TAS2R38 bitter taste genetics, dietary vitamin C, and both natural and synthetic dietary folic acid predict folate status, a key micronutrient in the pathoaetiology of adenomatous polyps
Mark
Lucock
*a,
Xiaowei
Ng
a,
Lyndell
Boyd
a,
Virginia
Skinner
b,
Ron
Wai
b,
Sa
Tang
a,
Charlotte
Naylor
a,
Zoë
Yates
a,
Jeong-Hwa
Choi
a,
Paul
Roach
a and
Martin
Veysey
b
aSchool of Environmental & Life Sciences, University of Newcastle, PO Box 127, Brush Rd, Ourimbah, NSW 2258, Australia
bTeaching & Research Unit, Northern Sydney Central Coast Health, PO Box 361, Gosford, NSW 2250, Australia. E-mail: Mark.Lucock@newcastle.edu.au; Fax: +61 2 4348 4145; Tel: +61 2 4348 4109
First published on 18th July 2011
Abstract
Taste perception may influence dietary preferences and nutrient intakes contributing to diet-related disease susceptibility. This study examined bitter taste genetics and whether variation in the TAS2R38 gene at three polymorphic loci (A49P, V262A and I296V) could alter dietary and systemic folate levels and dietary vitamin C intake, and whether a nutrigenetic circuit existed that might link bitter taste, folate/antioxidant status and risk for a colonic adenomatous polyp. TAS2R38 diplotype predicted bitter taste (PROP) phenotype (p value <0.00001) and red cell folate status (p = 0.0179) consistent with the diplotype that has the broadest range of bitter perception (AVI/PAV) also possessing the highest average red cell folate value. However, TAS2R38 diplotype did not predict dietary intake of methylfolic acid, pteroylmonoglutamic acid or total folic acid. Neither did it predict dietary intake of vitamin C. Despite this, intake of dietary folate predicts red cell folate with analysis pointing to a key nutrient-nutrient interaction between vitamin C intake and systemic folate status. Analysis of 38 patients with an adenomatous polyp and 164 controls showed that individually, dietary nutrient intake, nutrient status and taste diplotype did not influence polyp risk. However, red cell folate status (in individuals below the population median value) did interact with bitter taste diplotype (AVI/PAV) to predict polyp risk (p = 0.0145). Furthermore, synthetic folic acid (below median intake) was statistically associated with adenoma occurrence (p = 0.0215); individuals with adenomatous polyps had a 1.77× higher intake than controls. Additionally, stepwise regression taking account of all dietary nutrients showed a tight relationship between methylfolic acid (but not pteroylmonoglutamic acid) intake and red cell folate level in those with a low folate status and occurrence of an adenomatous polyp (p = 0.0039). These findings point to a role for folate in the pathoaetiology of adenomatous polyps, with the natural and synthetic vitamers not necessarily having the same biological effect.
Introduction
Human taste can be broken down into five categories: salty, sour, sweet, umami and bitter.1,2 Of these, bitter taste is an important determinant in the rejection of diverse food products.3 In particular, the bitterness of phytoprotectant and folate-rich cruciferous vegetables has repeatedly been linked to their low acceptance.3 As such, variations in taste perception may influence dietary preferences and nutrient intakes. This may contribute to diet-related disease susceptibility. Specifically, it may alter risk for adenomatous polyps/colorectal cancer, a disease spectrum in which the genetics and pathoaetiology are often related to folate metabolism.4–12Humans perceive bitterness as a consequence of signalling mediated by transmembrane G protein-coupled receptors. The 7 genes encoding these proteins are termed TAS2Rs and are co-expressed in taste receptor cells of the palate epithelium and tongue.2,3,13,14 An established marker of genetic variation in bitter taste is the perceived bitterness of 6-n-propylthiouracil (PROP). PROP perception may vary from relatively tasteless (non-tasters) to very bitter (super-tasters). Recent research into bitter taste has focused on genetic variation in the TAS2R38 gene,1 a polymorphic locus known to alter bitter perception. Three single nucleotide polymorphisms (SNPs) occur in this gene;15,16 the two most common haplotypes are designated proline-alanine-valine (PAV) and alanine-valine-isoleucine (AVI), and possess a strong correlation with taste status, with PAV being strongly correlated to taster phenotypes and AVI to non-taster phenotypes.1,15–19 Recent studies show that ability to perceive bitterness as tested with PROP sensitivity is significantly correlated with adenomatous polyp number19 and reduced vegetable consumption.19,20
While studies have independently shown that PROP sensitivity and SNPs in key folate-metabolising enzymes are associated with altered risk of colorectal neoplasms, to date, no study has investigated whether bitter taste perception determines dietary patterns that modify folate status and hence folate nutrient-gene interactions important in the development of adenomas. This study reports on the relationship between three polymorphisms of the TAS2R38 gene (A49P, V262A and I296V) and red cell folate status as a surrogate measure of folate-rich foods. It additionally examines the relationship between bitter taste genetics, and dietary intake of folic acid (natural methylfolic acid and synthetic pteroylmonoglutamic acid) in the occurrence of colonic adenomatous polyp, a recognised antecedent of colon cancer.
Vitamin C, like folate, is largely a plant-based vitamin. It is an antioxidant known to interact synergistically with natural dietary methylfolic acid in the stomach lumen to improve the reduced status and hence bioavailability of this folyl vitamer. Vitamin C however, does not influence synthetic dietary pteroymonoglutamic acid in the same way.21,22 This study therefore additionally explores whether dietary vitamin C influences folate status (nutrient-nutrient effect), and if bitter taste genetics can modify vitamin C intake via altered bitter taste perception and hence food preference. As an important antioxidant and scavenger of free radicals, dietary vitamin C's relationship to occurrence of colonic adenomatous polyp is also examined.
Materials and methods
Subjects
Participants (n = 202; 116♀ and 86♂) were volunteers from a gastroenterology practice (Gosford, NSW) undergoing colonoscopy as a screening protocol for colonic pathology: Subjects were between 40 and 89 years of age at time of colonoscopy (overall mean age 63.2; mean age adenoma group 65.7; mean age control group 62.6); had a complete colonoscopy; were mentally competent to complete the food frequency interview; and did not have a cancer or adenoma diagnosis prior to the date of colonoscopy. Local Human Research Ethics Committee approval was given and informed consent obtained prior to volunteers being recruited into the study. Sampling on this population took place up to the phasing in of mandatory fortification of the Australian diet with pteroylmonoglutamic acid at the end of 2009. Following examination, 38 individuals were found to have an adenomatous polyp.Bitter taste phenotype
Bitter taste perception was assessed by asking each subject to rate the intensity of seven different concentrations of PROP. Separate 10 ml solutions of water, 17, 56, 180, 560, 1800 and 3200 μmol L−1PROP were provided.23 Participants were asked to swirl the full contents of each sample in their mouth for a few seconds and then expectorate into a plastic cup. They then rated the sample ‘bitterness’ by using a labelled magnitude scale (LMS), marking a cross (X) on a 10 cm line (i.e. anywhere on the line from barely detectable to the strongest imaginable based on how bitter they perceived the sample to be).24 After each sample, they rinsed their mouth with water and allowed 3 min to pass before tasting the next sample. Based on the overall average index from all seven LMS measurements, the different taster groups were defined as Super Taster (ST), Medium Taster (MT) and Non Taster (NT) where ST ≥ 5.82 cm; MT = 1.16–5.81 cm; NT ≤ 1.15 cm. These taster group cut-off values were ascertained by adherence to the methodology described by Prescott et al.24 based on the lower and upper 25% of values from the distribution of large data sets as described by Rankin et al.25Bitter taste genetics
Genomic DNA was extracted from whole blood by standard procedures using the QIAamp DNA Blood Mini Kit (QIAGEN, Hilden, Germany). The P49A variant of the TAS2R38 gene was amplified using the polymerase chain reaction (PCR). Forward primer = 5′-CCTTCGTTTTCTTGGTGAATTTTTGGGATGTAGTGAAGAGGCGG-3′ and reverse primer = 5′-AGGTTGGCTTGGTTTGCAATCATC-3′. PCR conditions involved denaturation at 94 °C for 2 min, followed by 35 cycles of denaturation at 90 °C for 30 s, annealing at 64 °C for 45 s, and extension at 72 °C for 45 s with a final extension at 72 °C for 7 min. PCR products were subsequently digested with HaeIII (Promega, Madison, WI, USA).In order to score the remaining two TAS2R38 gene variants, primers were designed using Primer3 (http://frodo.wi.mit.edu/primer3/) to cover both V262A and I296V polymorphic sites as follows: forward primer = 5′-GGAAGGCACATGAGGACAAT-3′ and reverse primer = 5′-GTGGTCGGCTCTTACCTTCA-3′. PCR amplification was carried out in a total volume of 20μl containing 10μl of iQsupermix (Bio-Rad Laboratories, USA), 2μl of each 5 pmol μl−1 primer, 50ng of template genomic DNA, and 3μl of nuclease-free water by using a Bio-Rad iCycler iQ system (Bio-Rad, Hercules, CA). The PCR conditions included denaturation at 94 °C for 5 min, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 60 °C for 30 s, and extension at 72 °C for 30 s with a final extension at 72 °C for 7 min. PCR products were purified using QIAquick PCR purification Kit (QIAGEN, USA) according to the manufacturer's instructions. Sequencing was carried out on an ABI PRISM 377 DNA Sequencer at the commercial SUPAMAC Facility (Supamac, The University of Sydney, NSW, Australia).
Food frequency questionnaire for native and synthetic folic acid and vitamin C
The estimated daily intake of nutrients was assessed by an interviewer administered food frequency questionnaire. The questionnaire was extensive, covering 225 food items and all food groups. Subjects were also asked to provide a list of all supplements they were taking, and were asked about these during the food frequency questionnaire interview.The food frequency questionnaires were analysed using Foodworks™ version 3.02 (Xyris Software, Brisbane, QLD, Australia). This package uses a number of food databases to cover the majority of foods consumed by Australians. These include; AusFoods (brands), Aus Nut (base foods) and the New Zealand Vitamin and Mineral Supplements 1999 databases.
The average daily intake of all vitamers of folic acid included the average daily intake from foods and supplements and is referred to as ‘dietary folate’. Total dietary folate intake (μg per day) was calculated by adding the daily average value from Foodworks™ with any additional amounts from supplemental sources. Synthetic folic acid in the form of pteroylmonoglutamic acid was estimated by adding any folic acid containing vitamin supplements with breakfast cereal sources of pteroylmonoglutamic acid and any other known sources of pteroylmonoglutamic acid, such as Sustagen® powder or fortified drinks/juices. Natural folate as 5-methylH4folic acid was estimated by adding together only natural forms of folate from foods such as fruits, vegetables and grains. Note: All samples were collected prior to the mandatory folate fortification of bread flour, which started on 19th September 2009 in Australia.
This methodology has been published previously,26 and has been shown to be valid from the point of view of folic acid intake. Not only is intake (natural and synthetic folic acid) highly correlated to plasma and red cell folate [see ref. 26 and the results section below], but the intake values shown in the results section match typical norms for Australian consumers. This is evident by comparing intake by gender for the present study with that reported for the same gender for the years 1992–1994, 1997–1999 and 2002–2004 as reported by Flood et al.27 Our data for 2007–2009* fits the curvi-linear increase in intake described by this study. Mean values being 325, 384, 403 and 433* DFE (dietary folate equivalents) respectively. A similarity between the dietary vitamin C levels in the present study and published values is also evident.28
The Foodworks™ database package was used for estimation of vitamin C intake. Three sources for vitamin C intake were measured to give a total amount per day; neutraceuticals, fruit juices and natural sources. Each participant's supplementary intake was reviewed for vitamin C content, and included for example, multivitamins and iron and vitamin C tablets.29 As Foodworks™ only measures the natural (endogenous) form of vitamin C in foods, and since all commercial fruit juices contain added (exogenous) ascorbic acid, where participants had noted specific fruit juice brands in their food frequency questionnaire, a separate vitamin C measurement for these fruit juices was calculated. As a consequence of this, for each participant, a total vitamin C intake value (exogenous plus endogenous), a total neutraceutical intake value and a total commercial fruit juice intake value was calculated. The exogenous value was subtracted from the total (exogenous plus endogenous) value in order to calculate the vitamin C intake from natural sources.
Folate assay
Serum and red cell folate were measured using a chemiluminescent immunoassay (Access Immunoassay System, Beckman Instruments, Inc). The laboratory normal reference range was 370–1050 nmol L−1 for red cell folate and 5–21 nmol L−1 for serum folate. With increased discretionary use of folic acid in supplemental form over recent years, an increasing number of individuals present with blood folate values at or above the upper calibration range of 2500 nmol L−1 for red cell folate and 45 nmol L−1 for serum folate. Therefore, where appropriate, data was analysed excluding individuals with these extraordinarily high folate values as it would not be possible to achieve these levels through consumption of natural sources of the vitamin, and also because an accurate value above the calibration range was not available.Statistics
All statistical analysis was performed using the JMP program for Windows (version 8.0; SAS Institute Inc., Cary, NC, U.S.A). Relationships between key variables and related parameters were analysed by either standard least squares, stepwise or ordinal/nominal logistic regression analysis as appropriate. Stepwise regression was performed in a mixed direction with significant probability [0.250] for a parameter to be considered as a forward step and entered into the model or considered as a backward step and removed from the model. Mallow's Cp criterion was used for selecting the model where Cp first approaches p variables. For ordinal/nominal data, logistic regression analysis which fits the cumulative response probabilities to the logistic distribution function of a linear model using maximum likelihood has been used. Outcomes have been assessed by the Wald χ2 test p value as a significance indicator for screening effects (p < 0.05 being considered significant). Descriptive statistics have been calculated with data tabulated and presented as appropriate.Results
The relationship between TAS2R38 gene variants and bitter taste phenotype is shown in both tabular and diagrammatic form. Fig. 1 clearly shows how the three main diplotypes AVI/AVI, AVI/PAV and PAV/PAV, representing 92.3% of all subjects, modify bitter taste phenotype. Table 1 provides the number and percentage of individuals with each of the three individual variants (A49P, V262A and I296V), while Table 2 gives the same data for all six possible diplotypes, including the three rarer ones (AAI/AVI, AAV/AVI and AAV/PAV). Nominal logistic regression analysis demonstrated that when examined individually, P49A, I296V and V262A variants, and taste diplotype all predicted bitter taste phenotype (in all cases Wald χ2 test p value <0.00001). The analysis was repeated excluding the three rare diplotypes to ensure they did not unduly influence the statistical outcome. When this was done, taste diplotype still strongly predicted bitter taste phenotype (Wald χ2 test p value <0.00001). The data is therefore consistent with the two commonest haplotypes (PAV and AVI) possessing the strongest correlation with taste status, such that PAV is most strongly correlated to taster phenotype and AVI to non-taster phenotype.1,15–19 It is also consistent with I296V and V262A variants of the TAS2R38 gene being in total linkage disequilibrium as previously reported.30 Although the minor diplotypes AA*/PAV and AA*/AVI are considered to have intermediate and low sensitivity respectively, their observed phenotypic distribution (%) makes it problematic to accurately coalesce diplotypes for a simpler statistical assessment. For example, on an a priori basis, AA*/PAV might best fit with AVI/PAV, yet the % of super tasters in AA*/PAV is even higher than in the PAV/PAV diplotype. For this reason we have kept these fundamental genetic subcategories separate.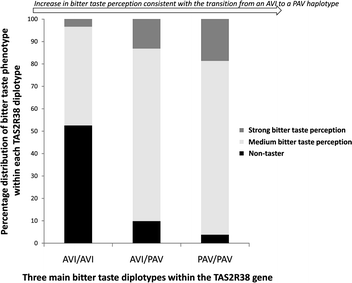 | ||
| Fig. 1 The three major TAS2R38 diplotypes; AVI/AVI, AVI/PAV, PAV/PAV, which represent 92.3% of all subjects, clearly modify bitter taste phenotype (p < 0.0001). | ||
| Number and percentage of individuals within each genotype for the three TAS2R38 gene variants according to PROP sensitivity | |||
|---|---|---|---|
| Bitter taste phenotype | P49A Variant | ||
| AA | AP | PP | |
| Non taster | 37 (53) | 8 (8) | 1 (4) |
| Medium taster | 30 (43) | 77 (79) | 21 (78) |
| Super taster | 3 (4) | 13 (13) | 5 (19) |
| Total | 70 | 98 | 27 |
| Bitter taste phenotype | I296V Variant | ||
|---|---|---|---|
| II | IV | VV | |
| Non taster | 33 (53) | 12 (12) | 1 (3) |
| Medium taster | 27 (44) | 73 (75) | 28 (78) |
| Super taster | 2 (3) | 12 (12) | 7 (19) |
| Total | 62 | 97 | 36 |
| Bitter taste phenotype | V262A Variant | ||
|---|---|---|---|
| VV | VA | AA | |
| Non taster | 32 (52) | 13 (13) | 1 (3) |
| Medium taster | 27 (44) | 73 (74) | 28 (78) |
| Super taster | 2 (3) | 12 (12) | 7 (19) |
| Total | 61 | 98 | 36 |
| Number and percentage of individuals for each of the six TAS2R38 diplotypes | ||||||
|---|---|---|---|---|---|---|
| Phenotype/Diplotype | AAI/AVI | AAV/AVI | AAV/PAV | AVI/AVI | AVI/PAV | PAV/PAV |
| Non taster | 1 (100) | 3 (60) | 0 (0) | 32 (52) | 9 (10) | 1 (4) |
| Medium taster | 0 (0) | 2 (40) | 7(78) | 27 (44) | 71 (77) | 21 (78) |
| Super taster | 0 (0) | 0 (0) | 2 (22) | 2 (3) | 12 (13) | 5 (19) |
| Total | 1 | 5 | 9 | 61 | 92 | 27 |
Fig. 2 shows the mean red cell folate concentration for each of the three main diplotypes (AVI/AVI, AVI/PAV and PAV/PAV). Clearly, the diplotype with the broadest range of bitter perception (AVI/PAV) has the highest average red cell folate value. These values are presented in Table 3 along with the standard error of the mean (SEM). Standard least squares regression shows that taste diplotype (taking account of all six haplotype combinations) predicts red cell folate; p = 0.0179 (r2 = 0.0700). Similarly, taste diplotype predicts serum folate; p = 0.0267 (r2 = 0.0678). However, taste diplotype does not predict dietary intake of folic acid (methylfolic acid, pteroylmonoglutamic acid or total folic acid), which in all cases gave p > 0.05. Neither does taste diplotype predict dietary intake of vitamin C (natural, supplemental sources, or total). Table 4 provides the mean and SEM for intake of methylfolic acid, pteroylmonoglutamic acid and total vitamin C.
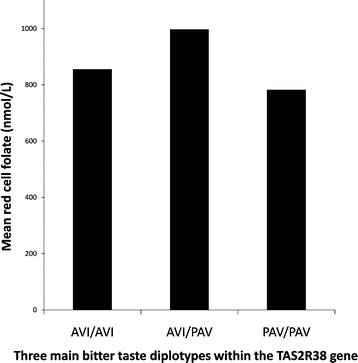 | ||
| Fig. 2 Mean red cell folate concentration for each of the three main TAS2R38 diplotypes showing that the diplotype with the widest range of bitter taste perception (AVI/PAV) has the highest average red cell folate value. Regression analysis shows taste diplotype significantly predicts red cell folate concentration (p = 0.0179). | ||
| Mean and SEM for red cell and serum folate within each of the six TAS2R38 diplotypes | ||||||
|---|---|---|---|---|---|---|
| Minor taste diplotypes | Major taste diplotypes | |||||
| Folate/Diplotype | AAI/AVI | AAV/AVI | AAV/PAV | AVI/AVI | AVI/PAV | PAV/PAV |
| n/a implies n = 1. | ||||||
| Serum folate (nmol L−1) | 6.0 (n/a) | 28 (5.0) | 19 (2.9) | 22 (1.4) | 18 (1.1) | 19.0 (1.6) |
| Red cell folate (nmol L−1) | 2022 (n/a) | 947 (147) | 1024 (176) | 856 (52) | 997 (48) | 782 (68) |
| Mean and SEM for intake of methylfolic acid, pteroylmonoglutamic acid, total folic acid and vitamin C within each of the six TAS2R38 diplotypes | |||||||
|---|---|---|---|---|---|---|---|
| Minor taste diplotypes | Major taste diplotypes | ||||||
| Nutrient/Diplotype | AAI/AVI | AAV/AVI | AAV/PAV | AVI/AVI | AVI/PAV | PAV/PAV | All subjects |
| n/a implies n = 1. | |||||||
| Methylfolic acid (μg/d) | 240 (n/a) | 398 (40) | 275 (16) | 331 (15) | 321 (12) | 316 (18) | 323 (8) |
| Pteroylmonoglutamic acid (μg/d) | 150 (n/a) | 137 (77) | 103 (52) | 152 (21) | 144 (26) | 167 (29) | 148 (15) |
| Total folic acid (μg/d) | 390 (n/a) | 535 (84) | 378 (49) | 483 (30) | 465 (28) | 483 (33) | 470 (17) |
| Vitamin C (mg/d) | 468 (n/a) | 405 (95) | 176 (26) | 259 (37) | 237 (16) | 263 (50) | 251 (16) |
Although bitter taste genetics did not influence vitamin C intake, a major question based on well established studies showing vitamin C is critical for dietary methylfolate stability and hence bioavailability21,22 justifies an examination of whether dietary vitamin C can influence blood folate status (nutrient-nutrient effect). When total vitamin C was examined to see if it could predict total red cell folate or serum folate, standard least squares regression gave a p value of 0.0630 (r2 = 0.0182) and 0.0012 (r2 = 0.0567) respectively, suggesting a key nutrient-nutrient interaction did indeed exist. This raises the interesting question of the extent to which vitamin C intake contributes to a given systemic folate level, and is one that certainly merits further study. As might be expected, both dietary intake of methylfolic acid and pteroylmonoglutamic acid showed a strong association with red cell folate status (p = 0.0035; r2 = 0.0437 and 0.0004; r2 = 0.0629 respectively) and with serum folate (p = 0.0286; r2 = 0.0259 and <0.0001; r2 = 0.0925 respectively).
The remaining results focus on the relationship between the TAS2R38 bitter taste gene, folate intake and status, and dietary vitamin C in the context of colonic adenomatous polyp aetiology. Of the 202 individuals examined, 38 had a diagnosis of adenomatous polyp, leaving 164 controls. Nominal logistic regression showed that neither age or gender, and no form of dietary folic acid (methylfolic acid, pteroylmonoglutamic acid, total folic acid) or dietary vitamin C predicted occurrence of an adenomatous polyp (in all cases Wald χ2 test p value >0.05). The same non-significant outcome was achieved when red cell and serum folate, and bitter taste diplotype were individually examined as potential predictors of adenomatous polyp occurrence. However, when data was subdivided according to folate status (i.e. above and below the median red cell folate level of 848 nmol L−1), certain relationships became significant. Individuals with a low red cell folate (below the median value) were examined to see if bitter taste diplotype could influence the relationship between red cell folate level and adenoma risk. Nominal logistic regression showed that red cell folate predicted adenomatous polyp risk only within the AVI/PAV diplotype: Wald χ2 test p value = 0.0145; r2 = 0.2593 (Effect Likelihood ratio test p = 0.0028). With age and gender adjustment, this result remains significant (Wald χ2 test p = 0.0246). Within this low folate status-AVI/PAV group, adenomatous polyp patients had a lower red cell folate value (454.8 nmol L−1) than control subjects (647.1 nmol L−1). This diplotype has the broadest range of bitter taste perception and represents the largest cohort with 92 individuals altogether, of which 42 exhibited a folate status below the population median value. This significant relationship between red cell folate status and adenomatous polyp occurrence was not maintained in the AVI/PAV diplotype when subjects with a red cell folate status above the median value were examined (Wald χ2 test p value = 0.6262), or when all red cell folate values were examined (Wald χ2 test p value = 0.9192).
While red cell folate acts as a surrogate for overall folate status, the same is not true for serum folate which is a far more dynamic index and a less suitable marker of this parameter. Nominal logistic regression showed that serum folate did not predict adenomatous polyp risk when examined by taste diplotype. The same is true for dietary vitamin C and dietary folic acid (methylfolic acid, pteroylmonoglutamic acid, and total folic acid). In all cases Wald χ2 test p value > 0.05.
Given the well reported link between dietary folic acid and neoplastic growth in the large bowel,4–12 we subdivided data according to methylfolic acid intake (i.e. above and below the median intake of 319 μg day−1) and examined the relationship with adenoma risk (with and without taste diplotype taken into account). The same was done for pteroylmonoglutamic acid intake (median intake of 91.3 μg day−1). Neither showed any effect according to taste diplotype, with the only point of significance being that, on its own, pteroylmonoglutamic acid intake did predict adenomatous polyp occurrence in individuals with an intake below the median value (Wald χ2 test p value = 0.0215; r2 = 0.0502. With age and gender adjustment, this result remains significant at p = 0.0381). In this case, it would appear that pteroylmonoglutamic acid intake has a possible negative impact in that on average individuals with polyps had a 1.77× higher intake than those without polyps (37.8 vs. 21.3 μg day−1 respectively).
Basic descriptive data is given in Table 5, with mean and SEM for the major indices measured in subjects with and without an adenomatous polyp. The difference between clinical phenotypes was, in all cases, not significant (Student’s t-test p value >0.05).
| Mean and SEM for for the major indices measured in subjects with and without an adenomatous polyp | ||
|---|---|---|
| Control | Adenomatous polyp | |
| No significant difference between clinical phenotypes (P > 0.05). | ||
| Red cell folate (nmol L−1) | 914 (33) | 990 (87) |
| Serum folate (nmol L−1) | 19.5 (0.8) | 20.2 (1.9) |
| Pteroylmonoglutamic acid (μg/d) | 157 (18) | 108 (19) |
| Methylfolic acid (μg/d) | 323 (9) | 323 (18) |
| Total folic acid (μg/d) | 480 (20) | 431 (28) |
| Vitamin C (mg/d) | 259 (19) | 215 (22) |
Stepwise regression analysis using a model that takes account of dietary methylfolic acid, pteroylmonoglutamic acid and total ascorbic acid suggests that the relationship between intake of methylfolic acid is most significantly linked to red cell folate status for those individuals with an adenomatous polyp and who also have a low folate status (below the median red cell folate level of 848 nmol L−1), while the relationship between intake of pteroylmonoglutamic acid is most significantly linked to red cell folate status for those individuals with no polyp and who are not subdivided according to folate status (p for methylfolic acid= 0.0039 and for pteroylmonoglutamic acid= 0.0019 – see Table 6). What is particularly interesting is that this therefore implies methylfolate may be an important vitamer where folate status tends to be lower, particularly in subjects who have had an adenomatous polyp. While more detailed work is required to support this, it is consistent with the a priori view that supplemental pteroylmonoglutamic acid leads to a typically higher folate status than would occur with natural sources of the vitamin alone.
| Parameter estimate table generated by stepwise regression taking account of dietary methylfolic acid, pteroylmonoglutamic acid and total vitamin C with respect to red cell folate for individuals with or without an adenomatous polyp | ||||||
|---|---|---|---|---|---|---|
| Term | Polyp/no polyp | Folate status | Estimate | t-ratio | p | r2 for whole model |
| Dietary methylfolic acid (μg/d) | Yes | Below median red cell folate | 0.8366 | 3.45 | 0.0039 | 0.5829 |
| Pteroylmonoglutamic acid (μg/d) | Yes | Below median red cell folate | 0.6127 | 2.27 | 0.0394 | — |
| Vitamin C (mg/d) | Yes | Below median red cell folate | — | — | — | — |
| Dietary methylfolic acid (μg/d) | No | All subjects | 0.6399 | 0.29 | 0.0288 | 0.0955 |
| Pteroylmonoglutamic acid (μg/d) | No | All subjects | 0.4542 | 3.15 | 0.0019 | — |
| Vitamin C (mg/d) | No | All subjects | — | — | — | — |
The finding of a tight relationship between methylfolic acid intake and red cell folate level in those with a low folate status and occurrence of an adenomatous polyp, combined with a negative effect of pteroylmonoglutamic acid intake in the same low folate status/polyp group, is a very interesting finding that points to a clear role for folate in the pathoaetiology of adenomatous polyps. However, while pteroylmonoglutamic acid appears to be a concern in this group, little difference exists in the mean level of intake of methylfolic acid within this group suggesting this natural vitamer may not have the same biological effect as the synthetic form of this vitamin (mean methylfolic acid intake for subjects with an adenomatous polyp and those without was 225.7 and 237.2 μg day−1 respectively).
Discussion
These data provide new insight into a nutrigenetic circuit that may well impact on human health. Genetic variation in the TAS2R38 gene,1 a polymorphic locus known to alter bitter taste perception has been shown to align with phenotype as assessed by sensitivity to PROP (p < 0.00001). But more interestingly, the data supports our original hypothesis that bitter taste genetics may alter dietary pattern and hence modify red blood cell folate status. However, while taste diplotype clearly predicts red cell folate level (p = 0.0179), it did not predict intake of dietary methylfolic acid, the vitamer that would in all likelihood have been the one modified by PAV or AVI haplotype. Despite this finding, and consistent with expectation, intake of dietary methylfolic acid strongly predicts red cell folate status (p = 0.0035) suggesting the dietary assessment methodology is sound. We did not expect any effect of bitter taste genetics on pteroylmonoglutamic acid intake, as was found to be the case. The reason for this is that while methylfolic acid is found in plant foods, including many bitter tasting plant food sources, pteroylmonoglutamic acid is artificially supplemented into the diet in a more general fashion. The lack of correlation between dietary methylfolic acid and taste diplotype is likely to stem from this vitamer not being limited to bitter tasting foods, while the direct correlation between bitter taste diplotype and red cell folate may be a consequence of several other interactive factors such as the specific folate vitamer(s) in the food (methyl/formyl/unsubstituted derivatives) and B-vitamin sparing co-nutrients, particularly antioxidant phytoprotectants. Although having said this, taste diplotype did not predict vitamin C intake, a nutrient well recognised as an antioxidant that protects methylfolic acid in the gastric lumen.21,22Therefore, although we have shown that AVI/PAV diplotype, which has the widest range of bitter taste perception, also has the highest folate status, a definitive explanation for this finding remains. It could simply reflect more eclectic eating habits within this diplotype given the broader taste preferences this gene imparts. However, there may be other factors such as unknown gene-gene or gene-nutrient interactions at play. For example, several common polymorphisms of folate metabolism are known to influence folate status and, as a consequence, clinical phenotype.9 This includes variants such as C677T-MTHFR which is often cited as a component in gene-gene interactions.9 Therefore, future research may well be rewarded by looking at how common folate SNPs interact with TAS2R38. Additionally, while antioxidant phytoprotectants like vitamin C have been discussed and examined in this paper, other important nutrient-nutrient interactions involving folate also exist. This is particularly true for vitamins B2, B6 and B12. Interestingly, the metabolic dependence of folate for these three micronutrients implicates an even wider range of dependent genes that exist in variant form. Clearly, folate nutritional genetics are complex, and it would seem that TAS2R38 is a factor in this complexity. Of course, it should also be noted that since the AVI/PAV group was the largest group in the study it was the most likely to show any association with the outcome variables examined. Others have also looked at genetic variation in bitter taste and plasma markers of anti-oxidant status, but not of folate status. Tepper and colleagues31 looked at PROP classification of taster status and blood vitamin C, β-carotene, α-tocopherol, lycopene, uric acid and total peroxyl-trapping antioxidant capacity. The only point of significance was plasma α-tocopherol, which was lower in super-tasters compared with non-tasters. This suggests PROP status does not associate with overall antioxidant status, supportive of our findings relating to vitamin C. The Tepper study does however suggest that the α-tocopherol intake, derived principally from vegetable oils and green vegetables, may be related to PROP status. This finding more clearly supports the idea that PROP tasters, especially super-tasters, are less accepting of cruciferous and other green vegetables, bitter citrus, added fats and chili pepper, than do our results relating to bitter taste genetics and folate status. Nevertheless, the present data is both interesting and relevant.
Given that the two most common haplotypes, proline-alanine-valine (PAV) and alanine-valine-isoleucine (AVI), clearly possess a strong correlation with taste status (PAV being strongly correlated to taster phenotype and AVI to non-taster phenotype1,15–19) it is clear from Fig. 2 that the broadest spectrum of bitter taste perception (AVI/PAV diplotype) leads to the highest systemic folate level, as given by the best indicator of this measure – red cell folate status. Serum folate by comparison is a dynamic measure and as such a poorer index of status. Given this finding, and the well described relationship between dietary intake of folic acid and the occurrence of colonic adenomatous polyps, a recognised antecedent of colon cancer, an obvious question arises: ‘can bitter taste genetics influence the risk for adenomatous polyps, and if so is this mediated viafolate metabolism/status/diet?’
The results presented here should be considered preliminary, as the number of adenomatous polyp patients was quite low at 38 compared to 164 controls. However, analysis does provide some interesting outcomes. Looking at the complete data set, no dietary component, blood index or measure of bitter taste predicted polyp risk. However, when the data was subdivided according to folate status (above/below median value), this changed. A red cell folate status below the median value modified adenomatous polyp risk within the broad spectrum AVI/PAV diplotype (p = 0.0145) such that adenomatous polyp patients had a 30% lower mean red cell folate value than controls. When a similar analysis was performed according to dietary intake of folic acid vitamers (i.e. above/below median intake value), no effect of taste diplotype was found. However, when analysed separately (independent of taste genetics), pteroylmonoglutamic acid intake did predict adenomatous polyp occurrence in individuals with an intake below the median intake value of 91.3 μg day−1 (p = 0.0215). In this case, pteroylmonoglutamic acid seems to confer a possible negative effect as on average, subjects with a polyp had a 1.77x higher intake than those without polyps (37.8 vs. 21.3 μg day−1 respectively). On the face of it, a lower red cell folate and higher intake of synthetic folic acid in adenomatous polyp patients may appear contradictory. However, this may point to a potential differential biological effect between the natural and supplemental forms of the vitamin, a phenomenon that has concerned others.9,32,34–36
There has been mixed reporting over the last few years regarding the health benefits of pteroylmonoglutamic acid supplements,9,32 with the present findings therefore particularly interesting, if not slightly worrying. Clearly there is much to learn about the relationship between folate and colorectal neoplasia. The data here does support such a relationship, and certainly could imply a differential effect between synthetic and natural forms of the vitamin, although this remains to be proven beyond any doubt. Analysed with step-wise regression, the present data does point to a tight relationship between dietary methylfolic acid (but not pteroylmonoglutamic acid) and red cell folate level in subjects with a low (below median) folate status who also have an adenomatous polyp (p = 0.0039). This finding once again, when combined with the seemingly negative effect of dietary pteroylmonoglutamic acid in the same low folate status/polyp group (see paragraph above), is interesting and supportive of a differential role for these two dietary folyl vitamers in the pathology of adenomatous polyps.
To understand how this might be, it is necessary to consider the role of cellular folate in both deoxythymidine monophosphate (dTMP) and methionine biosynthesis. Folate (as the intracellular coenzyme 5,10-methylene-H4folate) is critical for maintaining the structural integrity of DNA; strand breaks occur if folate depletion causes uracil (as dUMP) to be misincorporated into DNA in place of thymine. These strand breaks are a recognised antecedent of malignant transformation. Additionally, folate dependent methionine biosynthesis contributes methyl groups to the methylome which governs gene expression. Some of these epigenetic DNA modifications (epimutations) may be heritable, and some are critical in the pathoaetiology of cancer development.32 Roughly half our methionine requirement is met viafolate metabolism at the level of methylfolate,33 and so dietary intake is clearly important. Interestingly, there has been some concern that synthetic pteroylmonoglutamic acid might act as an antimetabolite at key folate enzymes where the Km for the synthetic analogue is higher than for the natural substrate leading to potential competition in substrate binding. This is particularly true for interactions involving dihydrofolate which has great structural similarity to pteroylmonoglutamate. DHFR and MTHFR enzymes use dihydrofolate as substrate and allosteric ligand respectively, and so may be modulated in an unnatural fashion by pteroylmonoglutamic acid.9,32,34–36 Additionally, synthetic pteroylmonoglutamate also undergoes UV induced molecular scission, yielding a potentially noxious pterin that causes DNA damage in in vitro studies, although methylfolate does not.37
The present data widens the search for nutritional factors that might modulate neoplastic change in colonic tissue, and while supporting a role for folate – possibly one that affords a differential effect by different folyl vitamers, it also introduces a possible new factor – taste genetics. These findings are extremely novel and should be taken account of in future work. The authors recognise the important modulatory role of common folate gene polymorphisms in maintaining folate status and altering risk for specific disease phenotypes9 and the next phase of this research program will examine how taste genes interact with folate status and genetics to modify clinical phenotype.
The significance of variation in bitter taste perception is interesting and may have been especially important to early man in alerting us to the many phytotoxins which tend to have a bitter taste – for example, many β-glucopyranosides exhibit cyanogenic toxicity, and are common plant constituents. It is therefore reasonable to postulate that those humans with an acute sense of bitter taste may have had a better ability to survive in primitive environments.2,3 Signatures of positive selection for bitter taste genes have been detected, and in one case, the origin of this trait to detect harmful cyanogenic glycosides is estimated at 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700–791
700–791![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 years BP. This places it in the Middle Pleistocene before early humans expanded out of Africa.2 Compared to other mammals, humans have attenuated sensory traits, but despite this, preservation of specific sensory functions via positive selection was seemingly still crucial during the earliest stages of human evolution.2 As a putative example, most populations harboring the low sensitivity K172 allele of the bitter tasteTAS2R16 gene are found within areas of Africa in which malaria is endemic. A postulate has been developed that individuals with the low sensitivity K172 allele might be predisposed to consuming more plants rich in bitter tasting alkaloids, and which hence might afford a greater degree of protection against the malaria parasite. If this assumption is correct, any disadvantage of consuming these toxic substances is likely offset by the therapeutic effects of these bitter alkaloids upon malarial parasites.2 Of course, tolerance to bitter compounds would also open up a wider repertoire of foods at times of food stress/shortage.
000 years BP. This places it in the Middle Pleistocene before early humans expanded out of Africa.2 Compared to other mammals, humans have attenuated sensory traits, but despite this, preservation of specific sensory functions via positive selection was seemingly still crucial during the earliest stages of human evolution.2 As a putative example, most populations harboring the low sensitivity K172 allele of the bitter tasteTAS2R16 gene are found within areas of Africa in which malaria is endemic. A postulate has been developed that individuals with the low sensitivity K172 allele might be predisposed to consuming more plants rich in bitter tasting alkaloids, and which hence might afford a greater degree of protection against the malaria parasite. If this assumption is correct, any disadvantage of consuming these toxic substances is likely offset by the therapeutic effects of these bitter alkaloids upon malarial parasites.2 Of course, tolerance to bitter compounds would also open up a wider repertoire of foods at times of food stress/shortage.
A central theme in this paper has been how bitter taste genes affect folate intake and status, and whether this has any bearing on neoplastic change in the colon. However, intake of other potential phytoprotectants will also be modified by bitter taste preferences, and so may also be relevant to this nutrigenetic circuit.
Conclusion
Bitter taste perception significantly modifies red cell folate status, with the diplotype having the broadest range of bitter perception (AVI/PAV) also possessing the highest average red cell folate value. This may be a consequence of altered dietary preferences and nutrient intakes, although this was not proven. A low red cell folate and possession of the AVI/PAV taste diplotype predicts polyp risk with some evidence that natural and synthetic vitamers of dietary folic acid play an important, but possibly differing role in the pathoaetiology of adenomatous polyps. The evidence presented here justifies further research into this nutrigenetic circuit involving TAS2R38, folate-rich foods and disease risk. This data was collected pre-mandatory fortification, but in an environment where discretionary use was quite high. An interesting question is whether post-mandatory fortification data would differ.References
- U. K. Kim and D. Drayna, Clin. Genet., 2004, 67, 275–280 CrossRef.
- N. Soranzo, B. Bufe, P. C. Sabeti, J. F. Wilson, M. E. Weale, R. Marguerie, W. Meyerhof and D. B. Goldstein, Curr. Biol., 2005, 15, 1257–1265 CrossRef CAS.
- A. Drewnowski and C. Gomez-Carneros, Am. J. Clin. Nutr., 2000, 72, 1424–1435 CAS.
- Y. I. Kim, Cancer Epidemiol. Biomarkers Prev., 2004, 13, 511–519 CAS.
- U. B. Fallon, Int. J. Epidemiol., 2003, 32, 67–70 CrossRef.
- J. D. Potter, J. Natl. Cancer Inst., 1999, 91, 916–932 CrossRef CAS.
- M. Lucock, Mol. Genet. Metab., 2000, 71, 121–138 CrossRef CAS.
- S. Sibani, S. Melnyk, I. P. Pogribny, W. Wang, F. Hiou-Tim, L. Deng, J. Trasler, S. J. James and R. Rozen, Carcinogenesis, 2002, 23, 61–65 CrossRef CAS.
- M. Lucock and Z. Yates, Curr. Opin. Clin. Nutr. Metab. Care, 2009, 12, 555–564 CrossRef CAS.
- C. M. Ulrich and J. D. Potter, JAMA, J. Am. Med. Assoc., 2007, 297, 2408–2409 CrossRef CAS.
- E. G. Luebeck, S. H. Moolgavkar, A. Y. Liu, A. Boynton and C. M. Ulrich, Cancer Epidemiol., Biomarkers Prev., 2008, 17, 1360–1367 CrossRef CAS.
- S. Hirsch, H. Sanchez, C. Albala, M. P. de la Maza, G. Barrera, L. Leiva and D. Bunout, Eur. J. Gastroenterol. Hepatol., 2009, 21, 436–439 CrossRef.
- J. Chandrashekar, K. L. Mueller, M. A. Hoon, E. Adler, L. Feng, W. Guo, C. S. Zuker and N. J. Ryba, Cell, 2000, 100, 703–711 CrossRef CAS.
- E. Adler, M. A. Hoon, K. L. Mueller, J. Chandrashekar, N. J. Ryba and C. S. Zuker, Cell, 2000, 100, 693–702 CrossRef CAS.
- S. Wooding, U. K. Kim, M. J. Bamshad, J. Larsen, L. B. Jorde and D. Drayna, Am. J. Hum. Genet., 2004, 74, 637–646 CrossRef CAS.
- U. K. Kim, E. Jorgenson, H. Coon, M. Leppert, N. Risch and D. Drayna, Science, 2003, 299, 1221–1225 CrossRef CAS.
- B. Bufe, P. A. Breslin, C. Kuhn, D. R. Reed, C. D. Tharp, J. P. Slack, U. K. Kim, D. Drayna and W. Meyerhof, Curr. Biol., 2005, 15, 322–327 CrossRef CAS.
- V. B. Duffy, A. C. Davidson, J. R. Kidd, K. K. Kidd, W. C. Speed, A. J. Pakstis, D. R. Reed, D. J. Snyder and L. M. Bartoshuk, Alcohol: Clin. Exp. Res., 2004, 28, 1629–1637 CrossRef CAS.
- M. D. Basson, L. M. Bartoshuk, S. Z. Dichello, L. Panzini, J. M. Weiffenbach and V. B. Duffy, Dig. Dis. Sci., 2005, 50, 483–489 CrossRef CAS.
- M. E. Dinehart, J. E. Hayes, L. M. Bartoshuk, S. L. Lanier and V. B. Duffy, Physiol. Behav., 2006, 87, 304–313 CrossRef CAS.
- M. D. Lucock, M. Priestnall, I. Daskalakis, C. J. Schorah, J. Wild and M. I. Levene, Biochem. Mol. Med., 1995, 55, 43–53 CrossRef CAS.
- X. Ng, M. D. Lucock and M. Veysey, Food Chem., 2008, 106, 200–210 CrossRef CAS.
- J. A. Anliker, L. Bartoshuk, A. M. Ferris and H. D. Hooks, Am. J. Clin. Nutr., 1991, 54, 316–320 CAS.
- J. Prescott, N. Ripandelli and I. Wakeling, Chem. Senses, 2001, 26, 993–1003 CrossRef CAS.
- K. M. Rankin, N. Godinot, C. M. Christensen, B. J. Tepper and S. V. Kirkmeyer, in Genetic Variation in Taste Sensitivity, ed. J. Prescott and B. J. Tepper, 2004, pp. 63–88. Marcel Dekker, New York Search PubMed.
- L. Dufficy, N. Naumovski, X. Ng, B. Blades, Z. Yates, C. Travers, P. Lewis, J. Sturm, M. Veysey, P. Roach and M. Lucock, Life Sci., 2006, 79, 957–966 CrossRef CAS.
- V. M. Flood, G. Burlutsky, K. L. Webb, J. J. Wang, W. T. Smith and P. Mitchell, Eur. J. Clin. Nutr., 2010, 64, 603–613 CrossRef CAS.
- A. G. Tan, P. Mitchell, V. M. Flood, G. Burlutsky, E. Rochtchina, R. G. Cumming and J. J. Wang, Am. J. Clin. Nutr., 2008, 87, 1899–1905 CAS.
- J. E. Castelao, J. M. Yuan, M. Gago-Dominguez, P. L. Skipper, S. R. Tannenbaum, K. K. Chan, M. A. Watson, D. A. Bell, G. A. Coetzee, R. K. Ross and M. C. Yu, Int. J. Cancer, 2004, 110, 417–423 CrossRef CAS.
- N. J. Timpson, J. Heron, I. N. Day, S. M. Ring, L. M. Bartoshuk, J. Horwood, P. Emmett and G. Davey-Smith, BMC Genet., 2007, 8, 51 CrossRef.
- B. J. Tepper, T. Z. Williams, J. R. Burgess, C. J. Antalis and R. D. Mattes, Int. J. Food Sci. Nutr., 2009, 60, 35–45 CrossRef CAS.
- D. A. Smith, Y. I. Kim and H. Refsum, Am. J. Clin. Nutr., 2008, 87, 517–533 Search PubMed.
- M. D. Lucock, Curr. Opin. Clin. Nutr. Metab. Care, 2006, 9, 748–756 CrossRef CAS.
- M. Lucock, Br. Med. J., 2004, 328, 211–214 CrossRef CAS.
- R. G. Matthews and B. J. Haywood, Biochemistry, 1979, 18, 4845–4851 CrossRef CAS.
- M. D. Lucock, eBMJ, 1999, electronic response 23 July 1999, http://www.bmj.com/cgi/eletters/319/7202/92.
- T. Offer, B. N. Ames, S. W. Bailey, E. A. Sabens, M. Nozawa and J. E. Ayling, FASEB J., 2007, 21, 2101–2107 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
