Effects of chitosan-oil coating on blue mold disease and quality attributes of jujube fruits
Yage
Xing
*a,
Qinglian
Xu
a,
Zhenming
Che
a,
Xihong
Li
b and
Wenting
Li
a
aKey Laboratory of Food Bio-technology under the supervision of Sichuan Province, School of Bioengineering, Xihua University, Chengdu, 610039, China. E-mail: xingyage1@yahoo.com.cn; Fax: +86 2887720552; Tel: +86 2887720502
bKey Laboratory of Food Nutrition & Safety (Tianjin University of Science & Technology), Ministry of Education, Tianjin, 300457, China
First published on 11th August 2011
Abstract
The effects of chitosan coating enriched with cinnamon oil on blue mold disease and quality attributes were investigated. In the in vitro experiment, the results demonstrated that the antifungal activity against P. citrinum improved with increasing concentration of chitosan or cinnamon oil. In the in vivo study, chitosan-oil treatments significantly reduced fungal decay caused by P. citrinum and all compounds with cinnamon oil at 2.0% showed complete control of the growth of P. citrinum on wound-inoculated fruits. High chitosan-oil concentrations correlated with low disease incidence regardless of storage temperature. Treatments of chitosan-oil coating also inhibited the activity of polyphenol oxidase and maintained vitamin C and phenolic compounds in wounded jujube fruits. Results suggested that the effect of chitosan coating (1.0%) enriched with cinnamon oil (0.75%) on blue mold in jujube fruits may be associated with fungitoxic properties against the pathogen and the elicitation of biochemical defense responses in fruits.
Introduction
Lingwu long jujube fruit is a local colored favorable fresh-eat fruit in Ningxia province of China. These jujube fruits have special flavor, great quality, sweet taste, and also provide various nutrients such as minerals and vitamins for human.1 Their economic benefit is several times more than that of other kind of fruits in China, such as table grapes and Prunus persica. However, Lingwu long jujube fruit has a short shelf-life and suffers severe postharvest losses, and therefore its commercial degree is usually low and its further development is also limited in many industries.1,2 Fungi, such as Penicillium species, may cause the production of mycotoxins and off-flavor formation, which affect the quality and shorten the shelf-life of fruits and vegetables.3,4 Blue mold disease caused by Penicillium species is one of the most important issues for the postharvest fruits, such as Lingwu long jujube fruits.5 Although the utilization of fungicides is one of the primary means to control postharvest diseases, the problems of resistant strains and fungicides residues have emerged recently.6 Moreover, its adverse impacts on human health and environment prompt public awareness.7 Hence there is a growing demand for the search of safer alternatives and an increasing interest for the non-chemically treated fruit.8In recent years, the possibility of edible coatings to carry antimicrobials has been investigated by several researchers.9 Edible coatings can keep the quality of fruits and vegetables by functioning as solute, gas, and vapor barriers.10 The antimicrobial agents in edible coatings can extend the shelf life of fruits by releasing them slowly onto the surface of the products and maintaining the high concentration in the packaging system during storage.11,12 Chitosan (poly-b-(1–4)-D-glucosamine) is a versatile biopolymer and has a broad range of applications on the storage of fruits and vegetables because of its film-forming, antimicrobial activity and elicitation of defense responses in plant tissues.13–16 Recent studies indicated that blue mold rots caused by Penicillium species in sweet cherry fruits and citrus fruits were reduced by postharvest dipping or coating with chitosan.17,18 Chitosan coating incorporated with essential oils may not only reduce water vapor permeability but also greatly enhance the coating's antimicrobial property.14,19 Essential oils as natural compounds have gained a great scientific interest recently.20Cinnamomum zeylanicum (L.), commonly known as cinnamon, is rich in cinnamaldehyde as well as caryophyllene, linalool and other terpenes.10 The US Food and Drug Administration lists it as “Generally Recognized as Safe – GRAS”.21 Several reports indicated that some substances in cinnamon oil inhibited the growth of bacteria, yeasts and moulds.22–24 Spraying cinnamon extracts on Embul banana prior to storage, controlled crown rot and extended shelf-life.25,26 Cinnamon oil at the concentration of 5.0 g L−1 completely inhibited conidial germination and mycelial growth of all fungi on banana.19 As reported by Ojagh et al. (2010), chitosan coating incorporated with cinnamon oil on fish samples maintained good quality characteristics and extended its shelf life during refrigerated storage.10
Development of natural preservative coatings enriched with natural antimicrobial agents is desirable for prolonging the shelf-life of fruits because of the unsafe aspects of chemical preservatives.20 Chitosan-oil coating has become a promising alternative treatment for keeping the quality of fruits and vegetables. Several studies indicated that the antimicrobial activity of chitosan coating was greatly enhanced by the addition of essential oils.14,27 Chitosan coating exhibits great potential for inducing defense-related enzymes and phenolic contents in plants.16,28 And they may be also be affected by the addition of cinnamon oil because of its antioxidant activity.29 Nevertheless, there is no published investigation regarding the effect of combinations of chitosan coating and cinnamon oil on blue mold disease caused by P. citrinum and quality attributes of jujube fruits.
Therefore, the objectives of this study were to investigate the effects of chitosan coating enriched with cinnamon oil on blue mold disease of Lingwu long jujube fruits caused by P. citrinum. In addition, vitamin C content, total soluble phenolic compounds and polyphenol oxidase (PPO) activity by coating treatments in jujube fruits were also investigated.
Materials and methods
Preparation of coating solutions
The method to prepare the coating solutions was developed from method that described by Kyu Kyu Win et al. (2007) and Ojagh et al. (2010).10,19Food grade cinnamon oil was purchased from Xianghui Bio-technology Co. Ltd. (Shanghai, China). Chitosan (deacetylated ≥95%, and viscosity ≤30 mPas) was purchased from Jinan Haidebei Marline Bioengineering Co. Ltd. (Jinan, China). The chitosan (0, 0.5%, 0.75%, and 1.0%) solution contained acetic acid (0.5%) as a media to dissolve chitosan and glycerol (0.75%) as a plasticizer was stirred by a magnetic stirrer at room temperature for 1 h to achieve complete dispersion. Then the cinnamon oil (0, 0.5%, 0.75%, 1.0%, and 2.0%), mixed with Tween 80 (0.2%), to help distribute and completely incorporate the cinnamon oil, was added to the chitosan solution and then stirred using a magnetic stirrer for 30 min. To the final solution was added 0.1% food grade dimethyl silicon oil as an antifoaming agent and then was homogenized under aseptic conditions at 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 rpm for 1 min (FLUKO Equipment Shanghai Co. Ltd., Shanghai, China). The obtained solution can be used after standing for 1 h and removing the residual bubbles using a small spatula.
600 rpm for 1 min (FLUKO Equipment Shanghai Co. Ltd., Shanghai, China). The obtained solution can be used after standing for 1 h and removing the residual bubbles using a small spatula.
Determination of antifungal activity of coating solutions
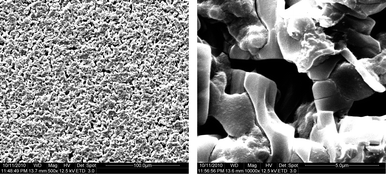
The agar diffusion method was used to determine antifungal activity of chitosan coating with cinnamon oil against P. citrinum.30P. citrinum strain (CICC 2478) was obtained from China Center of Industrial Culture Collection. The disks (10 mm in diameter) impregnated with 10 μl chitosan-oil solution and 10 μL of water as the control. Then disks were placed on PDA plate which had been spread by 100 μL of inoculum containing approximately 105–106 conidia per mL of tested fungus P. citrinum. The plates were incubated at 28 °C for 72 h in the incubation chamber, and then the diameters of the “inhibition zone” of the film disks were measured with a calliper. Experiments were done in triplicate.Morphology of chitosan-oil coating by scanning electron microscopy (SEM)
The chitosan-oil coating were placed on the SEM stub using two-sided adhesive tapes and then SEM analyses by using a LEO Gemini 1530 field emission SEM at a voltage of 5 kV acceleration after Pt sputtering.Preparation of jujube fruits samples
Lingwu jujube fruits (Ziziphus jujuba Mill cv.) were harvested at the mature red stage and sorted based on size and absence of physical injuries or disease infection. Before treatments, fruit were surface-disinfected with 2% (w/v) sodium hypochlorite for 3 min then rinsed with tap water, and air-dried. The fruit were randomly distributed into groups of 50 fruit and three replicates were used for each treatment. The method of in vivo antifungal assay of cinnamon oil in wounded fruit was developed from the method that described by Kyu Kyu Win et al.(2007) and Badawy and Rabea (2009).16,19 The surface of each fruit was re-cut using a sterilized knife to make three fresh wounds in the surface for the in vivo antifungal test (4–5 mm deep and 3 mm wide for each wound). Then 20 μL of the chitosan-oil coating or the control solution was placed into each wound using a micropipette. After airing and drying for 1 h, 20 μL of a conidial suspension (1.0 × 105 conidia per mL) of P. citrinum was added to each wound, respectively. After 2–3 h, the air-dried fruits were put in the 200 mm × 130 mm × 50 mm plastic boxes and put five filter papers with 25 mL sterile water into each box (50 fruit: 25 mL water) in order to maintain a high relative humidity (90–95%). The fruits were stored at 25 °C for 3 d and at 4 °C for 20 d, respectively. Disease incidence (%) was calculated as the percentage of the number of wounds that showed disease symptoms.Sensory acceptability
The sensory acceptability of each replicate jujube fruits was evaluated by visual appearance, taste, flavor and acceptability. Samples of fruit pulp were cut the wounded part completely and were surfaced-disinfected with 2% (w/v) sodium hypochlorite for 3 min, then rinsed with tap water and air-dried. Samples presented in random order to six panelists for sensory evaluations. They were rated on a nine-point hedonic scale (9, excellent; 7, very good; 5, good; 3, fair and 1, poor) for visual appearance, off-flavor and acceptability increased with the numerical value.31Vitamin C content
The determination of the vitamin C concentration in fruit tissue was performed by HPLC-UV as the method reported by Odriozola-Serrano et al. (2007) with minor modification.32 A portion of 2.5 g of fruit tissue was added to 25 mL of a solution containing 45 g L−1 of metaphosphoric acid and 7.2 g L−1 of DL-1,4-dithiothreitol (DTT). The mixture was stirred and centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 10 min using a centrifuge (Eppendorf China Ltd., Beijing, China) at 4 °C. The sample was passed through a Millipore 0.45 μm membrane and injected into the HPLC system. The HPLC system was equipped with a Varian 325-LC UV-Detector (Varian, Inc. USA) working at 245 nm. Duplicates of 20 μL of each extract were injected into a reverse-phase C18 Varian HPLC column (250 mm × 4.6 mm) (Varian, Inc. USA), used as stationary phase. A 0.01% solution of sulfuric acid adjusted to pH 2.6 was used as the mobile phase. The flow rate was fixed at 1 mL min−1 at room temperature. Results were expressed as mg vitamin C of per gram jujube fruit tissue.
000 × g for 10 min using a centrifuge (Eppendorf China Ltd., Beijing, China) at 4 °C. The sample was passed through a Millipore 0.45 μm membrane and injected into the HPLC system. The HPLC system was equipped with a Varian 325-LC UV-Detector (Varian, Inc. USA) working at 245 nm. Duplicates of 20 μL of each extract were injected into a reverse-phase C18 Varian HPLC column (250 mm × 4.6 mm) (Varian, Inc. USA), used as stationary phase. A 0.01% solution of sulfuric acid adjusted to pH 2.6 was used as the mobile phase. The flow rate was fixed at 1 mL min−1 at room temperature. Results were expressed as mg vitamin C of per gram jujube fruit tissue.
Total soluble phenolics content
Total soluble phenolics content in Jujube fruit tissue was determined according to McCue et al. (2000).33 For each sample, 10 g of fruit tissue was placed in 25 mL of 95% ethanol and was frozen for 48–72 h. Samples were homogenized with a tissue tearor and centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 10 min. One millilitre of the resulting supernatant was combined with 1 mL 95% ethanol, 5 mL distilled water, and 0.5 mL of 50% (1N) Folin-Ciocalteu phenol reagent. After 5 min of incubation at room temperature, 1 mL of 5% (w/v) sodium carbonate was added followed by brief vortexing to mix. The reaction mixture was incubated for 1 h in the dark at room temperature. After briefly vortexing, the absorbance at 725 nm was then determined. The analysis was performed using UV-VIS spectrometry. A standard curve was established using gallic acid in 95% ethanol. Total phenolics content was standardized against gallic acid and absorbance values were converted to mg of phenolics per gram of fresh weight tissue. Each value reported is the average of three replicates.
000 × g for 10 min. One millilitre of the resulting supernatant was combined with 1 mL 95% ethanol, 5 mL distilled water, and 0.5 mL of 50% (1N) Folin-Ciocalteu phenol reagent. After 5 min of incubation at room temperature, 1 mL of 5% (w/v) sodium carbonate was added followed by brief vortexing to mix. The reaction mixture was incubated for 1 h in the dark at room temperature. After briefly vortexing, the absorbance at 725 nm was then determined. The analysis was performed using UV-VIS spectrometry. A standard curve was established using gallic acid in 95% ethanol. Total phenolics content was standardized against gallic acid and absorbance values were converted to mg of phenolics per gram of fresh weight tissue. Each value reported is the average of three replicates.
Polyphenol oxidase (PPO) activity
PPO activity was assayed spectrophotometrically by a modified method based on Galeazzi et al. (1981).34 Ten grams of fruit tissue was homogenized with extraction buffer (0.2 mol L−1, pH 6.8, sodium phosphate buffer, stored at 4 °C) at the ratio of 2.0 mL:1.0 g (buffer: fruit) in an external ice bath for 3 min including 20 g kg−1 polyvinylpolypyrrolidone (PVPP, insoluble). The homogenates were centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 10 min using a centrifuge (Eppendorf China Ltd., Beijing, China) at 4 °C. The supernatants were collected and measured for PPO activity. The reaction mixture contained 0.1 mL crude extract and 2.9 mL substrate solution (0.020 mol L−1catechol in 0.05 mol L−1phosphate buffer, pH 6.5). The rate of catechol oxidation was followed at 420 nm for 2 min at 25 °C. An enzyme activity unit was defined as an increase of 0.0001 in absorbance per minute.
000 × g for 10 min using a centrifuge (Eppendorf China Ltd., Beijing, China) at 4 °C. The supernatants were collected and measured for PPO activity. The reaction mixture contained 0.1 mL crude extract and 2.9 mL substrate solution (0.020 mol L−1catechol in 0.05 mol L−1phosphate buffer, pH 6.5). The rate of catechol oxidation was followed at 420 nm for 2 min at 25 °C. An enzyme activity unit was defined as an increase of 0.0001 in absorbance per minute.
Statistical analysis
SPSS 13.0 software was used for data analysis. The mean values were calculated and reported as the mean ± standard deviation (SD) (n = 3). The data of in vivo experiments were analyzed by one-way analysis of variance (ANOVA). Results were performed by Student–Newman–Keuls (SNK) test and differences at P < 0.05 were considered as significant.Results and discussion
In vitro antifungal assay against P. citrinum
Investigation on the antifungal behavior of cinnamon oil against moulds was conducted by several investigators.10,19,26 However, few reports on inhibitory effects of chitosan-oil coating against P. citrinum were observed. As shown in Table 1, higher inhibitory effects of chitosan coating incorporated with cinnamon oil against P. citrinum were obtained. Chitosan coating at the concentration of 0.75% exhibited a clear inhibitory zone. The sample photo of antifungal activity of chitosan-oil coating can be seen from Fig. 1. The diameter of inhibition zone against P. citrinum increased from 10.4 mm to 48.3 mm with increasing chitosan concentration from 0.5% to 1.0% and the cinnamon oil concentration from 0.5% to 2.0%.| CC (%) | COC (%) | Diameter (D) of the inhibition zone/mm |
|---|---|---|
| Control water | 0.0 | 0.0i ± 0.0 |
| 0 | 0.5 | 16.5g ± 1.0 |
| 0.5 | 0.0 | 10.4h ± 0.16 |
| 0.5 | 17.2g ± 0.35 | |
| 0.75 | 22.1f ± 1.56 | |
| 1.0 | 26.2e ± 0.80 | |
| 2.0 | 36.1c ± 0.56 | |
| 0.75 | 0.0 | 15.9g ± 0.60 |
| 0.5 | 22.6f ± 0.60 | |
| 0.75 | 26.4e ± 0.98 | |
| 1.0 | 30.5d ± 0.95 | |
| 2.0 | 42.2b ± 1.11 | |
| 1.0 | 0.0 | 16.7g ± 0.80 |
| 0.5 | 26.3e ± 0.55 | |
| 0.75 | 30.3d ± 0.95 | |
| 1.0 | 37.2c ± 0.80 | |
| 2.0 | 48.3a ± 1.65 | |
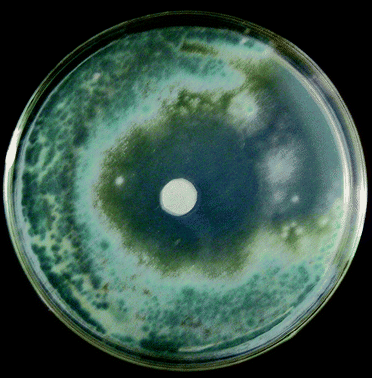 | ||
| Fig. 1 Sample photo of antifungal activity of chitosan-oil coating. | ||
The killing effect of chitosan-oil coating on P. citrinum should be contributed to the antifungal activity of chitosan and cinnamon oil. As reported by Liu et al. (2007), chitosan with low molecular weight at different concentrations (0.01–1%) markedly inhibited mycelial growth of P. expansum.35 For its antifungal activity, chitosan interferes with the negatively charged residues of macromolecules exposed on the fungal cell surface, and then changes the permeability of the plasma membrane.36,37 The inhibitory activity of cinnamon oil against fungi has also been proved by other researchers.38 Its antifungal activity may be due to the bioactivity of cinnamaldehyde.38 Chang and Cheng (2002) and Wang et al. (2005) reported that among congeners of cinnamaldehyde, cinnamaldehyde has a conjugated double bond and a long CH chain outside the ring and exhibited the strongest antifungal activity.38,39 It also suggested that hydroxyl groups in antimicrobial compounds could form hydrogen bonds with active enzymes affecting the biosynthesis of mycotoxins and resulting in deactivation.40 For the application of chitosan-oil coating, it is important to investigate the effect on postharvest blue mold disease and physiological attributes of jujube fruits.
Blue mold disease and sensory acceptability of jujube fruits
The in vivo effect of chitosan-oil coating on the postharvest decay of jujube fruits artificial inoculated by P. citrinum was investigated. After inoculation and storage at 25 °C for 3 days or 4 °C for 20 days, all the control fruits developed initially white lesions, which gradually turned into blue mold. Results also showed that the chitosan compounds at the concentrations of 0.5% to 2.0% inhibited fungal decay. Chitosan coating incorporated with cinnamon oil exhibited an excellent control for the blue mold disease on jujube fruits. The antifungal effect of chitosan coating against P. citrinum was greatly enhanced by the addition of essential oil. Disease incidence decreased with increasing the concentration of chitosan or cinnamon oil. If chitosan-oil coating is used as a natural biopreservative, it should not introduce any deleterious effects on the sensory acceptability of jujube fruits. Results shown in Table 2 indicated that chitosan coatings enriched with cinnamon oil at the concentrations of 0 to 0.75% did not produce undesirable sensory properties. However, the jujube fruits treated with cinnamon oil at concentrations of 1.0% and 2.0% showed the lower sensory acceptability. Results suggested that the treatment of chitosan coating (1.0%) with cinnamon oil (0.75%) could reduce the blue mold disease on jujube fruits and also keep good sensory acceptability.| CC (%) | COC (%) | Disease incidence (%) ± SD | Sensory acceptability (%) ± SD | ||
|---|---|---|---|---|---|
| 3 d (25 °C) | 20 d (4 °C) | 3 d (25 °C) | 20 d (4 °C) | ||
| Control | 0 | 100a ± 0.0 | 100a ± 0.0 | 6.60c ± 0.265 | 6.63cd ± 0.208 |
| 0 | 0.5 | 96.2ab ± 2.04 | 89.1b ± 1.02 | 5.60d ± 0.173 | 5.97e ± 0.306 |
| 0.5 | 0 | 100a ± 0.0 | 100a ± 0.0 | 7.03abc ± 0.153 | 7.07bc ± 0.252 |
| 0.5 | 93.4b ± 2.83 | 85.6b ± 5.09 | 6.63c ± 0.252 | 6.30de ± 0.265 | |
| 0.75 | 77.1d ± 2.53 | 69.6d ± 7.34 | 4.67fg ± 0.115 | 4.73g ± 0.208 | |
| 1.0 | 45.6g ± 1.93 | 33.1g ± 3.0 | 4.27gh ± 0.306 | 4.10h ± 0.173 | |
| 2.0 | 0.0j ± 0.0 | 0.0j ± 0.0 | 3.20i ± 0.300 | 3.40i ± 0.265 | |
| 0.75 | 0 | 100a ± 0.0 | 100a ± 0.0 | 7.27ab ± 0.153 | 7.37ab ± 0.252 |
| 0.5 | 83.1c ± 2.77 | 75.0c ± 0.0 | 6.83bc ± 0.115 | 6.67cd ± 0.115 | |
| 0.75 | 60.4f ± 5.5 | 42.4f ± 2.69 | 4.97ef ± 0.153 | 5.13f ± 0.208 | |
| 1.0 | 33.3h ± 0.0 | 20.7h ± 3.06 | 4.17h ± 0.115 | 4.33gh ± 0.252 | |
| 2.0 | 0.0j ± 0.0 | 0.0j ± 0.0 | 3.40i ± 0.300 | 3.30i ± 0.265 | |
| 1.0 | 0 | 92.2b ± 2.04 | 86.0b ± 4.00 | 7.33a ± 0.252 | 7.57a ± 0.306 |
| 0.5 | 66.4e ± 6.05 | 55.3e ± 3.05 | 6.60c ± 0.300 | 7.67a ± 0.115 | |
| 0.75 | 46.7g ± 1.34 | 33.1g ± 2.77 | 5.33de ± 0.252 | 5.30f ± 0.173 | |
| 1.0 | 27.6i ± 2.14 | 15.3i ± 0.0 | 4.43gh ± 0.153 | 4.63g ± 0.306 | |
| 2.0 | 0.0j ± 0.0 | 0.0j ± 0.0 | 3.30i ± 0.265 | 3.43i ± 0.115 | |
Various investigators have demonstrated that chitosan coating exhibited the potential for controlling decay and prolonging the shelf life of fruits such as strawberries and table grapes.8,9,13 Liu et al. (2007) reported that the control effect of chitosan on blue mold caused by P. expansum was observed in tomato fruit at 5000 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg L−1.35,41 On the other hand, chitosan coating resulted in decreased concentrations of oxygen and increased internal levels of carbon dioxide. This is because that micro-perforated panels in the chitosan-oil coating used to wrap jujube fruits could moderate probably letting O2 and CO2 to pass through (Fig. 2).42 Moreover, cinnamon oil has been previously proposed as an alternative postharvest treatment for fruit disease.19,25,26 Tzortzakis (2009) showed that cinnamon oil reduced spore germination and germ tube length in C. coccodes, B. cinerea and R. stolonifer with the effects were dependent on oil concentration.21 The antimicrobial properties of cinnamon are thought to be due to cinnamaldehyde (Chanthaphon et al., 2008). Expression of antifungal activity is often very clear and the mechanisms were also reported by others. It is conceivable that small differences in antimicrobial concentration internally may determine the biochemical event that dominates to inhibit cell growth or cause death.38 Gill & Holley (2003) showed that the difference in response to cinnamaldehyde may be due in part to differences in the solubility or permeability of the agents in the cell membrane and in part to the energy generation by the cells or the ability to partition in the lipid phase of the membrane.43 Wang et al. (2005) and Chang & Cheng (2002) suggested that a compound having a conjugated double bond and a long CH chain outside the ring possesses much stronger antifungal activity.38,39 It was also suggested that hydroxyl groups in antimicrobial compounds could form hydrogen bonds with active enzymes affecting the biosynthesis of mycotoxins and resulting in deactivation.21 Our investigation also indicated that the antifungal activity of chitosan coating against P. citrinum was enhanced by the addition of cinnamon oil, which was consistent with the results reported by Georgantelis et al. (2007) and Kanatt et al., (2008).14,27 This was because the pathogens would be less likely to develop resistance against the mixture of preservatives.44 These two synergistic effects between the preservatives may be also due to the chitosan coating. This combined coating could control the oil vapor diffusion and reduce the loss of cinnamon oil, which was more effective in inhibiting the growth of P. citrinum on fruits.45 The negative impacts of essential oil at higher concentration on the sensory acceptability of food products were also observed by other researchers. Valero and Giner (2006) observed a strong smell and flavor of thymol which greatly minimized the degree of acceptance.46 The strong effect of thyme on sensory quality of chopped bell peppers was also described by Uyttendaele et al. (2004).47 Undesirable flavors may be produced by the loss of organic acids during senescence or the change in carbohydrates, proteins, amino acids and phenolic compounds.48,49
000 mg L−1.35,41 On the other hand, chitosan coating resulted in decreased concentrations of oxygen and increased internal levels of carbon dioxide. This is because that micro-perforated panels in the chitosan-oil coating used to wrap jujube fruits could moderate probably letting O2 and CO2 to pass through (Fig. 2).42 Moreover, cinnamon oil has been previously proposed as an alternative postharvest treatment for fruit disease.19,25,26 Tzortzakis (2009) showed that cinnamon oil reduced spore germination and germ tube length in C. coccodes, B. cinerea and R. stolonifer with the effects were dependent on oil concentration.21 The antimicrobial properties of cinnamon are thought to be due to cinnamaldehyde (Chanthaphon et al., 2008). Expression of antifungal activity is often very clear and the mechanisms were also reported by others. It is conceivable that small differences in antimicrobial concentration internally may determine the biochemical event that dominates to inhibit cell growth or cause death.38 Gill & Holley (2003) showed that the difference in response to cinnamaldehyde may be due in part to differences in the solubility or permeability of the agents in the cell membrane and in part to the energy generation by the cells or the ability to partition in the lipid phase of the membrane.43 Wang et al. (2005) and Chang & Cheng (2002) suggested that a compound having a conjugated double bond and a long CH chain outside the ring possesses much stronger antifungal activity.38,39 It was also suggested that hydroxyl groups in antimicrobial compounds could form hydrogen bonds with active enzymes affecting the biosynthesis of mycotoxins and resulting in deactivation.21 Our investigation also indicated that the antifungal activity of chitosan coating against P. citrinum was enhanced by the addition of cinnamon oil, which was consistent with the results reported by Georgantelis et al. (2007) and Kanatt et al., (2008).14,27 This was because the pathogens would be less likely to develop resistance against the mixture of preservatives.44 These two synergistic effects between the preservatives may be also due to the chitosan coating. This combined coating could control the oil vapor diffusion and reduce the loss of cinnamon oil, which was more effective in inhibiting the growth of P. citrinum on fruits.45 The negative impacts of essential oil at higher concentration on the sensory acceptability of food products were also observed by other researchers. Valero and Giner (2006) observed a strong smell and flavor of thymol which greatly minimized the degree of acceptance.46 The strong effect of thyme on sensory quality of chopped bell peppers was also described by Uyttendaele et al. (2004).47 Undesirable flavors may be produced by the loss of organic acids during senescence or the change in carbohydrates, proteins, amino acids and phenolic compounds.48,49
Vitamin C content in jujube fruits
The effect of chitosan-oil coating on the vitamin C content in jujube fruits was evaluated during the storage time. Vitamin C value of the jujube fruits before the coating application (at day 0) was 4.21mg g−1. The results illustrated in Fig. 3 revealed that significant decrease in vitamin C values of jujube fruits was observed. However, the decrease rate of vitamin C in the treated fruits was significantly lower than that of the control samples. Vitamin C content in the fruits treated by the chitosan (1.0%)-cinnamon oil (0.75%) coating was the highest among all the treatment. The protection effect of chitosan coating on vitamin C was enhanced by the addition of essential oil. This result indicated that the cinnamon oil concentration was the component that affected vitamin C as the chitosan concentration, since an increase in cinnamon oil level promoted an increase in vitamin C retention. However, the lower levels of vitamin C were achieved when cinnamon oil was used at 1.0% and 2.0% either for chitosan concentration at 0.5, 0.75 or 1.0%. The treatment of chitosan coating (1.0%) with cinnamon oil (0.75%) could reduce the loss of vitamin C in jujube fruits.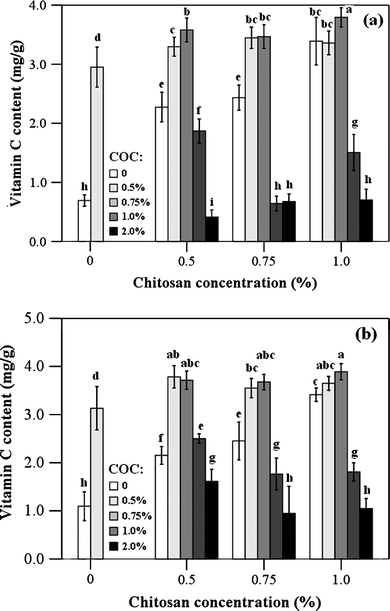 | ||
| Fig. 3 Vitamin C contents of fruit treated with different chitosan-oil coating. The fruits were stored at 25 °C for 3 d (a) and at 4 °C for 20 d (b), respectively. Different letters within columns indicate significant differences at P < 0.05, according to Student–Newman–Keuls (SNK) test (COC: cinnamon oil concentration). | ||
Chitosan coating and the addition of certain levels of cinnamon oil protected vitamin C from destruction.50,51 This is because that chitosan coating greatly slowed the ripening rate of jujube fruits and the antibrowning agent protected vitamin C in jujube fruits.42,52 These results were similar to those already reported for other cultivars by other researchers.53,54Vitamin C in jujube fruits may be also protected by the antioxidant phenolics in cinnamon oil.29,50,51 Cinnamon oil inhibited vitamin C loss by acting as an elicitor generating reactive oxygen species (ROS), which were always scavenged by antioxidant compounds such as vitamin C. The antioxidant activity of chitosan coating was improved by the addition of essential oils.14,27 This synergistic effect may be also due to the function of chitosan coating. This combined coating could reduce the loss of cinnamon oil, which was more effective in protecting the loss vitamin C in fruits. Possible synergism between chitosan coating and cinnamon oil could be beneficial for keeping quality of jujube fruits. However, the treatment of chitosan coating enriched with cinnamon oil at the concentration of 2.0% showed the lower vitamin C content. This result may be due to the adverse impact of higher concentration of cinnamon oil. The negative effect of essential oils on the food products was also reported by Bauer et al. (2001) and Serrano et al. (2005).55,56 As reported by Serrano et al. (2005), cherries packaged with eucalyptol behaved even worse than control cherries.56 These results were opposite to Ranasinghe et al. (2003) and Kyu Kyu Win et al. (2007), who reported that there were no significant adverse effects on the quality of banana. This beneficial effect may be due to the low concentration of essential oils or the different treatment method.19,26 The cultivar of fruit was also important for the use of essential oils because of their different tolerance or sensitiveness to essential oils. The lower concentration in the coating could maintain vitamin C in jujube fruits. This protection effect might be disturbed when the higher concentration of essential oil was used. On the other hand, interactions between essential oils or their components and other food ingredients need to be considered and investigated.57 As reported by Bauer et al. (2001), a reaction occurred and a dark pigmentation was acquired when clove oil contacted with iron. However, the mechanism is not completely understood and further research is needed.55
Total soluble phenolics content in jujube fruits
The changes of the total soluble phenolics content in jujube fruits treated with chitosan-oil coating are shown in Fig. 4. When fruits were stored at 25 °C for 3 d and 4 °C for 21 d, respectively, the content of total phenolics in all treatments gradually accumulated with increasing the concentration of chitosan or cinnamon oil. The increased total phenolics content (71.13 mg g−1 tissue) was observed in the jujube fruits treated with the chitosan (1.0%)-oil (0.75%) coating. However, the treatments of chitosan coating with cinnamon oil at 2.0% showed the similar content of total soluble phenolics as the control samples.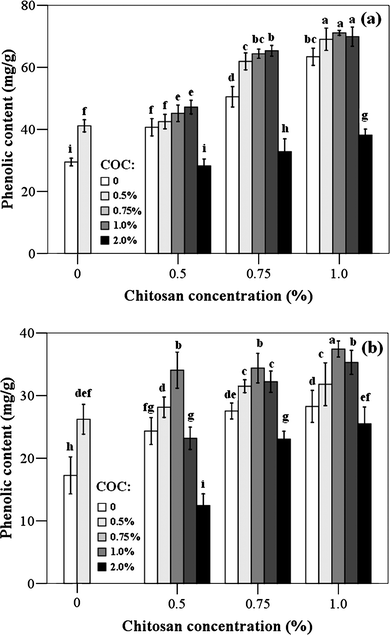 | ||
| Fig. 4 Total soluble phenolic content of fruit treated with different chitosan-oil coating. The fruits were stored at 25 °C for 3 d (a) and at 4 °C for 20 d (b), respectively. Different letters within columns indicate significant differences at P < 0.05, according to Student–Newman–Keuls (SNK) test (COC: cinnamon oil concentration). | ||
Besides its antifungal activity, chitosan also has a potential for inducing phenolic contents in fruits.16 Our study indicated that the total soluble phenolic compounds in chitosan-treated fruits raised significantly with increasing the chitosan concentration. This finding was also in agreement with the result that obtained by Benhamou and Thériault (1992) and Liu et al. (2007), who reported that the production of phenolic compounds was induced in tomato plants and fruits treated with chitosan.35,58 The increasing concentration of cinnamon oil in the chitosan solution also affected the content of total phenolics in jujube fruits. This may be due to the antioxidant property of cinnamon oil. Antioxidants are able to prevent or delay oxidation processes by reacting with free radicals, chelating metals and by acting as oxygen scavengers, triplet as well as singlet form and transferring hydrogen atoms to the free radical structure.59,60Antioxidant properties of cinnamon compounds has been studied by several researchers.29,60 The addition of essential oils may improve the antioxidant activity of chitosan coating.14,27 The protection of phenolic compounds in the fruits may be also due to the chitosan coating, which reduces the loss of cinnamon oil and then increases its antioxidant activity. However, the negative effect occurred and the defense system was disturbed when a high concentration (2.0%) of essential oil was used. The damage effects on fruits were also observed by other researchers.56,61 Results reported by Liet al. (2008) indicated that fruit injuries induced by essential oils mainly reflected by the browning on the surface of fruit, and then the browning extended from outside to inside, which was always associated with the content of phenolic compound.61 Higher concentration of cinnamon oil induced or accelerated the oxidation process, with occurrence of phytotoxicity, and acceleration of the physic-chemical and physiological changes related to fruit ripening and senescence.56 As reported by Serrano et al. (2005), the damage effect was due to the antioxidant property of essential oil, which led to the highest CO2 and lowest O2 concentrations and accelerated the oxidative metabolism.56 However, the mechanism for the reduction of phenolics content is still unknown and further research is needed.
Polyphenol oxidase (PPO) activity in jujube fruits
PPO activity of jujube fruits treated by chitosan-oil coating after 3 and 20 d of storage at 25 °C and 4 °C, respectively, is presented in Fig. 5. Low PPO activity in the jujube fruit was observed before wounded (10.54 Unit/g). While after being wounded, the activities were increased significantly, which were 27.64 Unit/g and 27.95 Unit/g in the fruits prepared to storage at 25 °C and 4 °C, respectively. In general, PPO activity in fruit stored at 4 °C for 20 d was lower than that of fruits stored at 25 °C for 3 d. Our study also demonstrated that the PPO activity was chitosan or cinnamon oil concentration dependent. The treatment of the combination of chitosan (1.0%) and cinnamon oil (0.75%) was the most active one in inhibiting the PPO activity in jujbe fruits. However, the treatment of chitiosan coating with cinnamon oil at 2.0% showed the similar PPO activities as the control samples.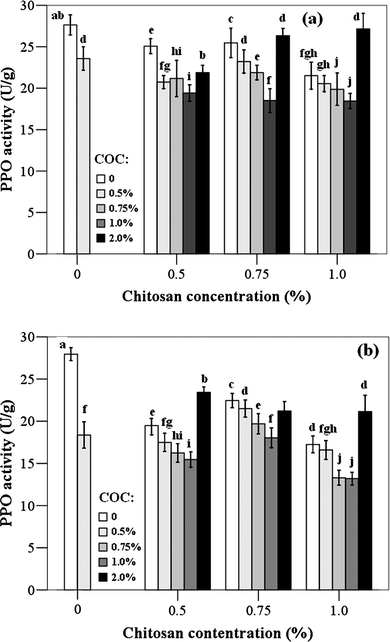 | ||
| Fig. 5 Polyphenol oxidase (PPO) activity of fruit treated with different chitosan-oil coating. The fruits were stored at 25 °C for 3 d (a) and at 4 °C for 20 d (b), respectively. Different letters within columns indicate significant differences at P < 0.05, according to Student–Newman–Keuls (SNK) test (COC: cinnamon oil concentration). | ||
Various techniques have been developed for the control of this undesirable enzyme activity. These techniques attempt to eliminate one or several essential components, such as oxygen, enzyme, copper, or substrate, from the reaction.16,62 Enzymes generally possess metal ions, such as copper and iron, at their active sites and chelating agents can remove these ions, thus the enzymes can be rendered inactivated.63 The result obtained in our study revealed that the chitosan treatments on wounded jujube fruits reduced the PPO activity, which was consistent with the observations of Zhang and Quantick (1997), and Badawy and Rabea (2009).16,64 They indicated that chitosan coating had a potential inhibitory effect on PPO activity in litchi fruit. The result about the PPO activity of jujube fruits in our study was also supported by the observations of the in vivo experiment on total phenolic content. The treatment of chitosan (1.0%)-oil (0.75) coating was the most active one in decreasing PPO activity. This result may be due to chitosan inhibitory effect and the antioxidant property of cinnamon compounds. As reported by Badawy and Rabea (2009), chitosan inhibitory effect was probably a consequence of the ability to remove the metal ions such as copper and iron found at enzyme active sites, to adsorb suspended PPO, its substrates, or products.16 On the other hand, cinnamon is a good source of antioxidant phenolics.29 The lower PPO activity treated by chitosan-oil coating may be also due to the antioxidant property of cinnamon compounds.60 The inhibitory effect on PPO activity may be improved by the synergistic effect between chitosan coating and essential oil. This is because the loss of cinnamon oil was reduced by the chitosan coating, and then the antioxidant activity of the combined coating was also improved. A high concentration of cinnamon oil (2.0%) could induce the higher PPO activity, which was also associated with the lower lever of phenolic compounds in injured fruits. Further research is underway aiming at answering this question.
Conclusions
Natural preservative coatings with low toxicity to mammals and high safety to environment have been paid increasing attention. This study showed that high concentration of chitosan and cinnamon oil could directly inhibit the growth of P. citrinum in in vitro and in vivo fruits test. The antifungal effects were chitosan or cinnamon oil concentration dependent. Moreover, chitosan potently induced defense reactions in jujube fruits. Chitosan coating enriched with cinnamon oil at a proper concentration improved resistance of jujube fruits against blue mold caused by P. citrinum and showed good sensory acceptability. This combined coating is promising to partially substitute for the utilization of synthetic fungicides in fruit and vegetables. Further investigations on the effect of chitosan coating (1.0%) with essential oil at the appropriate dose (0.75%) on the quality of whole jujube fruits and the mechanism for the detrimental effects of high concentration oil are needed.Acknowledgements
This study was supported by the Key Research Foundation Program of Xihua University and by the National Natural Science Foundation of China (31000826).References
- H. Han, Y. Zhang, X. Shen, Y. Li and J. Zhao, Effects of different atmosphere compositions on physiological changes of Lingwu long jujube during storage, Trans. Chin. Soc. Agric. Eng., 2007, 10, 246–250 Search PubMed.
- J. Zhao, Y. Su, X. Shen, H. Wang and Y. Zhang, Effects of Different Fresh-keeping Agents on Storage Quality of Lingwu Long Jujube, Chin. Agric. Sci. Bull., 2009, 7, 86–88 Search PubMed.
- P. Wanchaitanawong, P. Chaungwanit, N. Poovarodom and S. Nitisinprasert, In vitro antifungal activity of Thai herb and spice extracts against food spoilage fungi, Kasetsart J. (Natural Sci.), 2005, 39, 400–405 Search PubMed.
- X. Li, Y. Xing, Y. Jiang, Y. Ding and W. Li, Antimicrobial activities of ZnO powder-coated PVC film to inactivate food pathogens, Int. J. Food Sci. Technol., 2009, 44, 2161–2168 CrossRef CAS.
- N. F. Sommer, R. J. Fortlage and D. C. Edwards, Postharvest diseases of selected commodities, in Postharvest Technology of Horticultural Crops, ed. A. A. Kader, 2nd edn, University of California Press, USA, vol. 3311, 1992, pp. 117–160 Search PubMed.
- M. Mari, P. Bertolini and G. C. Pratella, Non-conventional methods for the control of postharvest pear diseases, J. Appl. Microbiol., 2003, 94, 761–766 CrossRef CAS.
- P. Houeto, G. Bindoula and J. R. Hoffman, Ethylenebisdithiocarbamates and ethylenethiourea: possible human health hazards, Environ. Health Perspect., 1995, 103, 568–573 CrossRef CAS.
- X. H. Meng, B. Q. Li, J. Liu and S. P. Tian, Physiological responses and quality attributes of table grape fruit to chitosan preharvest spray and postharvest coating during storage, Food Chem., 2008, 106, 501–508 CrossRef CAS.
- C. Han, Y. Zhao, S. W. Leonard and M. G. Traber, Edible coatings to improve storability and enhance nutritional value of fresh and frozen strawberries (Fragaria × ananassa) and raspberries (Rubus ideaus), Postharvest Biol. Technol., 2004, 33, 67–78 CrossRef CAS.
- S. M. Ojagh, M. Rezaei, S. H. Razavi and S. M. H. Hosseini, Effect of chitosan coatings enriched with cinnamon oil on the quality of refrigerated rainbow trout, Food Chem., 2010, 120, 193–198 CrossRef CAS.
- V. Coma, I. Sebti, P. Pardon, A. Deschamps and F. H. Pichavant, Antimicrobial edible packaging based on cellulosic ethers, fatty acids, and nisin incorporation to inhibit Listeria innocua and Staphylococcus aureus, J. Food Prot., 2001, 64, 470–475 CAS.
- B. Ouattara, R. E. Simard, G. Piette, A. Begin and R. A. Holley, Diusion of acetic and propionicacids from chitosan-based antimicrobial packaging films, J. Food Sci., 2000, 65, 768–773 CrossRef CAS.
- G. Romanazzi, F. Nigro, A. Ippolito, D. D. Venere and M. Salerno, Effects of Pre- and Postharvest chitosan treatments to control storage grey mold of table grapes, J. Food Sci., 2002, 67, 1862–1867 CrossRef CAS.
- S. R. Kanatt, R. Chander and A. Sharma, Chitosan and mint mixture: A new preservative for meat and meat products, Food Chem., 2008, 107, 845–852 CrossRef CAS.
- C. L. Fisk, A. M. Silver, B. C. Strik and Y. Zhao, Postharvest quality of hardy kiwifruit (Actinidia arguta ‘Ananasnaya’) associated with packaging and storage conditions, Postharvest Biol. Technol., 2008, 47, 338–345 CrossRef CAS.
- M. E. I. Badawy and E. I. Rabea, Potential of the biopolymer chitosan with different molecular weights to control postharvest gray mold of tomato fruit, Postharvest Biol. Technol., 2009, 51, 110–117 CrossRef CAS.
- G. Romanazzi, F. Nigro and A. Ippolito, Short hypobaric treatments potentiate the effect of chitosan in reducing storage decay of sweet cherries, Postharvest Biol. Technol., 2003, 29, 73–80 CrossRef CAS.
- P. J. Chien, F. Sheu and H. R. Lin, Coating citrus (Murcott tangor) fruit with low molecular weight chitosan increases postharvest quality and shelf life, Food Chem., 2007, 100, 1160–1164 CrossRef CAS.
- N. Kyu Kyu Win, P. Jitareerat, S. Kanlayanarat and S. Sangchote, Effects of cinnamon extract, chitosan coating, hot water treatment and their combinations on crown rot disease and quality of banana fruit, Postharvest Biol. Technol., 2007, 45, 333–340 CrossRef.
- S. Chanthaphon, S. Chanthachum and T. Hongpattarakere, Antimicrobial activities of essential oils and crude extracts from tropical Citrus spp. against food-related microorganisms, Songklanakarin J. Sci. Technol., 2008, 30, 125–131 Search PubMed.
- N. G. Tzortzakis, Impact of cinnamon oil-enrichment on microbial spoilage of fresh produce, Innovative Food Sci. Emerging Technol., 2009, 10, 97–102 CrossRef CAS.
- D. E. Conner and L. R. Beuchat, Effect of esential oils from plants on growth of food spoilage yeasts, J. Food Sci., 1984, 49, 429–434 CrossRef.
- K. M. Soliman and R. I. Badeaa, Effect of oil extracted from some medicinal plants on different mycotoxigenic fungi, Food Chem. Toxicol., 2002, 40, 1669–1675 CrossRef CAS.
- N. Matan, H. Rimkeeree, A. J. Mawson, P. Chompreeda, V. Haruthaithanasan and M. Parker, Antimicrobial activity of cinnamon and clove oils under modified atmosphere conditions, Int. J. Food Microbiol., 2006, 107, 180–185 CrossRef CAS.
- L. Ranasinghe, B. Jayawardena and K. Abeywickrama, Fungicidal activity of essential oils of Cinnamomum zeylanicum (L.) and Syzygium aromaticum (L.) Merr et L.M. Perry against crown rot and anthracnose pathogens isolated from banana, Lett. Appl. Microbiol., 2002, 35, 208–211 CrossRef CAS.
- L. Ranasinghe, B. Jayawardena and K. Abeywickrama, Use of waste generated from cinnamon bark oil (Cinnamomum zeylanicum Blume) extraction as a post harvest treatment for Embul banana, J Food Agri. Environ., 2003, 1, 340–344 CAS.
- D. Georgantelis, I. Ambrosiadis, P. Katikou, G. Blekas and S. A. Georgakis, Effect of rosemary extract, chitosan and a-tocopherolon microbiological parameters and lipid oxidation of fresh pork sausages stored at 4 °C, Meat Sci., 2007, 76, 172–181 CrossRef CAS.
- S. Bautista-Bañosa, A. N. Hernández-Lauzardoa, M. G. Velázquez-del Vallea, M. Hernández-Lópeza, E. Ait Barkab and E. Bosquez-Molinac, et al., Chitosan as a potential natural compound to control pre and postharvest diseases of horticultural commodities, Crop Prot., 2006, 25, 108–118 CrossRef.
- G. K. Jayaprakasha, P. S. Negi, B. S. Jena and L. J. M. Rao, Antioxidant and antimutagenic activities of Cinnamomum zeylanicum fruit extracts, J. Food Compos. Anal., 2007, 20, 330–336 CrossRef CAS.
- A. Adiguzel, H. Ozer, M. Sokmen, M. Gulluce, A. Sokmen and H. Kilic, et al., Antimicrobial and antioxidant activity of the essential oil and methanol extract of Nepeta cataria, Pol. J. Microbiol., 2009, 58, 69–76 CAS.
- P. J. Chien, F. Sheu and F. H. Yang, Effect of edible chitosan on quality and shelf life of sliced mango fruit, J. Food Eng., 2005, 29, 23–29 Search PubMed.
- L. Odriozola-Serrano, T. Hernández-Jover and O. Martín-Belloso, Comparative evaluation of UV-HPLC methods and reducing agents to determine vitamin C in fruits, Food Chem., 2007, 105, 1151–1158 CrossRef.
- P. McCue, Z. Zheng, J. L. Pinkham and K. Shetty, A model for enhanced pea seedling vigour following low pH and salicylic acid treatments, Process Biochem., 2000, 35, 603–613 CrossRef CAS.
- M. A. M. Galeazzi, V. C. Sgarbieri and S. M. Constantinides, Isolation, purification and physicochemical characterization of poliphenolxidases (PPO) from dwarf variety of banana (Musa cavendishii, L), J. Food Sci., 1981, 46, 150–155 CrossRef CAS.
- J. Liu, S. P. Tian, X. H. Meng and Y. Xu, Effects of chitosan on control of postharvest diseases and physiological responses of tomato fruit, Postharvest Biol. Technol., 2007, 44, 300–306 CrossRef CAS.
- E. A. El-Ghaouth, J. Arul, C. Wilson and N. Benhamou, Ultrastructural and cytochemical aspects of the effect of chitosan on decay of bell pepper fruit, Physiol. Mol. Plant Pathol., 1994, 44, 417–422 CrossRef.
- E. I. Rabea, M. E. T. Badawy, C. V. Stevens, G. Smagghe and W. Steurbaut, Chitosan as antimicrobial agent: applications and mode of action, Biomacromolecules, 2003, 4, 1457–1465 CrossRef CAS.
- S. Y. Wang, P. F. Chen and S. T. Chang, Antifungal activities of essential oils and their constituents from indigenous cinnamon (Cinnamomum osmophloeum) leaves against wood decay fungi, Bioresour. Technol., 2005, 96, 813–818 CrossRef CAS.
- S. T. Chang and S. S. Cheng, Antitermitic activity of leaf essential oils and components from Cinnamomum osmophloeum, J. Agric. Food Chem., 2002, 50, 1389–1392 CrossRef CAS.
- S. Juglal, R. Govinden and B. Odhav, Spice oils for the control of co-occurring mycotoxin-producing fungi, J Food Prot., 2002, 65, 683–687 CAS.
- T. Reglinski, P. A. G. Elmer, J. T. Taylor, F. J. Parry, R. Marsden and P. N. Wood, Suppression of Botrytis bunch rot in Chardonnay grapevines by induction of host resistance and fungal antagonism, Aust. Plant Pathol., 2005, 34, 481–488 CrossRef.
- M. A. Rojas-Graüa, M. S. Tapiab and O. Martín-Bellosoa, Using polysaccharide-based edible coatings to maintain quality of fresh-cut Fuji apples, LWT–Food Sci. Technol., 2008, 41, 139–147 CrossRef.
- A. O. Gill and R. A. Holley, Interactive inhibition of meat spoilage and pathogenic bacteria by lysozyme nisin and EDTA in the presence of nitrite and sodium chloride at 24 degrees C, Int. J. Food Microbiol., 2003, 80, 251–259 CrossRef.
- P. Tripathi and N. K. Dubey, Exploitation of natural products as an alternative strategy to control postharvest fungal rotting of fruit and vegetables, Postharvest Biol. Technol., 2004, 32, 235–245 CrossRef.
- N. V. Yanishlieva, E. M. Marinova, M. H. Gordon and V. G. Raneva, Antioxidant activity and mechanism of action of thymol and carvacrolin two lipid systems, Food Chem., 1999, 64, 59–66 CrossRef CAS.
- M. Valero and M. J. Giner, Effects of antimicrobial components of essential oils on growth of Bacillus cereus INRA L2104 in and the sensory qualities of carrot broth, Int. J. Food Microbiol., 2006, 106, 90–94 CrossRef CAS.
- M. Uyttendaele, K. Neyts, H. Vanderswalmen, E. Notebaert and J. Debevere, Control of Aeromonas on minimally processed vegetables by decontamination with lactic acid, chlorinated water, or thyme essential oil solution, Int. J. Food Microbiol., 2004, 90, 263–271 CrossRef CAS.
- E. A. Baldwin, J. K. Burns, W. Kazokas, J. K. Brecht, R. D. Hagenmaier and R. J. Bender, et al., Effect of two coatings with different permeability characteristics on mango (Mangifera indica L.) ripening during storage, Postharvest Biol. Technol., 1999, 17, 215–226 CrossRef CAS.
- T. M. M. Malundo, E. A. Baldwin, M. G. Moshonas, R. A. Baker and R. L. Shewfelt, Method for the rapid headspace analysis of mango (Mangifera indica L.) homogenate volatile constituents and factors affecting quantitative results, J. Agric. Food Chem., 1997, 45, 2187–2194 CrossRef CAS.
- D. M. Hodges, C. J. Andrews, D. A. Johnson and R. I. Hamilton, Antioxidant compound responses to chilling stress in differentially sensitive inbred maize lines, Physiol. Plant., 1996, 98, 685–692 CrossRef CAS.
- A. D. N. Simões, J. A. Tudela, A. Allende, R. Puschmann and M. I. Gil, Edible coatings containing chitosan and moderate modified atmospheres maintain quality and enhance phytochemicals of carrot sticks, Postharvest Biol. Technol., 2009, 51, 364–370 CrossRef.
- N. A. Abbasi, Z. Iqbal, M. Maqbool and I. A. Hafiz, Postharvest Quality of mango (Mangifera Indica L.) fruits as affected by coating, Pakistan J. Botany, 2009, 41, 343–357 Search PubMed.
- S. N. Hajare, V. S. Dhokane, R. Shashidhar, A. Sharma and J. R. Bandekar, Radiation processing of minimally processed carrot (Daucus carota) and cucumber (Cucumis sativus) to ensure safety: effect on nutritional and sensory quality, J. Food Sci., 2006, 71, 198–203 CrossRef.
- H. P. Song, M. W. Byun, C. Jo, C. H. Lee, K. S. Kim and D. H. Kim, Effect of gamma irradiation on the microbiological, nutritional, and sensory properties of fresh vegetable juice, Food Control, 2006, 23, 372–378 CAS.
- K. Bauer, D. Garbe and H. Surburg, in Common Fragrance and Flavor Materials: Preparation, Properties and Uses, Wiley-VCH, Weinheim, 2001, p. 293 Search PubMed.
- M. Serrano, D. Martinez-Romero, S. Castillo, F. Guillen and D. Valero, The use of natural antifungal compounds improves the beneficial effect of MAP in sweet cherry storage, Innovative Food Sci. Emerging Technol., 2005, 6, 115–123 CrossRef CAS.
- I. Rasooli, Food Preservation–A Biopreservative Approach, Food, 2007, 1, 111–136 Search PubMed.
- N. Benhamou and G. Thériault, Treatment with chitosan enhances resistance of tomato plants to the crown and root pathogen Fusarium oxysporum f. sp. radicislycopersici, Physiol. Mol. Plant Pathol., 1992, 41, 33–52 CrossRef CAS.
- D. E. G. Torres, D. A. P. Mancini, R. P. Torres and J. Mancini-Filho, Antioxidant activity of macambo (Theobroma bicolor L) extracts, Eur. J. Lipid Sci. Technol., 2002, 104, 278–281 CrossRef CAS.
- E. R. Kitazuru, A. V. B. Moreira, J. Mancini-Filho, H. Delincée and A. L. C. H. Villavicencio, Effects of irradiation on natural antioxidants of cinnamon (Cinnamomum zeylanicum N.), Radiat. Phys. Chem., 2004, 71, 37–39 CrossRef.
- P. Li, X. Zhang, Y. Liu, W. Wang and Y. Wu, Preservative Activities of 36 Kinds of Essential oils on Post-harvest Tomatoes, J. Northwest Forestry University, 2008, 23, 156–159 Search PubMed.
- C. B. S. Tong and K. B. Hicks, Sulfated polysaccharides inhibit browning of apple juice and diced apples, J. Agric. Food Chem., 1991, 39, 1719–1722 CrossRef CAS.
- A. J. McEvily, R. Iyengar and W. S. Otwell, Inhibition of enzymatic browning in foods and beverages, Crit. Rev. Food Sci. Nutr., 1992, 32, 253–273 CrossRef CAS.
- D. Zhang and P. C. Quantick, Effects of chitosan coating on enzymatic browning and decay during postharvest storage of litchi (Litchi chinensis Sonn) fruit, Postharvest Biol. Technol., 1997, 12, 195–202 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |