pro-oxidant properties instead of antioxidant properties.47–49
The lowering blood pressure effect exhibited by CPE would be mainly due to the presence of flavan-3-ols. As mentioned before, the doses of both products, the original polyphenol rich cocoa powder and the extract used in the present work, are equivalent in (−)-epicatechin content, but the potential contribution for the antihypertensive effect of other polyphenolic compounds, and the synergy between them, cannot be ruled out. It has been also published that polyphenols are able of inducing a progressive, and sustained reduction in blood pressure when administered chronically to humans or to different rat models of hypertension, including SHR and L-NAME-treated rats.24–26 It is important to note that hypertension is a chronic pathology that requires chronic treatment, and the use of strategies with long lasting antihypertensive effects is always desirable. In this context, it is important to highlight that the decrease in arterial blood pressure caused by CPE lasted for a longer period of time than the antihypertensive effect previously reported for the original polyphenol rich cocoa powder. In accordance with this idea, the antihypertensive properties of CPE may be more favourable than the original cocoa powder for controlling high blood pressure levels.
The administration of 80 mg kg−1 of CPE to normotensive WKY rats did not change the arterial blood pressure of these animals. This indicates that the effect of CPE is specific for the hypertensive condition.
In this study we have also demonstrated that CPE presents an extraordinary in vitroantioxidant capacity (12134 μmol TE/g), which can be considered as a very high one when compared with the antioxidant capacity of other cocoa derivatives such as milk chocolate, dark chocolate or unsweetened chocolate (74, 219 and 490 μmol TE/g respectively), or when compared with the antioxidant capacity of a natural cocoa powder (820 μmol TE/g), all of them analyzed by the H-ORAC method.37 Moreover, the antioxidant capacity of CPE was much higher than those reported for other foods different from cocoa, such as cereals, legumes, vegetables, or fruits.50 This suggests that an antioxidant mechanism could be implicated in the antihypertensive effect observed when CPE is administered. However, we cannot discard that other mechanisms of action are involved in this effect, and more studies are necessary in order to elucidate the blood pressure lowering mechanisms of CPE.
In this paper, the in vitroantioxidant capacity and antihypertensive effect of CPE in SHR has been demonstrated. Our results suggest that this extract could be used as a functional food ingredient with potential therapeutic benefit in the prevention and treatment of hypertension. In particular, CPE could be useful for prehypertensive patients who do not need the prescription of blood pressure lowering medications so far, and we want also to point out that our results clearly support that it can be consumed by normotensive subjects without promoting any change in arterial blood pressure. Undoubtedly clear advantages are applicable to CPE, and this extract could be used preferentially to the original polyphenol rich cocoa powder as a food antihypertensive ingredient. However, further studies after long-term treatment are necessary in animals and humans before to use CPE as antihypertensive functional ingredient. In addition, studies to elucidate the mechanisms implicated in the antihypertensive effect of CPE should also be carried out. In fact, we are currently conducting some studies using young SHR, in order to investigate if CPE is useful to prevent the development of hypertension and to clarify the mechanisms implicated in their antihypertensive effect.
Acknowledgements
This study was supported by Natraceutical Group (36/2007 U.C.M. Project). We also thank Manuel Bas Caro, Technician in Pharmacology, for his excellent care of the rats, and Yolanda Castilla for her helpful technical assistance. In addition, Miguel M. holds a Ramon and Cajal work contract.References
- American Heart Association, Heart Disease and Stroke Statistics: 2004 Update,Dallas, TX, American Heart Assosiation, 2003 Search PubMed.
- J. Müller-Nordhorn, S. Binting, S. Roll and S. N. Willich, An update on regional variation in cardiovascular mortality within Europe, Eur. Heart J., 2008, 29, 1316–26 CrossRef.
- K. L. Thomas, A. F. Hernandez, D. Dai, P. Heidenreich, G. C. Fonarow, E. D. Peterson and C. W. Yancy, Association of race/ethnicity with clinical risk factors, quality of care, and acute outcomes in patients hospitalized with heart failure, Am. Heart J., 2011, 161, 746–54 CrossRef.
- L. Yochum, L. H. Kushi, K. Meyer and A. R. Folsom, Dietary flavonoid intake and risk of cardiovascular disease in postmenopausal women, Am. J. Epidemiol., 1999, 149, 943–49 CAS.
- M. Galleano, O. Pechanova and C. G. Fraga, Hypertension, nitric oxide, oxidants, and dietary plant polyphenols, Curr. Pharm. Biotechnol., 2010, 11, 837–48 CAS.
- F. Perez-Vizcaino and J. Duarte, Flavonols and cardiovascular disease, Mol. Aspects Med., 2010, 3, 478–94 CrossRef.
- Z. Liu, L. P. Ma, B. Zhou, L. Yang and Z. L. Liu, Antioxidative effects of green tea polyphenols on free radical initiated and photosensitized peroxidation of human low density lipoprotein, Chem. Phys. Lipids, 2000, 106, 53–63 CrossRef CAS.
- K. J. Joshipura, F. B. Hu, J. E. Manson, M. J. Stampfer, E. B. Rimm, F. E. Speizer, G. Colditz, A. Ascherio, B. Rosner, D. Spiegelman and W. C. Willett, The effect of fruit and vegetable intake on risk for coronary heart disease, Ann. Intern. Med., 2001, 134, 1106–14 CAS.
- P. M. Kris-Etherton and C. L. Keen, Evidence that the antioxidant flavonoids in tea and cocoa are beneficial for cardiovascular health, Curr. Opin. Lipidol., 2002, 13, 41–9 CrossRef CAS.
- M. Miguel, B. Muguerza, E. Sánchez, M. A. Delgado, I. Recio, M. Ramos and M. A. Aleixandre, Changes in arterial blood pressure in hypertensive rats caused by long-term intake of milk fermented by Enterococcus faecalis CECT 5728, Br. J. Nutr., 2005, 94, 36–43 CrossRef CAS.
- Z. Y. Chen, C. Peng, R. Jiao, Y. M. Wong, N. Yang and Y. Huang, Anti-hypertensive nutraceuticals and functional foods, J. Agric. Food Chem., 2009, 57, 4485–99 CrossRef CAS.
- K. W. Lee, Y. J. Kim, H. J. Lee and C. Y. Lee, Cocoa has more phenolic phytochemicals and higher antioxidant capacity than teas and red wines, J. Agric. Food Chem., 2003, 51, 7292–95 CrossRef CAS.
- C. Manach, A. Scalbert, C. Morand, C. Rémésy and L. Jiménez, Polyphenols: food sources and bioavailability, Am. J. Clin. Nutr., 2004, 79, 727–47 CAS.
- E. L. Ding, S. M. Hutfless, X. Ding and S. Girotra, Chocolate and prevention of cardiovascular disease: a systematic review, Nutr. Metab., 2006, 3, 2 Search PubMed.
- K. A. Cooper, J. L. Donovan, A. L. Waterhouse and G. Williamson, Cocoa and health: a decade of research, Br. J. Nutr., 2008, 99, 1–11 CrossRef CAS.
- M. Galleano, P. I. Oteiza and C. G. Fraga, Cocoa, chocolate, and cardiovascular disease, J. Cardiovasc. Pharmacol., 2009, 54, 483–90 CrossRef CAS.
- N. K. Hollenberg, G. Martinez, M. McCullough, T. Meinking, D. Passan, M. Preston, A. Rivera, D. Taplin and M. Vicaria-Clement, Aging, acculturation, salt intake, and hypertension in the Kuna of Panama, Hypertension, 1997, 29, 171–76 CAS.
- B. Buijsse, E. J. Feskens, F. J. Kok and D. Kromhout, Cocoa intake, blood pressure, and cardiovascular mortality: the Zutphen Elderly Study, Arch. Intern. Med., 2006, 166, 411–17 CrossRef.
- P. J. Mink, C. G. Scrafford, L. M. Barraj, L. Harnack, C. P. Hong, J. A. Nettleton and D. R. Jacobs, Flavonoid intake and cardiovascular disease mortality: a prospective study in postmenopausal women, Am. J. Clin. Nutr., 2007, 85, 895–909 CAS.
- C. G. Fraga, M. C. Litterio, P. D. Prince, V. Calabró, B. Piotrkowski and M. Galleano, Cocoa flavanols: effects on vascular nitric oxide and blood pressure, J. Clin. Biochem. Nutr., 2011, 48, 63–7 CrossRef CAS.
- J. Wollgast and E. Anklam, Review on polyphenols in Theobroma cacao: changes in composition during the manufacture of chocolate and methodology for identification and quantification, Food Res. Int., 2000, 33, 423–47 CrossRef CAS.
- I. C. Arts, P. C. Hollman and D. Kromhout, Chocolate as a source of tea flavonoids, Lancet, 1999, 354, 488 CrossRef CAS.
- K. W. Lee, Y. J. Kim, H. J. Lee and C. Y. Lee, Cocoa has more phenolic phytochemicals and higher antioxidant capacity than teas and red wines, J. Agric. Food Chem., 2003, 51, 7292–95 CrossRef CAS.
- D. Taubert, R. Roesen, C. Lehmann, N. Jung and E. Schömig, Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide, JAMA, J. Am. Med. Assoc., 2007, 298, 49–60 CrossRef CAS.
- S. Desch, D. Kobler, J. Schmidt, M. Sonnabend, V. Adams, M. Sareban, I. Eitel, M. Blüher, G. Schuler and H. Thiele, Low vs. higher-dose dark chocolate and blood pressure in cardiovascular high-risk patients, Am. J. Hypertens., 2010, 23, 694–700 CrossRef CAS.
- M. O. Kane, N. Etienne-Selloum, S. V. Madeira, M. Sarr, A. Walter, S. Dal-Ros, C. Schott, T. Chataigneau and V. B. Schini-Kerth, Endothelium-derived contracting factors mediate the Ang II-induced endothelial dysfunction in the rat aorta: preventive effect of red wine polyphenols, Pfluegers Arch., 2010, 459, 671–79 CrossRef CAS.
- J. Wollgast and E. Anklam, Review on polyphenols in Theobroma cacao: changes in composition during the manufacture of chocolate and methodology for identification and quantification, Food Res. Int., 2000, 33, 423–47 CrossRef CAS.
- E. Cienfuegos-Jovellanos, M. Quiñones, B. Muguerza, L. Moulay, M. Miguel and A. Aleixandre, Antihypertensive effect of a polyphenol-rich cocoa powder industrially processed to preserve the original flavonoids of the cocoa beans, J. Agric. Food Chem., 2009, 57, 6156–62 CrossRef CAS.
- M. Quiñones, D. Sánchez, B. Muguerza, L. Moulay, S. Laghi, M. Miguel and A. Aleixandre, Long-term intake of a soluble cocoa fiber product attenuates the development of hypertension in spontaneously hypertensive rats, Food Chem., 2010, 122, 1013–19 CrossRef.
- M. Quiñones, D. Sánchez, B. Muguerza, M. Miguel and A. Aleixandre, Mechanisms for antihypertensive effect of CocoanOX, a polyphenol-rich cocoa powder, in spontaneously hypertensive rats, Food Res. Int., 2011, 44, 1203–08 CrossRef.
- I. Andujar, M. C. Recio, R. M. Giner, E. Cienfuegos-Jovellanos, S. Laghi, B. Muguerza and J. L. Ríos, Inhibition of Ulcerative Colitis in Mice after Oral Administration of a Polyphenol-Enriched Cocoa Extract Is Mediated by the Inhibition of STAT1 and STAT3 Phosphorylation in Colon Cells, J. Agric. Food Chem., 2011, 59, 6474–83 CrossRef CAS.
- S. Ramos, L. Moulay, A. B. Granado-Serrano, O. Vilanova, B. Muguerza, L. Goya and L. Bravo, Hypolipidemic effect in cholesterol-fed rats of a soluble fiber-rich product obtained from cocoa husks, J. Agric. Food Chem., 2008, 56, 6985–93 CrossRef CAS.
- R. D. Buñag, Validation in awake rats of a tail-cuff method for measuring systolic pressure, J. Appl. Physiol., 1973, 34, 279–82 Search PubMed.
- F. A. Tomás-Barberán, E. Cienfuegos-Jovellanos, A. Marin, B. Muguerza, A. Gil-Izquierdo, B. Cerda, P. Zafrilla, J. Morillas, J. Mulero, A. Ibarra, M. A. Pasamar, D. Ramón and J. C. Espín, A new process to develop a cocoa powder with higher flavonoid monomer content and enhanced bioavailability in healthy humans, J. Agric. Food Chem., 2007, 55, 3926–35 CrossRef.
- G. Schinella, S. Mosca, E. Cienfuegos-Jovellanos, P. A. Pasamar, B. Muguerza, D. Ramón and J. L. Ríos, Antioxidant properties of polyphenol-rich cocoa products industrially processed, Food Res. Int., 2010, 43, 1614–23 CrossRef CAS.
- J. A. Vinson, J. Proch and L. Zubik, Phenol antioxidant quantity and quality in foods: cocoa, dark chocolate, and milk chocolate, J. Agric. Food Chem., 1999, 47, 4821–24 CrossRef CAS.
- L. Gu, S. E. House, X. Wu, B. Ou and R. L. Prior, Procyanidin and catechin contents and antioxidant capacity of cocoa and chocolate products, J. Agric. Food Chem., 2006, 54, 4057–61 CrossRef CAS.
- K. B. Miller, D. A. Stuart, N. L. Smith, C. Y. Lee, N. L. McHale, J. A. Flanagan, B. Ou and W. J. Hurst, Antioxidant activity and polyphenol and procyanidin contents of selected commercially available cocoa-containing and chocolate products in the United States, J. Agric. Food Chem., 2006, 54, 4062–68 CrossRef CAS.
- K. A. Cooper, E. Campos-Giménez, D. Jiménez-Alvarez, A. Rytz, K. Nagy and G. Williamson, Predictive relationship between polyphenol and nonfat cocoa solids content of chocolate, J. Agric. Food Chem., 2008, 56, 260–65 CrossRef CAS.
- D. Rein, S. Lotito, R. R. Holt, C. L. Keen, H. H. Schmitz and C. G. Fraga, Epicatechin in human plasma: in vivo determination and effect of chocolate consumption on plasma oxidation status, J. Nutr., 2000, 130, 2109S–14S CAS.
- M. Serafini, R. Bugianesi, G. Maiani, S. Valtuena, S. De Santis and A. Crozier, Plasma antioxidants from chocolate, Nature, 2003, 424, 1013 CrossRef CAS.
- H. Schroeter, C. Heiss, J. Balzer, P. Kleinbongard, C. L. Keen, N. K. Hollenberg, H. Sies, C. Kwik-Uribe, H. H. Schmitz and M. Kelm, (−)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 1024–29 CrossRef CAS.
- M. Quiñones, B. Muguerza, M. Miguel and A. Aleixandre, Evidence that nitric oxide mediates the blood pressure lowering effect of a polyphenol-rich cocoa powder in spontaneously hypertensive rats, Pharmacol. Res., 2011, 64, 478–81 CrossRef.
- I. Ramirez-Sanchez, L. Maya, G. Ceballos and F. Villarreal, (−)-Epicatechin activation of endothelial cell endothelial nitric oxide synthase, nitric oxide, and related signaling pathways, Hypertension, 2010, 55, 1398–1405 CrossRef CAS.
- C. J. Kelly, Effects of theobromine should be considered in future studies, Am. J. Clin. Nutr., 2005, 82, 486–87 CAS.
- B. van den Bogaard, R. Draijer, B. E. Westerhof, A. H. van den Meiracker, G. A. van Montfrans and B.-J. H. van den Bor, Effects on peripheral and central blood pressure of cocoa with natural or high-dose theobromine, Hypertension, 2010, 56, 839–846 CrossRef CAS.
- N. Cotelle, Role of flavonoids in oxidative stress, Curr. Top. Med. Chem., 2001, 1, 569–90 CrossRef CAS.
- S. Azam, N. Hadi, N. U. Khan and S. M. Hadi, Prooxidant property of green tea polyphenols epicatechin and epigallocatechin-3-gallate: implications for anticancer properties, Toxicol. in Vitro, 2004, 18, 555–61 CrossRef CAS.
- M. Lahouel, S. Amedah, A. Zellagui, A. Touil, S. Rhouati, F. Benyache, E. Leghouchi and H. Bousseboua, The interaction of new plant flavonoids with rat liver mitochondria: relation between the anti- and pro-oxidant effect and flavonoids concentration, Therapie, 2006, 61, 347–55 CrossRef.
- G. E. Adamson, S. A. Lazarus, A. E. Mitchell, R. L. Prior, G. Cao, P. H. Jacobs, B. G. Kremers, J. F. Hammerstone, R. B. Rucker, K. A. Ritter and H. H. Schmitz, HPLC method for the quantification of procyanidins in cocoa and chocolate samples and correlation to total antioxidant capacity, J. Agric. Food Chem., 1999, 47, 4184–88 CrossRef CAS.
Footnote |
| † Present Address: Dpto. Bioquímica y Biotecnología, U. Rovira i Virgili, C/ Marcel.li Domingo s/n 43007 Tarragona, Spain. |
| This journal is © The Royal Society of Chemistry 2011 |
