Effects of O-methylated metabolites of quercetin on oxidative stress, thermotolerance, lifespan and bioavailability on Caenorhabditis elegans†
Felipe
Surco-Laos
a,
Juan
Cabello
b,
Eva
Gómez-Orte
b,
Susana
González-Manzano
a,
Ana M.
González-Paramás
a,
Celestino
Santos-Buelga
*a and
Montserrat
Dueñas
a
aGrupo de Investigación en Polifenoles, Unidad de Nutrición y Bromatología, Facultad de Farmacia, Universidad de Salamanca, Campus Miguel de Unamuno, 37007, Salamanca, Spain. E-mail: csb@usal.es; Fax: +00 34 923294515; Tel: +00 34 923294537
bCenter for Biomedical Research of La Rioja (CIBIR), Logroño, Spain
First published on 20th July 2011
Abstract
Quercetin is a major flavonoid in the human diet and the most commonly used in studies of biological activity. Most of the knowledge about its biological effects has originated from in vitro studies while in vivo data are scarce. Quercetin mostly occurs in foodstuffs as glycosides that are deglycosylated during absorption and further submitted to different conjugation reactions. Methylation to isorhamnetin (quercetin 3′-O-methylether) or tamarixetin (quercetin 4′-O-methylether) seems to be an important conjugation process in quercetin metabolism. In this work, the effects of quercetin and its 3′- and 4′-O-methylated metabolites on the phenotypic characteristics, stress oxidative resistance, thermotolerance and lifespan of the model organism Caenorhabditis elegans have been assessed. The three assayed flavonols significantly prolonged the lifespan of this nematode with an increase from 11% to 16% in the mean lifespan with respect to controls. However, only quercetin significantly increased the reproductive capacity of the worm and enlarged the body size. Exposure to the assayed flavonols also increased significantly the resistance against thermal and juglone-induced oxidative stress, although differences were found depending on the stage of development of the worm. Thus, quercetin offered greater protection when thermal stress was applied in the 1st day of adulthood, whereas tamarixetin was more efficient in worms submitted to stress in the 6th day of adulthood. Similarly, significantly greater protection was provided by quercetin than by its methylated derivatives at the 1st day of adulthood, whilst quercetin and isorhamnetin were equally efficient when the oxidative stress was induced in the 6th of day of adulthood. Further evidence of antioxidant protection was obtained checking the oxidation status of proteins by the OxyBlot™ detection kit. Analyses by HPLC-DAD-ESI/MS confirmed that the three flavonols were taken up by C. elegans leading to the formation of some glycosylated, sulfated and methylated metabolites, and that demethylation of these latter to quercetin was also produced. Quantification of the levels of quercetin, isorhamnetin and tamarixetin, as well as their detected metabolites indicated a greater uptake of quercetin than its methylated derivatives by the nematode.
1. Introduction
A large number of epidemiological studies have demonstrated that the intake of dietary flavonoids is inversely associated with the risk of coronary heart disease, cancer and immuno disfunctions.1–4Quercetin is the main flavonol in the human diet and the most commonly used in studies of biological activity. Much attention has been paid to its antioxidant and free radical scavenging properties5 and biological activities, such as antithrombotic and anti-carcinogenic activities.6 Also quercetin is an effective inhibitor of xanthine oxidase and lipoxygenase, enzymes involved in processes such as inflammation, atherosclerosis, cancer and ageing.7,8 Most of the knowledge about quercetin has originated from in vitro studies while in vivo data that take into consideration the complex interplay of diverse processes like uptake, metabolism and organ and tissue interactions within a whole animal are more limited.9 In order to overcome this limitation and to gain additional knowledge about the effects of flavonoids in an in vivo model, some studies have been carried out with the nematode Caenorhabditis elegans, especially with respect to stress resistance and ageing.10–19 Beside others, the reasons for using C. elegans as an in vivo model are the ease of handling, its well-defined cell lineages and development stages that make it an attractive model animal to identify physiological roles of chemicals, and the fact of having its complete genome sequence and the vast genetic information about it.20 Furthermore, the multicellularity of this organism with the presence of tissue and organ systems raises the possibility to consider the metabolism of compounds. The worms exhibit many behavioural phenotypes including egg laying, locomotion, chemotaxis, stress response etc., which are well employed in developmental biology, genetics and neurobiology studies.12 In past decades, many key discoveries with relevance for mammals have been made in C. elegans. This was possible because there is a strong correlation between C. elegans and mammals in cellular and molecular principles and for 60–80% of the human genes homologues have been identified in C. elegans.20,21Studies with blueberry and Ginkgo biloba extracts rich in polyphenols have provided evidences that C. elegans responds upon exposure to these extracts with increased stress oxidative and thermal resistance and even extended lifespan in wild type worms.10,11,13,22,23 It has been observed that thermal stress increased reactive oxygen species (ROS) accumulation in worms in a time dependent manner, which suggests that an important component of the toxicity of heat could be the elevated generation of ROS.14Flavonols such as quercetin, kaempferol, fisetin and rutin reduced the intracellular ROS accumulation at thermal stress.14,15Quercetin shows higher antioxidant activity than other flavonols, which may explain its greater protective effects against lethal thermal stress and the reduction in accumulation of the ageing marker lipofuscin,14,16 a pigment resulting from oxidative degeneration of cellular components and also correlated with the process of ageing in C. elegans.24,25 Other results suggested that in addition to these beneficial effects, quercetin influences the expression of the phase II metabolism enzyme glutathione S-transferase GST-4, a marker for intracellular oxidative stress, and also affects cellular signalling.14 Various studies have shown that the protective and life prolonging action of quercetin in C. elegans was not only due to its strong antioxidant capacity but it may also be mediated by modulation of the FoxO transcription factor DAF-16, which could have a crucial function in several signalling cascades controlling the stress response, the process of ageing and other important biological functions in C. elegans.15–18
The uptake of compounds is an essential prerequisite for the ability to cause systemic effects. However, few studies exist about the metabolism and bioavailability of flavonoids by C. elegans. As far as we know, the only study available concerning quercetin was carried out in liquid medium by Kampkötter et al.,16 who observed that quercetin was taken up by C. elegans although no possible metabolites generated by the worm were detected.
Flavonols like quercetin mostly occur in foodstuffs as glycosides and, in general, in humans the first step in their metabolism is likely to be deglycosylation before absorption in the small intestine.26,27 During transfer across the enterocyte, and, subsequently, in the liver, quercetin undergoes O-methylation and other conjugation reactions, namely glucuronidation and sulfation by phase II enzymes. Although it has never been shown, some of these reactions might also take place in C. elegans, once the major pathways of intermediary metabolism found in heterotrophic organisms are also present in C. elegans,28 and databases such as Wormbase, Reactome and KEGC show that C. elegans possesses orthologs for most of the enzymes involved in the main pathways of intermediary metabolism. Even though the nematode has no distinct adipose tissue or liver, some of their functions might be expressed in other tissues such as the intestine.29 For instance, glycosyltransferases, which function in carbohydrate metabolism, appear to be expressed in the digestive tract of the worm.30,31
Methylation to isorhamnetin (quercetin 3′-O-methylether) or tamarixetin (quercetin 4′-O-methylether) seems to be an important step in quercetin metabolism. Conjugated metabolites are likely to possess different biological properties than parent compounds do. A decrease in the in vitroantioxidant activity of quercetin following methylation of the hydroxyl groups was found in different studies.32–34 However, to our knowledge, no studies exist concerning the effect of methylated metabolites of quercetin on C. elegans.
In the present work, the effects of quercetin and its 3′- and 4′-O-methylethers (Fig. 1) on the phenotypic characteristics, stress oxidative resistance, thermotolerance and lifespan of C. elegans have been assessed. Furthermore, the uptake of the assayed compounds by the worm was confirmed and some metabolites of their biotransformation identified by HPLC-DAD-ESI/MS.
 | ||
| Fig. 1 Structures of quercetin, isorhamnetin and tamarixetin. | ||
2. Experimental
2.1. Standards and reagents
Quercetin was purchased from Sigma-Aldrich (Madrid, Spain). Ampicillin sodium salt, nistatine, agar, yeast extract, juglone and cholesterol were purchased from Sigma-Aldrich (St. Louis, USA). Isorhamnetin, tamarixetin were purchased from Extrasynthèse (Genay, France). HPLC-grade methanol, acetonitrile, dimethyl sulfoxide (DMSO), calcium chloride and magnesium sulfate were purchased from Merck KGaA (Darmstadt, Germany), CarloErba (Rodano, Italy), and Scharlau Chemie (Barcelona, Spain), respectively. Peptone from soybean and tryptone medium were purchased from Fluka (Switzerland).2.2. Strains and maintenance conditions
C. elegans strains wild type N2 were obtained from the Caenorhabditis Genetics Centre at the University Minnesota (Minneapolis, USA). All strains were routinely propagated at 20 °C on nematode growth medium (NGM) plates with Escherichia coli strain OP50 as a food source.35 Some assays were also performed using heat killed bacteria (30 min at 65 °C).36Synchronisation of worm cultures was achieved by treating gravid hermaphrodites with bleach (12% sodium hypochlorite). The eggs were incubated in buffer M9 (3 g KH2PO4, 6 g Na2HPO4, 5 g NaCl, 1 mL 1M MgSO4, H2O to 1 L) for one day up to the embryos hatched at the L1 stage, and were then transferred to NGM plates. For the assays in the presence of the different assayed flavonols, stock solutions (200 mM) of each compound were prepared in DMSO and incorporated into the culture medium to a final concentration of 200 μM. Since the studied flavonols tended to partially precipitate during the preparation of the plates, these were placed on an ice bath so quick solidification of the agar was produced, which avoided compound separation. NGM plates containing DMSO at the same final concentration as the one used in the assays with flavonols (i.e., 0.2% DMSO, v/v) were also prepared and used as control assays.
2.3. Lifespan assay
Age synchronized young larvae (L1) were transferred to NGM agar plates (Ø 96 mm) containing quercetin, isorhamnetin or tamarixetin (200 μM) and grown at 20 °C, the optimal culture temperature for C. elegans. Once the worms reached the last larval stage (L4) they were transferred with a platinum wire to new treatment plates (Ø 35 mm). This moment was considered as the first time point for the counting of surviving worms. Fifteen animals were transferred per plate. Assays for each compound were performed in at least 100 nematodes. For six consecutive days until the cessation of egg laying the worms were transferred daily to avoid overlapping generations; further the worms were transferred every two days. Worms were scored as dead if they did not respond to touch stimulus with the platinum wire. Three independent assays were carried out with each flavonol.2.4. Body length measurement
Synchronized young larvae (L1) were transferred to treatment plates and grown at 20 °C for 6 days to adulthood. Nematode length was determined from about 10 individuals per trial by means of a microscope (Leica M205 FA, Germany) equipped with a camera (Leica DFC 420), coupled to an Leica Application Suite V3 data processing software, after killing by heat treatment at 45 °C for 2 h. Each assay was performed at least three times.2.5. Reproduction assays
L4 larvae pre-treated in flavonol containing plates (10 per treatment) were transferred individually to treatment and control plates and moved to each day until reproduction ceased. The offspring of each animal was counted at the L2 or L3 stage to verify that eggs were fertile. Assays for each compound were performed three times.2.6. Assessment of resistance to oxidative stress
Immediately after the bleaching procedure, N2 eggs were incubated in buffer M9 until the hatching of the eggs (L1 stage). L1 larvae were transferred to NGM agar plates containing the different flavonols at a concentration of 200 μM and cultivated to adulthood. Afterwards, adult worms were transferred to NGM agar plates containing 150 μM juglone, a redox cycler that generates an intracellular oxidative stress,37 and cultivated for 24 h at 20 °C. In order to evaluate if the stage of development of the worm had an influence in the resistance against oxidative stress, two different assays were performed, in the first one the animals were transferred to the juglone medium the 1st day of adulthood (young adults), and in the second one the 6th day of adulthood. Control assays in plates prepared with only the solvent (0.2% DMSO) without flavonols, were also performed in parallel. The survival of the worms was monitored every 8 h and after 24 h by touch-provoked movement. Worms that reacted to the mechanical stimulus were scored as alive whereas non-responding worms were considered to be dead. All assays were performed three times using about 100 individuals per treatment and controls.2.7. Assessment of resistance to thermal stress
The survival of the worms at the lethal temperature 35 °C was also evaluated at two stages of development (1st and 6th day of adulthood). L1 nematodes were placed onto treatment and control plates at 20 °C until they reached the first day of adulthood, in which they were switched to 35 °C for 8 h. Besides, a further assay was made with worms that were kept on the flavonol treatments five days more (6th day of adulthood) prior to induce the thermal stress. Afterwards, dead and alive nematodes were counted. Assays were performed with approximately 100 nematodes per treatment.2.8. Uptake of flavonols by C. elegans
After synchronisation, L1 larvae (wild type N2 worms) were incubated for 4–6 days on NGM agar plates supplemented with the different flavonols at a final concentration of 200 μM using either live or dead E. coliOP50 as food source for the worms. Plates prepared with 0.2% DMSO were employed as controls. After the incubation, the worms were washed successively once with water, followed by twice with PBST (PBS + 0.01% Tween 20), once with PBS and at lastly with water. The remaining worm pellet was resuspended in methanol and kept at −20 °C for 12 h. Afterwards, samples were homogenised on a Genius 3 vortex for 1 min, and sonicated for 5 min with a Labsonic 1510 (Braun, Germany). Samples were then centrifuged for 10 min at 13,000 g and 4 °C, the supernatant was collected and the process of extraction repeated three times. The methanolic extracts were combined and dried in a centrifugal concentrator micVac (GeneVac, Ipswich, United Kingdom) The residue was dissolved in 100 μL methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (20
water (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80, v/v) for HPLC-DAD-ESI/MS analysis.
80, v/v) for HPLC-DAD-ESI/MS analysis.
The remaining worm pellet after the extraction process was also collected and submitted to digestion according to Reinhart and Ruvkun38 so as to determine the protein content by the Bradford method.39
2.9. HPLC-DAD-ESI/MS analyses
Analyses were carried out in a Hewlett-Packard 1100 chromatograph (Agilent Technologies, Waldbronn, Germany) with a quaternary pump and a diode array detector (DAD) coupled to an HP Chem Station (rev. A.05.04) data-processing station. An Ascentis ™ RP-Amide 3 μm (2.1 × 150 mm) column thermostatted at 30 °C was used. The solvents used were: (A) 0.1% formic acid, and (B) acetonitrile. A elution gradient was established from 15 to 50% B over 15 min, isocratic 50% B for 10 min, from 50 to 75% B over 3 min, isocratic 75% B for 10 min, and re-equilibration of the column, at a flow rate of 0.2 mL min−1. Double online detection was carried out in the DAD using 370 nm as a preferred wavelength and in a mass spectrometer (MS) connected to an HPLC system via the DAD cell outlet. MS detection was performed in an API 3200 Qtrap (Applied Biosystems, Darmstadt, Germany) equipped with an ESI source and a triple quadrupole-ion trap mass analyzer that was controlled by the Analyst 5.1 software. Zero grade air served as the nebulizer gas (30 psi) and turbo gas for solvent drying (400 °C, 40 psi). Nitrogen served as the curtain (20 psi) and collision gas (medium). The quadrupols were set at unit resolution. The ion spray voltage was set at −4500 V in the negative mode. Precursor ion analysis was employed to detect all the precursor ions that fragment to a common product ion (i.e., m/z 301 corresponding to quercetin). Settings used were: declustering potential (DP) −40 V, entrance potential (EP) −10 V, collision energy (CE) −50 V, and cell exit potential (CXP) −3 V. Enhanced product ion (EPI) mode was further performed in order to obtain the fragmentation pattern of the parent ion(s) of the studied transition in the previous experiment using the following parameters: DP −50 V, EP −6 V, CE −25 V, and collision energy spread (CES) 0 V.Quantitative analysis of the assayed flavonols and detected metabolites was performed from their chromatographic peaks recorded at 370 nm by comparison with calibration curves obtained by injection of increasing concentrations of quercetin, isorhamnetin and tamarixetin. The results were expressed referred to worm protein as μg of compound/mg protein.
2.10. Quantification of mRNAs by real-time PCR
L1 larvae were transferred to NGM agar plates 8 plates, Ø 96 mm) containing quercetin, isorhamnetin or tamarixetin (200 μM) and grown at 20 °C; worms cultivated in plates without flavonols were used as controls. After 6 days they were collected from plates, washed with water three times, frozen with liquid N2 and homogenized in a mortar maintained in liquid N2. Total RNA was isolated from the homogenate using RNA easy kit from Qiagen. Reverse transcription was performed using Transcriptor First Strand cDNA Synthesis Kit (Roche, Mannheim, Germany) and cDNA was produced by random priming. The RT-PCR primers were as follow: let-767, 5′-CGGATACATCACGCATCAAC, 5′-TCTTTCTGAGAGCAGCAGCA; act-1, 5′- CCAGGAATTGCTGATCGTATG, 5′-GGAGAGGGAAGCGAGGATAG; ama-1, 5′-CTGACCCAAAGAACACGGTGA, 5′-TCCAATTCGATCCGAAGAAGC; 18s, 5′-TTCTTCCATGTCCGGGATAG, 5′-CCCCACTCTTCTCGAATCAG; mRNA expression was performed in triplicate in an Applied Biosystem 7300 real time PCR detection system. The dissociation curve was determined to confirm a unique amplification; act-1, ama-1 and 18s were taken as the internal controls. The differences of expression were determined by relative quantification method taking act-1, ama-1 and 18smRNA as the normalizers.2.11. OxyBlot analysis
The oxidation status of proteins was determined using the commercial OxyBlot™ protein oxidation detection kit (Millipore, USA). L1 larvae were transferred to NGM agar plates (8 plates, Ø 96 mm) containing quercetin, isorhamnetin or tamarixetin (200 μM) and grown at 20 °C; worms grown in plates without flavonols were used as controls. After 6 days they were collected from plates and washed with water three times. The worm pellet was resuspended in 2 volumes of ice-cold lysis buffer (150 mM NaCl, 50 mM Tris-HCl pH 8, 1% Nonidet P40 and protease inhibitor cocktail), and the mixture was homogenized on a vortex during 30 min to allow cuticle breakdown. Lysates were centrifuged at 10,000 g for 30 min and the supernatant collected for protein analysis. Protein concentrations were determined by the BCA method (BCA™ Protein Assay Kit, Pierce, Rockford, USA). Afterwards, the derivatization of protein carbonyls with DNPH solution and subsequent detection using the Oxyblot™ protein oxidation detection kit was performed. Assays were carried out in duplicate, using two amounts of proteins: 15.5 and 6.5 μg. The protein was derivatized and separated on SDS polyacrylamide. Following separation, the gels were transferred to a PVDF membrane, probed with the primary and secondary antibodies provided in the kit and developed using enhanced chemiluminescence (ECL Plus Western Blotting Detection System, Amersham™ Buckinghamshire, UK). Membrane was stripped and reprobed using Actin (H-300):sc-10731 antibody (Santa Cruz Biotechnology, inc., CA, USA).2.12. Statistical analysis
The data of the lifespan assays were processed using the Kaplan-Meier survival analysis using the PC software package SPSS (version 13.0; SPSS Inc., Chicago). The p values were calculated by Kaplan-Meier long-rank pair-wise comparison between the control and the treated flavonol groups. Statistical analysis was performed by one-way analysis of variance (ANOVA). Significant differences were assessed with an LSD test (p < 0.05).3. Results
3.1. Preliminary assays
Prior to the studies with C. elegans, the stability of the assayed compounds in the culture media was checked. With this aim, NGM media containing each of the flavonols were prepared, distributed in plates and kept at 20 °C for 30 days. The compounds extracted from the plates with methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (80
water (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, v/v) were analysed every 5 days by HPLC-DAD-ESI/MS. The results obtained showed that the compounds remained stable in the media during the assayed period, and that no significant decrease in their concentrations was produced (neither products of their possible degradation were formed).
20, v/v) were analysed every 5 days by HPLC-DAD-ESI/MS. The results obtained showed that the compounds remained stable in the media during the assayed period, and that no significant decrease in their concentrations was produced (neither products of their possible degradation were formed).
3.2. Effects of isorhamnetin, tamarixetin and quercetin on C. elegans
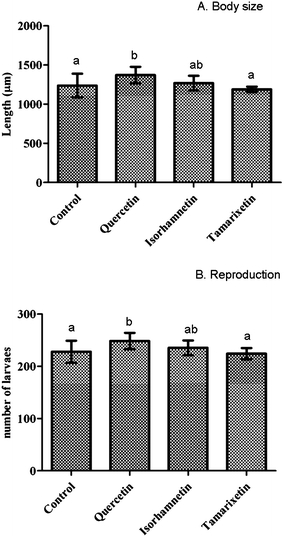 | ||
| Fig. 2 Influence of isorhamnetin, tamarixetin and quercetin on nematodes length (A), and reproduction(B). Results were evaluated at the 6th day of worm adulthood. Assays were performed at a concentration of 200 μM of the assayed flavonols in the culture media. The error bars represent standard deviation (n = 20). Different letters indicate the existence of significant differences (p < 0.05). | ||
Exposure to quercetin (200 μM) slightly but significantly increased (p < 0.05) the reproductive capacity of C. elegans (9%) with respect to the controls, whereas no significant differences in total reproduction output were induced by isorhamnetin and tamarixetin compared to the untreated worms (Fig. 2B). In all cases the reproduction started on the first day of adulthood and was completed on the 4th day.
In order to check if this effect on reproduction may be due to an interference of the estrogenic metabolism, the influence of the studied flavonols on the expression of LET-767, an enzyme that shares the highest homology with human 17-beta-hydroxysteroid dehydrogenases (types 3 and 12), was explored. It has been shown that mutations that inactivate LET-767 affect growth, reproduction and development in C. elegans.40 Quantification of mRNA of LET-767 enzyme by real time PCR was performed using three internal controls (act-1, ama-1 and 18s). The obtained results suggested that quercetin and isorhamnetin could significantly up-regulate the expression of the estrogenic metabolism genelet-767 with respect to worms without treatment when act-1 was used as internal control. However, no relevant differences in the expression of genelet-767 with respect to the untreated worms were observed when the other two internal controls (ama-1 and 18s) were used.
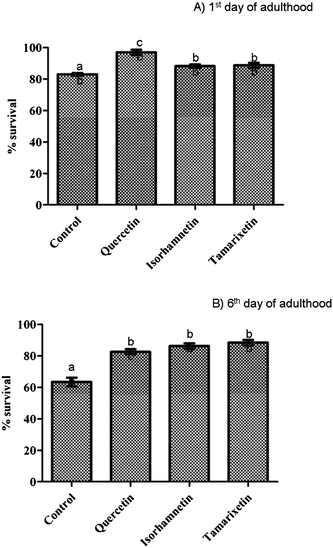 | ||
| Fig. 3 Resistance against thermal stress after pre-treatment with isorhamnetin, tamarixetin or quercetin (200 μM). Results obtained at the 1st day (A), and 6th day of worm adulthood (B). The error bars represent standard deviation (n = 20). Different letters indicate the existence of significant differences (p < 0.05). | ||
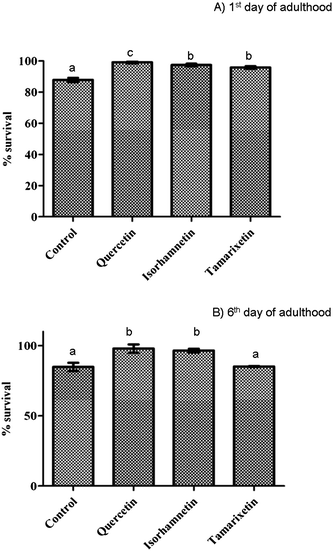 | ||
| Fig. 4 Resistance against juglone-induced oxidative stress after pre-treatment with isorhamnetin, tamarixetin or quercetin (200 μM). Results obtained at the 1st day (A), and 6th day of worm adulthood (B). The error bars represent standard deviation (n = 20). Different letters indicate the existence of significant differences (p < 0.05). | ||
To verify the oxidation status of proteins in C. elegans treated with the different flavonols and controls, an assay using the commercial OxyBlot™ kit was performed. Negative controls for each of the samples were used (proteins that have not been derivatized). Fig. 5 shows that the worms treated with flavonols presented a marked decreased in the oxidation of proteins with respect to untreated animals. Lower density was found in the bands of the worms treated with quercetin, suggesting that it provided greater protection against protein oxidation in the worms than its methylated metabolites.
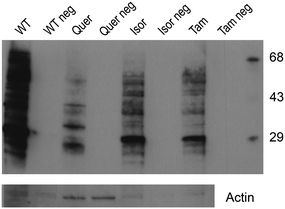 | ||
| Fig. 5 Influence of quercetin, isorhamnetin and tamarixetin in the protein oxidation evaluated by the OxyBlot™ kit. Non-derivatized proteins were used as negative controls for each sample. Membrane was re-probed with actin antibody to allow loading variation. | ||
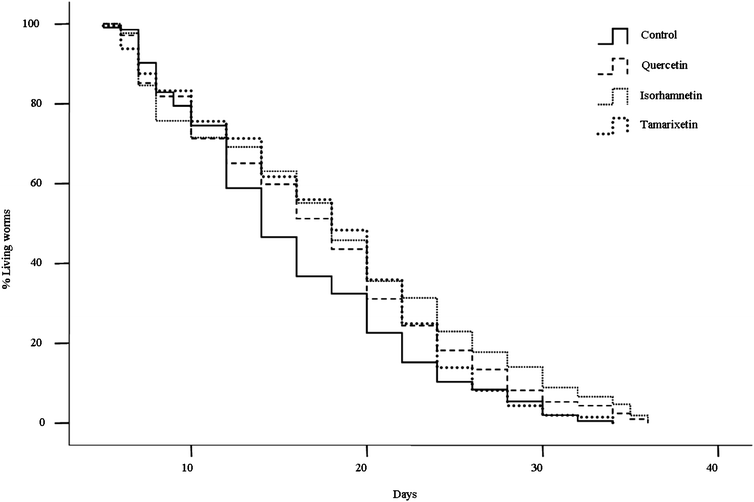 | ||
| Fig. 6 Rates of C. elegans survival obtained in culture media containing 200 μM of isorhamnetin, tamarixetin or quercetin, compared to a control of untreated worms. | ||
| Treatment | Mean (days) | Median (days) | Maximum (days)a | n | p vs. control |
|---|---|---|---|---|---|
| a Maximum lifespan was determined as the mean life span of the longest living 10% of each population. b Mean ± standard deviation (n = 3). Statistical significance was calculated by long-rank testing, changes in mean lifespan are considered significant at p < 0.05. | |||||
| Control | 15.7 ± 0.5 | 14.0 ± 0.6 | 29.3 ± 1.0 | 304 | |
| Quercetin | 17.4 ± 0.5 | 18.0 ± 0.8 | 30.8 ± 2.0 | 309 | 0.008 |
| Isorhamnetin | 18.2 ± 0.6 | 18.0 ± 0.7 | 34.0 ± 0.6 | 314 | 0.000 |
| Tamarixetin | 17.5 ± 0.5 | 18.0 ± 0.7 | 28.7 ± 0.5 | 309 | 0.018 |
3.3. Uptake of quercetin, isorhamnetin and tamarixetin by C. elegans
The uptake of the studied flavonols by C. elegans was assayed in media containing either live or dead E. coliOP50 as a food source for the worms. After 4–6 days of incubation at −20 °C, the worms were collected and homogenised in methanol as described in the experimental section, and the obtained extracts analysed by HPLC-DAD-ESI/MS. In all cases the presence of assayed flavonols and some related metabolites was observed in the chromatograms (Fig. 7), confirming the incorporation of the compounds by the worm. Identical results were obtained in the assays performed with live and dead bacteria, confirming that the latter were not influencing the uptake of the flavonols by the nematodes and that the metabolites observed resulted from biotransformation by C. elegans and not by the bacteria.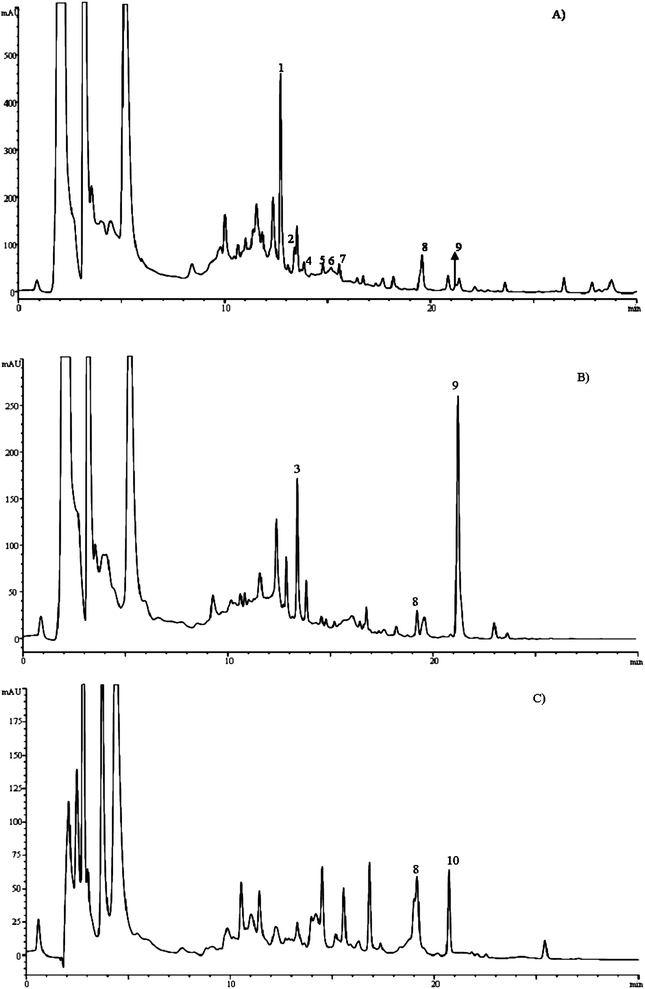 | ||
| Fig. 7 HPLC chromatograms recorded at 370 nm of extracts of C. elegans obtained after incubation of the worms for 5 days in culture media containing quercetin(A), isorhamnetin(B) or tamarixetin(C). Peaks identified as metabolites of the assayed flavonols are marked with numbers. See Table 2 for peak identities. | ||
Table 2 shows the results of the HPLC-DAD-ESI/MS analyses in the assays with the different flavonols and the tentative identification of the metabolites detected. None of the metabolites was observed in the control assays (worms grown in media containing 0.2% DMSO without flavonols), which confirmed that they actually derived from transformation of the assayed flavonols.
In the assays carried out with quercetin (chromatogram in Fig. 7A) eight peaks corresponding to quercetin-related metabolites were unequivocally detected. Peaks 1, 2 and 4 showed λmax at 354 nm and a precursor ion at m/z 463, releasing one fragment ion at m/z 301 (quercetin) by loss of a hexose moiety (−162 amu), indicating that they were quercetin glycosides. Only peak 1 could be fully identified as quercetin 3-O-glucoside by comparison of its retention, UV and mass spectral characteristics with our data library and a commercial standard. Peaks 6, 7 and 8 showed a precursor ion at m/z 543, and a daughter ion at m/z 301 (quercetin), suggesting the loss of one hexose (−162 amu) and one sulfate (−80 amu) moieties. This was confirmed by analysis in enhanced product ion (EPI) mode, where the parent ion (m/z at 543) yielded two fragments at m/z 381 ([M-H-162]−) and at m/z 301 ([M-H-162-80]−). Thus, these compounds were assigned as quercetin sulfate-glycosides. The successive losses of the sugar and sulfate moieties suggest that they were located at different positions on the quercetin, although the precise position of substitution could not be concluded. Peaks 8 and 9 corresponded to quercetin and isorhamnetin, respectively. The formation of this latter also suggests the existence of methyltransferase activity by the nematode.
| Peak | Rt (min) | λ max (nm) | Precursor ion (m/z) | Fragment ions (m/z) | Tentative identification | Assay |
|---|---|---|---|---|---|---|
| a The identity of these compounds was confirmed by comparison of their chromatographic, spectral and MS characteristics with those of real standards. | ||||||
| 1 | 12.7 | 355.2 | 463 | 301 | Quercetin 3-O-glucoside a | Q |
| 2 | 13.2 | 355.0 | 463 | 301 | Quercetin glycoside | Q |
| 3 | 13.3 | 354.0 | 625 | 301 | Quercetin diglycoside | Is |
| 4 | 13.8 | 355.0 | 463 | 301 | Quercetin glycoside | Q |
| 5 | 14.7 | 342.0 | 543 | 381,301 | Quercetin sulfate glycoside | Q |
| 6 | 15.1 | — | 543 | 381,301 | Quercetin sulfate glycoside | Q |
| 7 | 15.5 | 346.8 | 543 | 381,301 | Quercetin sulfate glycoside | Q |
| 8 | 19.5 | 372.0 | 301 | — | Quercetin a | Q, Is, Tm |
| 9 | 21.2 | 370.0 | 315 | 301 | Isorhamnetin a | Q, Is |
| 10 | 21.4 | 370.0 | 315 | 301 | Tamarixetin a | Tm |
In the assays carried out with isorhamnetin and tamarixetin, their presence was also detected in the chromatograms (Fig. 7B and 7C and Table 2), confirming their uptake by the nematode. In both cases, quercetin was also detected in the chromatograms, indicating a demethylating activity by C. elegans. Furthermore, the presence of some glycosyl and sulfate-glycosyl derivatives of these flavonols was also found (see Table 2), confirming that they are metabolized following similar biotransformation pathways as quercetin.
Peak 3, detected in the assays with isorhamnetin, showed a UV spectrum with λmax at 355 nm, similar to quercetin 3-O-glucoside, but eluted at a different retention time. This compound showed a precursor ion at m/z 625, and daughter ion at m/z 301, corresponding to loss of two hexoses moieties (M-H-162-162]− which allowed its tentative identification as a quercetin diglycoside.
In order to compare of the uptake of the different assayed flavonols by C. elegans, they were quantified from their chromatographic peaks and expressed as μg of compound per mg worm protein. More prominent metabolites were also quantified and expressed as quercetin equivalents. The obtained results are shown in Table 3. Although the quantified concentration of quercetin (19.85 μg mg−1protein) was much lower than those of isorhamnetin and tamarixetin (48.80 and 49.85 μg mg−1protein, respectively), the sum of the concentrations of quercetin plus its quantifiable metabolites (89.60 μg mg−1protein) was higher than those of isorhamnetin or tamarixetin plus their metabolites (56.95 or 52.95 μg mg−1protein). These results suggested that greater uptake of quercetin than its methylated derivatives by the nematode exists. As far as we know, the only estimation of the uptake of quercetin by C. elegans was made by Kampkötter et al.,16 although their results cannot be compared with ours, as the assays were carried out in liquid medium and expressed as nmol quercetin per 1000 worm instead of per protein. No quercetin metabolites were analysed in that study.
| Concentration (μg mg−1protein) | Assay | ||
|---|---|---|---|
| Quercetin | Isorhamnetin | Tamarixetin | |
| a nd: not detected. b Expressed as quercetin equivalents. c Mean ± standard deviation (n = 3). | |||
| Quercetin | 19.85 ± 2.05c | 1.95 ± 0.35 | 3.10 ± 0.42 |
| Isorhamnetin | 15.95 ± 2.47 | 48.80 ± 5.83 | nd |
| Tamarixetin | nd | nd | 49.85 ± 1.20 |
| Quercetin 3-O-glucoside b | 33.90 ± 2.83 | — | — |
| Other quercetin glycosidesb | 19.90 ± 2.55 | — | — |
| Quercetin diglycoside b | — | 6.20 ± 1.55 | — |
| Total | 89.60 | 56.95 | 52.95 |
4. Discussion
Quercetin significantly increased reproductive capacity of C. elegans and enlarged the body size, whereas no modification of these characteristics was induced by their methylated derivatives, isorhamnetin and tamarixetin.In a study carried out by Kaempkötter et al.13 with a Ginkgo biloba extract containing tamarixetin as a component, no effects on the reproduction of C. elegans were found, although the worm's body size decreased approximately 17%, which might agree with the slight (not significant) reduction in the worm length observed in our assays with tamarixetin. This effect could be a symptom of caloric restriction mechanisms and/or explained by the possible interaction of the compound with signalling processes that influence the worm's body size.17 Contrary to our observations, no differences in the reproductive output or body length of the nematodes exposed to 100 and 200 μM of quercetin were found by Piestch et al.18 This discrepancy with our results might be explained because the assays of those authors were performed with nematodes of the F1 generation whose parents had been exposed to quercetin.
Quantification of mRNA of let-767gene was performed by real time PCR in order to determine if the effects of quercetin and its methylated metabolites on the increase of the reproductive capacity of the worms may be related to estrogenic metabolism. As above indicated, whereas quercetin and isorhamnetin appeared to up-regulate the expression of the estrogenic metabolism genelet-767 in the worms in the assays using act-1 as an internal control, no clear differences in the expression of this gene with respect to untreated animals were observed in the assays performed using the other two controls (ama-1 and 18s). This discrepancy did not allow us to conclude that differences in the expression of let-767gene were responsible for the increase in the reproductive capacity of worms. Further studies are thus required to verify the mechanisms involved in the apparent increase of fertility in the worms treated with flavonols.
All the assayed flavonols significantly prolonged the lifespan of C. elegans with an increase from 11% to 16% in mean lifespan with respect to controls (17.5 to 18.2 days vs. 15.7 days), being the treatment with isorhamnetin the most effective. Wu et al.10 observed that tamarixetin prolonged the median lifespan of C. elegans by 25%, whereas kaempferol, quercetin or G. biloba extracts did not increase significantly the life of the nematode with respect to controls. An increase of 19% in the duration of C. elegans life was, however, observed by Kampkötter et al.,16 although this result can not be fully compared with ours, since their studies were performed in liquid medium.
Exposure to the three assayed flavonols also significantly increased the resistance of the worms to thermal stress, although differences were found depending on the stage of development. Thus, quercetin offered greater protection (+17% increase in the survival percentages in relation to controls) when thermal stress was applied in the 1st day of adulthood, whereas tamarixetin (+39%) was more efficient in worms submitted to stress in the 6th day of adulthood. The different results obtained at the two stages of development may be explained as due to worm ageing, since stress resistance and life expectancy are generally linked.10 Some studies have been published showing an improvement in thermal resistance of C. elegans by different flavonols such as quercetin, kaempferol, fisetin, and quercetin-3-O-rutinoside,14,15 as well as by Ginkgo biloba13 and blueberry flavonoid-rich extracts.11 It was reported that these compounds reduced the intracellular reactive oxygen species (ROS) accumulation produced by thermal stress, which is likely to be at least partially involved in the death of the worms.13,15 According to our knowledge, no previous studies have been published dealing with the influence of methylated forms of quercetin in thermal resistance of C. elegans, neither the effects at two stage of development have been checked.
The assayed compounds also proved efficient to protect the nematode against juglone-induced oxidative stress. A significantly greater protection was provided by quercetin (increase of around 12% in the survival rate compared to controls) than by its methylated derivatives (+9–10%) at the 1st day of adulthood, whilst quercetin and isorhamnetin were equally efficient (around +15% of survival in both cases), but not in tamarixetin (no significant improvement in relation to the controls), when the oxidative stress was induced in the 6th of day of adulthood. These results are consistent with the previously observed quercetin-mediated increase in oxidative stress resistance of C. elegans in studies carried out in liquid medium.16 Similar observations were also made in assays performed with a Ginkgo biloba extract containing quercetin and tamarixetin.10 Other flavonols such as kaempferol and fisetin were also showed to diminish the extent of oxidative stress in oxidatively challenged worms.15
In order to confirm the existence of an antioxidant protective effect, an OxyBlot analysis was performed to check the oxidation status of proteins in the worms treated with the different flavonols and controls. Carbonyl groups introduced into protein side chains during oxidative modification by ROS and other reactive species are a hallmark of the oxidation status of proteins, and can be analysed by immunodetection using the OxyBlot™ reaction Kit. The results obtained (Fig. 5) confirmed that the exposure to flavonols induced a marked decreased in the oxidation of proteins, which would confirm the antioxidant effect of the studied compounds, particularly higher in the case of quercetin,
The protective effects against thermal and oxidative stress provided by flavonoids have been suggested to be due to their ability to decrease intracellular ROS accumulation together with a parallel down-regulation of catalases.11,13,41 In previous in vitro studies carried out by our group30 it was also found that quercetin and its methylated derivatives behave as better radical scavengers and reducing compounds than usually recognised antioxidants like α-tocopherol. Indeed, flavonoids are widely recognised as antioxidant compounds, although they are also able to act as pro-oxidants in in vivo situations.42 Whereas pro-antioxidant activity is expected to produce toxic effects, in practice light pro-oxidant effects might also be beneficial, since by imposing a mild degree of oxidative stress, the levels of antioxidant defences and xenobiotic-metabolising enzymes might be raised, leading to overall cytoprotection.42,43
The concentrations of flavonols used in the culture media in our study (200 μM) are certainly high when compared with the levels of these compounds that might be found in human plasma and tissues (in the nanomolar to low micromolar range).44,45 However, we do not know the actual extent in which the assayed compounds are incorporated by the worm, which could be quite low and insufficient to cause toxic pro-oxidant effects but rather induce beneficial responses. The mechanisms of the protective effects observed in our studies remain, thus, to be established. Further studies are required to assess the bioavailability of the assayed flavanols in the worm model, as well as to conclude about the cellular and molecular mechanisms involved in observed effects.
With the aim to have a first approach to the bioavailability of flavonols in C. elegans, assays to evaluate their uptake by the worm were also performed. It could be demonstrated that the three studied flavonols were incorporated by the nematode and partially biotransformed to different Phase II conjugated metabolites (i.e., glycosylated and sulfated forms), some of which could be tentatively identified. The existence of glucosyltransferase and sulfotransferase enzymatic activities were described in the worm,46,47 which would explain the formation of these metabolites. In C. elegans, the glycosylation machinery is similar to that in mammals in that nematodes express glycosyltransferase enzymes involved in both N-linked and O-linked glycosylation pathways. The C. elegansgenome contains a total of nine genes that express thirteen glycosyltransferase mRNAs, all of which share significant sequence similarity and predicted structural topology with mammalian ppGaNTases.48Sulfation is catalyzed by the members of the cytosolic sulfotransferase (SULT) enzyme family, which in mammals have been classified into six families. C. elegans has only one SULT homologue in its genome, in contrast to the human genome that contains 17 SULT genes. Hattori et al.49 identified a gene (clone Y113G7A.11) from C. elegans, designed as ceST1 gene that was annotated in WormBase as a cytosolic sulfotransferase, and that was, in fact, the only such SULT homologue in the C. elegansgenome. According to Hattori et al.49 this sulfotransferase either forms part of the defence system against xenobiotics or regulates germ cell proliferation in C. elegans.
The sugar(s) used by the nematode for flavonol conjugation may derive from the hydrolysis of the polysaccharides present in the agar medium, and similarly the sulfate residues could derive from either the potassium sulfate added to the culture medium or the agar that contains sulfated polysaccharides. The identification of isorhamnetin in the assays carried out with quercetin also suggests a methyltransferase activity in the nematode, and the appearance of quercetin in the assays with isorhamnetin and tamarixetin, a demethylating activity.
Quantification of the studied flavonols and their majority metabolites revealed that higher levels of quercetin plus metabolites were present in the worms in worm's organism than those of isorhamnetin or tamarixetin plus their respective metabolites. This observation suggested that greater capacity of uptake of quercetin than of the methylated derivatives by the nematode exists, although quercetin is further biotransformed by C. elegans in a greater extent than isorhamnetin or tamarixetin.
5. Conclusions
The results obtained in this study demonstrated that quercetin, isorhamnetin and tamarixetin increase the resistance of C. elegans against thermal and oxidative stress and are also able to prolong the lifespan of the worm. The results also suggested that lifespan extension might be associated with the increased reproductive capacity of nematode. Although further studies are required in order to verify the mechanism involved in this effect. Analyses by HPLC-DAD-ESI/MS confirmed the uptake of the three studied compounds by the nematode and their biotransformation to different glycosylated, sulfated and methylated metabolites. Quantification of levels of the flavonols and their main metabolites suggested an increased bioavailability of quercetin in the nematode compared to its methylated derivatives.Acknowledgements
Financial support was obtained from the Spanish Ministerio de Ciencia e Innovación through the projects AGL2009-12001 and CSD2007-00063 (Fun-c-Food, Consolider-Ingenio 2010 Programme). Dr J. Cabello was funded by the Spanish Ministry of Science and Innovation (grant BFU2010-21794) and the Riojasalud Foundation. Dr M. Dueñas also thanks the Spanish “Ramón y Cajal” Program for a contract.References
- G. Block, Nutr. Rev., 1992, 50, 207–213 Search PubMed.
- M. G. L. Hertog, E. J. M. Feskens, P. C. H. Hollman and M. B. Katan, Lancet, 1993, 342, 1007–1011 CrossRef CAS.
- P. Knekt, R. Jarvinen, A. Reunanen and J. Maatela, Br. Med. J., 1996, 312, 478–481 CAS.
- L. Yochum, L. H. Kushi, K. Meyer and A. R. Folsom, Am. J. Epidemiol., 1999, 149, 943–949 CAS.
- C. A. Rice-Evans, N. J. Miller and G. Paganga, Free Radical Biol. Med., 1996, 20, 933–956 CrossRef CAS.
- N. C. Cook and S. Samman, J. Nutr. Biochem., 1996, 96, 66–76 CrossRef.
- P. Cos, L. Ying, M. Calomme, J. P. Hu, K. Cimanga, B. Van Poel, L. Pieters, A. J. Vlietinck and D. Vanden Berghe, J. Nat. Prod., 1998, 61, 71–76 CrossRef CAS.
- E. L. da Silva, T. Tsushida and J. Terao, Arch. Biochem. Biophys., 1998, 349, 313–320 CrossRef CAS.
- G. Williamson and C. Manach, Am. J. Clin. Nutr., 2005, 81, 243S–255S CAS.
- Z. Wu, J. V. Smith, V. Paramasivam, P. Butko, I. Khan, J. R. Cypser and Y. Luo, Cell Mol. Biol., 2002, 48, 725–731 CAS.
- M. A. Wilson, B. Shukitt-Hale, W. Kalt, D. K. Ingram, J. A. Joseph and C. A. Wolkow, Aging Cell, 2006, 5, 59–68 CrossRef CAS.
- M. K. Brown, J. L. Evans and Y. Luo, Pharmacol., Biochem. Behav., 2006, 85, 620–628 CrossRef CAS.
- A. Kaempkötter, T. Pielarski, R. Rohring, C. Timpel, Y. Chovolou, W. Watjen and R. Kahl, Pharmacol. Res., 2007, 55, 139–147 CrossRef.
- A. Kaempkötter, C. Gombitang-Nkwonkam, R. F. Zurawski, C. Timpel, Y. Chovolou, W. Watjen and R. Kahl, Toxicology, 2007, 234, 113–123 CrossRef.
- A. Kaempkötter, C. Gombitang-Nkwonkam, R. F. Zurawski, C. Timpel, Y. Chovolou, W. Watjen and R. Kahl, Arch. Toxicol., 2007, 81, 849–858 CrossRef.
- A. Kaempkötter, C. Timpel, R. F. Zurawski, S. Ruhl, Y. Chovolou, P. Proksch and W. Watjen, Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol., 2008, 149, 314–323 CrossRef.
- N. Saul, K. Pietsch, R. Menzel, S. R. Stürzenbaum and C. E. W. Steinberg, Mech. Ageing Dev., 2008, 129, 611–613 CrossRef CAS.
- K. Pietsh, N. Saul, R. Menzel, S. R. Stürzenbaum and C. E. W. Steinberg, Biogerontology, 2009, 10, 565–578 CrossRef.
- L. Zhang, G. Jie, J. Zhang and B. Zhao, Free Radical Biol. Med., 2009, 46, 414–421 CrossRef CAS.
- The C. elegans Sequencing Consortium, Science, 1998, 282, 2012–2018 CrossRef.
- T. Kaletta and M. O. Hengartner, Nat. Rev. Drug Discovery, 2006, 5, 387–399 CrossRef CAS.
- A. Strayer, Z. Wu, Y. Christen, C. D. Link and Y. Luo, FASEB J., 2003, 17, 2305–2307 CAS.
- Z. Cao, Y. Wu, K. Curry, Z. Wu, Y. Christen and Y. Luo, J. Gerontol. A Biol. Sci. Med. Sci., 2007, 62, 1337–1345 Search PubMed.
- D. Garigan, A. L. Hsu, A. G. Fraser, R. S. Kamath, J. Ahringer and C. Kenyon, Genetics, 2002, 161, 1101–1112 CAS.
- B. Gerstbrein, G. Stamatas, N. Kollias and M. Driscoll, Aging Cell, 2005, 4, 127–137 CrossRef CAS.
- J. P. Spencer, G. Chowrimootoo, R. Choudhury, E. S. Debnam, S. K. Srai and C. Rice-Evans, FEBS Lett., 1999, 458, 224–230 CrossRef CAS.
- A. J. Day, Y. Bao, M. R. A. Morgan and G. Williamson, Free Radical Biol. Med., 2000, 29, 1234–1243 CrossRef CAS.
- I. Vastrik, P. D'Eustachio, E. Schmidt, G. Joshi-Tope, G. Gopinath, D. Croft, B. de Bono, M. Gillespie, B. Jassal, S. Lewis, L. Matthews, G. M. Wu, E. Birney and L. Stein, GenomeBiology, 2007, 8, R39 CrossRef.
- S. J. Lee, T. M. Coleen and C. Kenyon, Cell Metab., 2009, 10, 379–391 CrossRef CAS.
- J. S. Griffitts, D. L. Huffman, J. L. Whitacre, B. C. Barrows, L. D. Marroquin, R. Muller, J. R. Brown, T. Hennet, J. D. Esko and R. V. Aroian, J. Biol. Chem., 2003, 278, 45594–45602 CrossRef CAS.
- S. J. McKay, R. Johnsen, J. Khattra, J. Asano, D. L. Baillie, S. Chan, N. Dube, L. Fang, B. Goszczynski, E. Ha, E. Halfnight, R. Hollebakken, P. Huang, K. Hung, V. Jensen, S. J. M. Jones, H. Kai, D. Li, A. Mah, M. Marra, J. McGhee, R. Newbury, R. Pouzyrev, D. L. Riddle, E. Sonnhammer, H. Tian, D. Tu, J. R. Tyson, G. Vatcher, A. Warner, K. Wong, Z. Zhao and D. G. Moerman, Cold Spring Harbor Symp. Quant. Biol., 2004, 68, 159–169.
- D. L. Crawford, R. O. Sinnhuber and H. Aft, J. Food Sci., 1960, 26, 139–145 Search PubMed.
- K. Lemanska, H. Van der Woude, H. Szymusiak, M. B. Boersma, A. Gliszczynska-Swiglo, I. M. C. M. Rietjens and B. Tyrakowska, Free Radical Res., 2004, 38, 639–647 CrossRef CAS.
- M. Dueñas, S. Gonzalez-Manzano, A. M. Gonzalez-Paramas and C. Santos-Buelga, J. Pharm. Biomed. Anal., 2010, 51, 443–449 CrossRef CAS.
- S. Brenner, Genetics, 1974, 77, 71–94 CAS.
- J. Gruber, S. Y. Tang and B. Halliwell, Ann. N. Y. Acad. Sci., 2007, 1100, 530–542 CrossRef CAS.
- E. de Castro, S. H. de Castro and T. E. Johnson, Free Radical Biol. Med., 2004, 37, 139–145 CrossRef CAS.
- B. J. Reinhart and G. Ruvkun, Genetics, 2001, 157, 199–209 CAS.
- M. M. Bradford, Anal. Biochem., 1976, 72, 248–254 CrossRef CAS.
- S. Desnoyers, P. G. Blanchard, J. F. St.-Laurent, S. N. Gagnon, D. L. Baillie and V. Luu-The, J. Endocrinol., 2007, 195, 271–279 Search PubMed.
- J. V. Smith and Y. Luo, J. Alzheimers Dis., 2003, 5, 287–300.
- B. Halliwell, Arch. Biochem. Biophys., 2008, 476, 107–112 CrossRef CAS.
- S. Y. Tang and B. Halliwell, Biochem. Biophys. Res. Commun., 2010, 394, 1–5 CrossRef CAS.
- J. P. E. Spencer, M. M. A. El Mohsen, A. M. Minihane and J. C. Mathers, Brit. J. Nutr., 2008, 99, 12–22 CAS.
- W. M. Loke, A. M. Jenner, J. M. Proudfoot, A. J. McKinley, J. M. Hodgson, B. Halliwell and K. D. Croft, J. Nutr., 2009, 139, 2309–2314 CrossRef CAS.
- T. Sherman, M. N. Chernova, J. S. Clark, L. Jiang, S. L. Alper and K. Nerke, Am. J. Physiol.: Cell Physiol., 2005, 289, C341–351 CrossRef CAS.
- P. M. Berninsone, in WormBook, ed. The C. elegans Research Community, WormBook, 2006, doi/10.1895/wormbook.1.125.1, http://www.wormbook.org Search PubMed.
- F. Hagen, M. Layden, K. Nehrke, K. Gentile, K. Berbach, C. C. Tsao and M. Forsythe, Trends Glycosci. Glycotechnol., 2001, 13, 463–479 Search PubMed.
- K. Hattori, M. Inoue, T. Innoue, H. Arai and H. Tamura, J. Biochem., 2006, 139, 355–362 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c1fo10049a |
| This journal is © The Royal Society of Chemistry 2011 |
