Raspberry juice consumption, oxidative stress and reduction of atherosclerosis risk factors in hypercholesterolemic golden Syrian hamsters†
Jin-Hyang
Suh‡
a,
Cindy
Romain‡
a,
Rocío
González-Barrio
b,
Jean-Paul
Cristol
a,
Pierre-Louis
Teissèdre
c,
Alan
Crozier
b and
Jean-Max
Rouanet
*a
aJoint Research Unit 204 NUTRIPASS, Prevention of Malnutritions & Linked Pathologies, University Montpellier South of France, Jean-Max Rouanet, UMR 204 NUTRIPASS, CC 023, Université Montpellier 2, Place E. Bataillon, 34095, Montpellier Cedex 05, France. E-mail: jm.rouanet@univ-montp2.fr; Fax: (+33) 04 67 14 35 21; Tel: (+33) 04 67 14 35 21
bPlant Products and Human Nutrition Group, Centre for Population and Health Sciences, School of Medicine, College of Medical, Veterinary and Life Sciences, University of Glasgow, Glasgow, G12 8QQ, UK
cUMR 1219 Œnologie, Institut de la Vigne et du Vin de Bordeaux-Aquitaine, Université Victor Ségalen Bordeaux 2, 210 chemin de Leysotte, CS 50008, 33882, Villenave d'Ornon Cedex, France
First published on 13th June 2011
Abstract
The effects of raspberries on early atherosclerosis in Syrian hamsters were investigated using three juices prepared from var. Cardinal, Glen Ample and Tulameen berries. The hamsters received an atherogenic diet for 12 weeks and at the same time a juice at a daily dose corresponding to the consumption of 275 ml by a 70 kg human. A control group received the same diet with water instead juice. The principal polyphenolic compounds in the juices were anthocyanins and ellagitannins, which were present at concentrations of 218–305 μg mL−1 and 45–72 μg mL−1, respectively. The three juices had similar but not identical effects. They all inhibited cardiac and aortic production of superoxide anion and increased hepatic glutathione peroxidase activity although only Tulameen juice brought about a significant increase in superoxide dismutase activity. Glen Ample was the only juice to significantly increase plasma paraoxonase activity. All the juices lowered plasma triglyceride level while consumption of Tulameen and Cardinal, but not Glen Ample, significantly lowered plasma total cholesterol and LDL-cholesterol. Cardinal was the sole juice to significantly increase HDL-cholesterol and likewise it also significantly reduced body weight. These findings suggest that moderate consumption of raspberry juices can help to prevent the development of early atherosclerosis, with the underlying mechanisms related to improved antioxidant status and serum lipid profiles.
1 Introduction
Dietary factors are thought to play a key role in the regulation of in vivo oxidant status. An imbalance between nutrients and, in particular, those involved in antioxidant status, could explain the onset of enhanced production of free radicals. A diet low in antioxidants contributes to the occurrence of an oxidative stress.1 Physiological production of reactive oxygen species (ROS) is regulated by enzymatic defense systems and by dietary antioxidants that keep transition metals in an inactive state thereby inhibiting the production of ROS. Several studies suggest that polymorphic variations in endogenous antioxidants are linked to increased risk for atherosclerosis,2 ROS-induced depletion of antioxidants is a key factor in the initiation of atherosclerosis which is thought to be closely dependent upon an imbalance between ROS generation and the antioxidant capacity of the cell in favor of the former.3The beneficial health effects of fruit consumption have been emphasized by epidemiological studies. Although the evidence is not unequivocal, some studies have shown a relationship between a reduced risk of cardiovascular disease (CVD) and fruit consumption.4,5 The protective effects could be derived from micronutrients and micro-constituents, in particular flavonoids and related phenolic compounds,6 which in general are powerful antioxidants.7
Red raspberries contain substantial amounts of polyphenolic compounds with the principal antioxidants being anthocyanins and the ellagitannins sanguin H-6 and lambertianin C, compounds which in vitro also induce vasorelaxation.8,9 Consumption of raspberry, strawberry and bilberry juices, as well as green and black tea, has been shown to bring about a marked reduction in aortic lipid deposition in hypercholesterolemic golden Syrian hamsters.10 In this paper we report on the impact of raspberry juice consumption on oxidative stress and early markers of atherosclerosis in hamsters.
2 Results
2.1 Raspberry juices and diets
Syrian golden hamsters, initially weighing 60–80 g, were maintained on a high fat diet for 12 weeks and fed daily by gavage either water or a raspberry juice prepared from either Cardinal, Glen Ample or Tulameen berries. The volume of juice/water fed was adjusted to the weight of hamsters and was equivalent to a consumption of 275 mL day−1 by a 70 kg human. The respective sugar levels of Cardinal, Glen Ample and Tulameen raspberry juices were 39.8, 48.1 and 32.2 g L−1 and the pH was 3.12, 3.14 and 2.97. Based on HPLC-PDA-MS analysis the three juices contained anthocyanins, ellagitannins and ellagic acid derivatives in the quantities listed in Table 1. Glen Ample juice had the lowest levels of anthocyanins, 218 μg mL−1, with 24% and 40% higher concentrations occurring in Cardinal and Tulameen juices, respectively. The lowest concentration of ellagitannins, 45 μg mL−1, was found in Tulameen juice with respective levels in Cardinal and Glen Ample being 29% and 60% higher. The juices also contained flavonols but they were very minor components and were not quantified.| Compound | Cardinal | Glen Ample | Tulameen |
|---|---|---|---|
| Cyanidin-3-O-sophoroside | 114 ± 3 | 134 ± 0.7 | 216 ± 3 |
| Cyanidin-3-O-(2′′-O-glucosyl)rutinoside/glucoside | 117 ± 2 | 65 ± 1.7 | 65 ± 1.4 |
| Pelargonidin-3-O-sophoroside | 6.2 ± 0.2 | 4.8 ± 1.3 | 8.4 ± 0.1 |
| Cyanidin-3-O-rutinoside | 27 ± 0.7 | 12 ± 0.1 | 13 ± 6.4 |
| Pelargonidin-3-O-(2′′-O-glucosyl)rutinoside | 6.9 ± 0.2 | 2.4 ± 0.0 | 2.9 ± 0.0 |
| Total anthocyanins | 271 ± 2 | 218 ± 2 | 305 ± 7 |
| Sanguin H-10 | 5.8 ± 0.5 | 5.4 ± 0.1 | 8.5 ± 0.3 |
| Sanguin H-6 | 52 ± 1 | 67 ± 2.1 | 36 ± 0.9 |
| Total ellagitannins | 58 ± 1 | 72 ± 2 | 45 ± 1 |
| Ellagic acid pentoside conjugate-1 | 1.3 ± 0.0 | 1.8 ± 0.6 | 3.2 ± 0.0 |
| Ellagic acidpentoside conjugate-2 | 1.3 ± 0.1 | 1.4 ± 0.7 | 1.1 ± 0.0 |
| Ellagic acid-like compounds | 0.4 ± 0.0 | 0.5 ± 0.1 | 1.6 ± 0.1 |
| Total ellagic acid-like compounds | 2.9 ± 0.1 | 3.7 ± 0.4 | 5.8 ± 0.0 |
| Total HPLC phenolics | 332 ± 2 | 294 ± 5 | 356 ± 8 |
2.2 Effects of juices on body weight
The food and energy intakes of the four groups of hamsters were very similar (Table 2). A statistically significant reduction in body weight was observed in animals that had consumed Cardinal raspberry juice. Lowered body weight was also associated with intakes of Glen Ample and Tulameen juices but in this case they were not statistically different from the controls (Table 2).| Diet | Initial BW (g) | Final BW (g) | Weight gain (g) | Food intake (g/d) | Energy intake (kJ/d) |
|---|---|---|---|---|---|
| a Values are the means ± SEM (n = 12). For each dietary treatment, mean values in a column with different superscripts (*) are significantly different from the controls, P < 0.05. | |||||
| Control | 79.6 ± 2.1 | 90.0 ± 2.3 | 10.3 ± 3.3 | 4.4 ± 0.5 | 96.8 ± 11.0 |
| Cardinal | 78.2 ± 1.8 | 80.3 ± 5.2* | 1.9 ± 2.8* | 4.0 ± 0.4 | 88.0 ± 8.8 |
| Glen Ample | 79.4 ± 2.0 | 85.3 ± 2.0 | 5.7 ± 1.8 | 4.1 ± 0.5 | 90.2 ± 11.0 |
| Tulameen | 77.4 ± 1.7 | 84.5 ± 2.9 | 7.1 ± 2.0 | 4.1 ± 0.5 | 90.2 ± 11.0 |
2.3 Effects of juices on total-, HDL-and LDL-cholesterol and triglycerides
Plasma glucose and lipid contents are presented in Table 3.Glycemia was not significantly different between the four groups, none of which exhibited hyperglycemia. Animals that were fed Tulameen and Cardinal juice showed a significant reduction in cholesterolemia. Although Glen Ample juice did not have a significant effect on total cholesterol, unlike the other juices, its consumption did result in a significant increase in HDL-cholesterol compared with control animals. Feeding Cardinal and Tulameen juices, but not Glen Ample, lowered LDL-cholesterol significantly compared with the control group while all three raspberry varieties significantly lowered triglyceride levels.| Groups | Glycemia | TC | HDL-C | LDL-C | TG |
|---|---|---|---|---|---|
| a Data are expressed as the mean values in mmol L−1 ± SEM (n = 12). For each dietary treatment, mean values in a column with different superscripts (*) are significantly different from the controls, P < 0.05. TC: total cholesterol; HDL-C: HDL-cholesterol; LDL-C: LDL-cholesterol; TG: triglycerides. | |||||
| Control | 8.44 ± 2.27 | 7.32 ± 1.42 | 2.77 ± 0.47 | 3.62 ± 0.90 | 2.04 ± 0.62 |
| Cardinal | 7.31 ± 2.05 | 6.35 ± 0.76* | 2.89 ± 0.32 | 2.96 ± 0.54* | 1.10 ± 0.38* |
| Glen Ample | 6.65 ± 1.37 | 7.73 ± 0.75 | 3.07 ± 0.20* | 4.05 ± 0.79 | 1.35 ± 0.24* |
| Tulameen | 8.00 ± 1.27 | 5.56 ± 0.77* | 2.93 ± 0.25 | 2.15 ± 0.55* | 1.05 ± 0.08* |
2.4 Effect of juices on parameters of oxidative stress
Table 4 provides data on the effects of raspberry juice consumption on oxidative stress parameters. All three juices resulted in a significant ∼30% increase in hepatic GSHPx activity in comparison with controls. However, Tulameen was the only juice to have a significant impact on liver SOD, increasing activity by ∼25% compared with control animals.| Diet | GSHPx (U/mg protein) | SOD (U/mg protein) | PON (U/mL) |
|---|---|---|---|
| a Data expressed as mean values ± SEM (n = 12). For each dietary treatment, mean values in a column with different superscripts (*, **) are significantly different from the controls, P < 0.05. GSHPx: glutathione peroxidase. SOD: superoxide dismutase. PON: paraoxonase. | |||
| Control | 949 ± 45* | 158 ± 13* | 60 ± 16* |
| Cardinal | 1412 ± 76** | 161 ± 9* | 70 ± 17* |
| Glen Ample | 1352 ± 72** | 144 ± 10* | 120 ± 29** |
| Tulameen | 1278 ± 68** | 203 ± 12** | 61 ± 13* |
Plasma PON activity was enhanced significantly by consumption of Glen Ample juice but not the other varieties of raspberry (Table 4). In plasma, PON is localized in HDL, and when PON activity is expressed as a ratio to HDL, Glen Ample remains the only effective juice (Fig. 1).
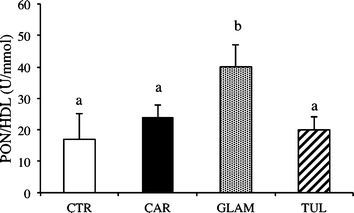 | ||
| Fig. 1 Ratio paraoxonase activity (PON)/HDL concentration in hamsters fed a high-fat diet (CTR), or a CTR diet plus either Cardinal (CAR), Glen Ample (GLAM) or Tulameen raspberry juice (TUL) for 12 weeks. Values are mean ± SEM (n = 6). For each dietary treatment, bars with different index letters are statistically significantly different (P < 0.05). | ||
2.5 Effects of juices on superoxide anion production
Fig. 2 and 3 show superoxide anion (O2˙−) production in the heart and thoracic aorta. Hamsters fed all three varieties of raspberry juice showed a significant decrease in cardiac O2˙− production (Fig. 2) and likewise with thoracic aorta O2˙−(Fig. 3).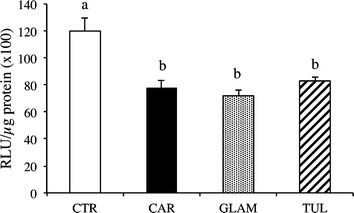 | ||
| Fig. 2 Cardiac superoxide anion production in hamsters fed a high-fat diet (CTR), or a CTR diet plus either Cardinal (CAR), Glen Ample (GLAM) or Tulameen raspberry juice (TUL) for 12 weeks. Values are expressed as mean ± SEM (n = 6). For each dietary treatment, bars with different index letters are statistically significantly different (P < 0.05). | ||
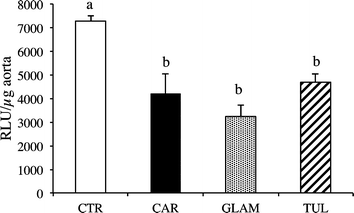 | ||
| Fig. 3 Thoracic aorta superoxide anion production in hamsters fed a high-fatdiet (CTR), or a CTR diet plus either Cardinal (CAR), Glen Ample (GLAM) or Tulameen raspberry juice (TUL) for 12 weeks. Values are expressed as mean ± SEM (n = 6). | ||
3 Discussion
The aim of the current study was to evaluate the impact of juices prepared from Cardinal, Glen Ample and Tulameen raspberries on prevention of the development of early atherosclerosis in hamsters receiving a high fat diet deficient in antioxidants. The data obtained are summarised in Table 5.| Juice | Anthocyanins (mg mL−1) | Ellagitannins (mg mL−1) | Body weight | TG | TC | LDL-C | HDL-C | Cardiac O2˙− | Thoracic O2˙− | GSHPx | SOD | PON |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a n.s.: effect not significant. TC: total cholesterol; HDL-C: HDL-cholesterol; LDL-C: LDL-cholesterol; TG: triglycerides. GSHPx: glutathione peroxidase. SOD: superoxide dismutase. PON: paraoxonase. | ||||||||||||
| Tulameen | 305 | 45 | n.s. | reduced | reduced | reduced | n.s. | reduced | reduced | increased | increased | n.s. |
| Cardinal | 271 | 58 | reduced | reduced | reduced | reduced | increased | reduced | reduced | increased | n.s. | n.s. |
| Glen Ample | 218 | 72 | n.s | reduced | n.s. | n.s. | n.s. | reduced | reduced | increased | n.s. | increased |
The main phenolic compounds of the three raspberry juices were anthocyanins and ellagitannins, and there were minor but not substantial differences in the levels of these compounds in the individual juices. Although they did not have identical effects, all the juices induced potentially protective effects when consumed by hamsters on a daily basis for 12 weeks (Table 5). There was, for instance, a trend towards reduced body weight with juice consumption but the effect was only statistically significant with the juice prepared from Cardinal berries (Table 2). Elevated LDL, triglycerides and total cholesterol are known to be risk factors for atherosclerosis. All three raspberry juices lowered triglyceride levels. However, Cardinal and Tulameen, but not Glen Ample, reduced both total cholesterol and LDL-cholesterol but did not impact on HDL-cholesterol which was increased significantly by ingestion of Glen Ample juice (Table 5).
All three juices also significantly reduced the production of cardiac and aortic O2˙− levels (Fig. 2 and 3, Table 5), via the decreased activity of NADPH oxidase and therefore the oxidative stress induced by the atherogenic diet. As noted above, the atherogenic diet led to high level of total and non-HDL-C (i.e. ≈ LDL-C) which is known to induce aortic fatty streak deposition.11 Interestingly, the diet-induced hypercholesterolemia was accompanied by an overproduction of O2˙− (data not shown), as previously shown in rat and hamster models of atherosclerosis.12,13 Thus, according to the oxidative hypothesis of atherosclerosis, it could be postulated that NADPH oxidase activity works with high LDL cholesterol to induce foam cells fatty streak14 and subsequent atherosclerosis.15
Consumption of all three raspberry juices resulted in significantly higher GSHPx activity than in control hamsters. In contrast, only intake of Talameen juice brought about a significant increase in SOD activity (Table 5). PON activity is another marker of oxidative stress. HDL has long been known to be antiatherogenic but the exact mechanism of action has yet to be identified although it has been attributed to a role in the reverse transport of cholesterol and to their antioxidant properties. It has been proposed that PON plays a crucial role in the antioxidant activity of HDL.16 It has been reported that PON is implicated in the protection of LDL and HDL from oxidation induced by copper ions and other free radical generators.17 This protection is most probably related to the ability of PON to hydrolyse oxidized phospholipids18 and/or lipid peroxide products.17 PON is therefore believed to be a protective factor against atherosclerosis.19 Some studies have shown that PON can reduce oxidative stress in aortic lesions.20,21 Moreover, a decrease in the specific anti-atherogenic activity of HDL might also contribute. In animal models, an impaired HDL antioxidant defense has been observed in dyslipidemic, obese mice.22In the current study, only Glen Ample was effective in increasing PON activity (Table 5). This is consistent with a lower production of cardiac O2°−in animals fed Glen Ample juice (Fig. 2).
Tulameen juice had the highest anthocyanin content of the three raspberry juices that were investigated, while Glen Ample juice contained the highest levels of ellagitannins. Regarding the bioactivity of the juices, Tulameen was the most efficient in modulating dyslipidemia (TC and LDL-C) and Glen Ample the least effective. In contrast, Glen Ample was the most efficient in reducing the induction of oxidative stress (O2˙− and PON activity) and Tulameen was the least effective juice. While it is tempting to speculate that the different effects of the juices are related to their differing anthocyanin and ellagitannin profiles, this would be premature. However, the protective effects of raspberries and the relationship between anthocyanins and ellagitannins are topics that merit further investigation. Neither anthocyanins nor ellagitannins per se are likely to enter the bloodstream in sufficient quantity to induce the protective effects observed with the hypercholesterolemic hamsters. In a separate study in which human volunteers consumed raspberries, it has been shown that the major components that enter the bloodstream are derived from the colon. Here ellagitannins are converted to urolithins, which are absorbed as O-glucuronides, and anthocyanins undergo C-ring fission yielding a number of phenolic acids.23 Urolithin aglycones and several phenolic acids have recently been shown in vitro to have anti-inflammatory properties,24 antiglycative effects and to protect neurons from oxidative stress.25
4 Experimental
4.1 Animals
Weanling male Syrian golden hamsters (Breeding Janvier, Le Genest-St-Isle, France) weighing 60–80 g were randomly separated into five groups of ten animals. They were maintained in plastic cages in a temperature-controlled environment (23 ± 1 °C) subjected to a 12 h light/dark cycle and allowed free access to both food and water. Hamsters were handled according to the guidelines of the Committee on Animal Care at the University of Montpellier and NIH guidelines.264.2 Raspberry juices
Three varieties of raspberry (Rubus idaeus), Cardinal, Glen Ample and Tulameen, obtained from growers around Blairgowrie (Perth and Kinross, Scotland, UK) were each converted to a juice (1 mL = 0.6 g of berries) by Ella Drinks Ltd (Alloa, Clackmannanshire, UK)4.3 Diets and feeding procedures
Hamsters were fed for 12 weeks on a semi-purified hyperlipidic diet (Table 6) in which the cholesterol content had been set at 0.5% and which was supplemented with 15% lard; no selenium, vitamin C, or vitamin E was added to this diet. Vitamin and mineral mixes were formulated according to AIN-93 guidelines27 and supplied by SSNIFF (Spezialdiaten GmbH, Soest, Germany). Uneaten food was weighed daily. The hamsters of each group also received daily by gavage either tap water (control) or juice from the three varieties of raspberry. The volume of solutions fed was adjusted daily to the weight of hamsters. The calculation is based on a consumption of 275 mL day−1 for a 70 kg human based on the US Food and Drug Administration Center for Drug Evaluation and Research dose calculator (http://www.accessdata.fda.gov/scripts/cder/onctools/animalquery.cfm).| Ingredients | Control |
|---|---|
a Mineral mixture contained (mg kg−1 of diet): CaHPO4, 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200; KCl, 4000; NaCl, 4000; MgO, 420; MgSO4, 2000; Fe2O3, 120; FeSO4·7H2O, 200; trace elements, 400 (MnSO4·H2O, 98; CuSO4. 5H2O, 20; ZnSO4·7H2O, 80; CoSO4·7H2O, 0,16; KI, 0.32; sufficient starch to bring to. 40 g (per kg of diet). Vitamin mixture contained (mg kg−1 of diet): retinol, 12; cholecalciferol, 0.125; thiamin, 40; riboflavin, 30; pantothenic acid, 140; pyridoxine, 20; inositol, 300; cyanocobalamin, 0.1; menadione, 80; nicotinic acid, 200; choline, 2720; folic acid, 10; p-aminobenzoic acid, 100; biotin, 0.6; sufficient starch to bring to 20 g (per kg of diet). 200; KCl, 4000; NaCl, 4000; MgO, 420; MgSO4, 2000; Fe2O3, 120; FeSO4·7H2O, 200; trace elements, 400 (MnSO4·H2O, 98; CuSO4. 5H2O, 20; ZnSO4·7H2O, 80; CoSO4·7H2O, 0,16; KI, 0.32; sufficient starch to bring to. 40 g (per kg of diet). Vitamin mixture contained (mg kg−1 of diet): retinol, 12; cholecalciferol, 0.125; thiamin, 40; riboflavin, 30; pantothenic acid, 140; pyridoxine, 20; inositol, 300; cyanocobalamin, 0.1; menadione, 80; nicotinic acid, 200; choline, 2720; folic acid, 10; p-aminobenzoic acid, 100; biotin, 0.6; sufficient starch to bring to 20 g (per kg of diet).
|
|
| Casein | 200 |
| DL-Methionine | 3 |
| Corn starch | 393 |
| Sucrose | 154 |
| Cellulose | 50 |
| Lard | 150 |
| Mineral mix | 35 |
| Vitamin mix | 10 |
| Cholesterol | 5 |
4.4 Analysis of phenolic compounds in raspberry juices by HPLC with PDA and MS detection
Raspberry juices were analyzed on a Surveyor HPLC system comprising of a HPLC pump, PDA detector, scanning from 200 to 700 nm and an autosampler cooled to 4 °C. (Thermo Fisher Corporation, San Jose, USA). Analyses were carried out at 40 °C using a 250 × 4.6 mm i.d. 4 μm Gemini C6-Phenyl column (Phenomenex, Macclesfield, UK) eluted with a 60 min linear gradient of 10–40% methanol in 1% aqueous formic acid at a flow rate of 1.0 mL/min. After passing through the flow cell of the PDA detector the column eluate split and 0.3 mL min−1 is directed to a LCQ Advantage ion trap mass spectrometer fitted with an electrospray interface (Thermo Fisher Corporation). Analyses utilised both the negative and positive ion mode. Samples were analysed in the mass spectrometer using full-scan data dependent MS2 scanning from m/z 100–2000. Capillary temperature was 150 °C, sheath gas and auxiliary gas were 40 and 20 units respectively, the source voltage was 3kV. Compounds that could not be identified by MS2 were further fragmented to produce MS3 spectra. The system was controlled by Xcalibur software (Thermo Fisher Corporation). Anthocyanins, ellagitannins and ellagic acid were quantified from their 520, 280 and 365 nm chromatographic peak areas and expressed as cyanidin-3-O-glucoside, gallic acid and ellagic acid equivalents, respectively.4.5 Other analytical procedures
At the end of 12-week experimental period, hamsters were deprived of food overnight and blood samples were collected under anaesthesia (Pentobarbital) by cardiac puncture.Plasma was prepared by centrifugation at 2000 g for 10 min at 4 °C, then stored at −80 °C until analysis. Plasma concentrations of total cholesterol (TC), HDL cholesterol (HDL-C) and triglycerides (TG) were measured by using commercially available enzymatic kits (respectively nos. CH 200, CH 203 and TR 1697, Randox Laboratories LTD, Crumlin, UK). Plasma non HDL-C (≈LDL-C) was calculated from the difference between TC and HDL-C. Paraoxonase activity (PON) was determined using paraoxon as a substrate and measured by increases in absorbance at 412 nm due to the formation of 4-nitrophenol, according to Jaouad et al.28 Briefly, the activity was measured at 25 °C, by adding 50 μL of plasma to 1 mL Tris/HCl buffer (100 mM, pH 8.0) containing 2mM CaCl2 and 5.5 mM of paraoxon. The rate of generation of 4-nitrophenol was determined at 412 nm. Enzymatic activity was calculated using the molar extinction coefficient 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 M−1 cm−1. One unit of paraoxonase activity is defined as 1 nM of 4-nitrophenol formed per minute under the above assay conditions.
100 M−1 cm−1. One unit of paraoxonase activity is defined as 1 nM of 4-nitrophenol formed per minute under the above assay conditions.
The liver was excised, weighed and sectioned for analyses and stored at −80 °C. Liver was homogenized in ice cold 0.1 mol L−1 potassium phosphate buffer (pH 7.4) and the homogenate was spun at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g for 15 min at 4 °C. Glutathione peroxidase (GSHPx) and superoxide dismutase (SOD) activities of the supernatant were assayed on an automat Pentra 400 (HORIBA ABX, Montpellier, France) using commercial kits (Ransod kit no. SD 125 and Ransel no. RS505, Randox Laboratories LTD, Crumlin, UK, respectively). The heart and the thoracic aorta were removedand stored at 4 °C in PBS for subsequent analysis.
000 g for 15 min at 4 °C. Glutathione peroxidase (GSHPx) and superoxide dismutase (SOD) activities of the supernatant were assayed on an automat Pentra 400 (HORIBA ABX, Montpellier, France) using commercial kits (Ransod kit no. SD 125 and Ransel no. RS505, Randox Laboratories LTD, Crumlin, UK, respectively). The heart and the thoracic aorta were removedand stored at 4 °C in PBS for subsequent analysis.
Following blood collection and tissues removal, the intact aortic cross of 6 hamsters per group were removed then immersed in Krebs buffer, and was cleaned of fat and connective tissue and cut into 2–3 mm wide. Superoxide anion (O2˙−) production was evaluated in thoracic aorta immersed and equilibrated in Krebs buffer containing lucigenin which enhanced luminescence, in the presence of 7.5 μL of NADPH (2 mM). The intensity of luminescence was recorded on a luminometer (Berthold Technology, France) for 30 min. Results were expressed as relative luminescence units (RLU/μg tissue). Cardiac superoxide anion production was also evaluated. Briefly, the left ventricle (150 mg) was homogenized with 10-fold volume of Krebs buffer and centrifuged.29 Supernatant was placed in a well with lucigenin (200 μM) and NADPH (2 mM). The intensity of luminescence was recordedand the results were expressed as relative luminescence units (RLU/mg of protein).
Protein content of tissues was determined by using a commercial protein assay (Sigma, Saint Quentin Fallavier, France) according to the method of Smith et al. and using bovine serum albumin as standard.30
4.6 Statistical analysis
Data are shown as the mean ± SEM. Statistical analysis of the data was carried out using the StatView IV software (Abacus Concepts, Berkeley, CA, USA) by one-way ANOVA followed by Fisher's protected least significant difference test. Differences were considered significant at P < 0.05.5 Conclusions
When fed daily to Syrian golden hamsters on an atherogenic diet for 12 weeks at a dose corresponding to the consumption of 275 mL by a 70 kg human, juices prepared from three varieties of raspberry, Cardinal, Glen Ample and Tulameen, lowered biomarkers of early atherosclerosis. Although the effects were not identical for all three juices, the findings suggest that moderate consumption of raspberry juices may help to prevent the development of early atherosclerosis, with the underlying mechanisms related to improved antioxidant status and serum lipid profiles. The main polyphenolic compounds in the juices were anthocyanins and ellagitannins, neither of which is likely to be absorbed into the bloodstream in sufficient quantity to induce the observed protective effects. The principal components entering the circulatory system from anthocyanins will be colonic microbiota-derived phenolic acid breakdown products while ellagitannins will be converted to urolithins in the large intestine and are which subsequently absorbed as O-glucuronides.23Acknowledgements
The investigation was funded by a grant from the Washington Red Raspberry Commission and Fundación Séneca (Spain) who provide Rocio González-Barrio with a postdoctoral fellowship.6 References
- J. Pincemail, K. Bonjean, K. Cayeux and J. O. Defraigne, Nutr. Clin. Metab., 2002, 16, 233–239 Search PubMed.
- S. Hiroi, H. Harada, H. Nishi, M. Satoh, R. Nagai and A. Kimura, Biochem. Biophys. Res. Commun., 1999, 261, 332–339 Search PubMed.
- B. Frei, Am. J. Med., 1994, 97, S5–S13 Search PubMed.
- S. Liu, J. A. E. Manson, I. M. Lee and S. R. Coler, Am. J. Clin. Nutr., 2000, 72, 922–928 Search PubMed.
- E. Strandhagen, P. O. Hansson, I. Bosaeus and B. Isaksson, Eur. J. Clin. Nutr., 2000, 54, 337–341 Search PubMed.
- S. Bradley and R. Shinton, J. Hum. Nutr. Diet., 1998, 11, 363–372 Search PubMed.
- A. R. Proteggente, A. S. Pannala, G. Paganga, L. Van Buren, E. Wagner, S. Wiseman, F. Van De Put, C. Dacombe and C. A. Rice-Evans, Free Radical Res., 2002, 36, 217–233 Search PubMed.
- W. Mullen, J. McGinn, M. E. J. Lean, M. R. MacLean, P. Gardner, G. G. Duthie, T. Yokota and A. Crozier, J. Agric. Food Chem., 2002, 50, 5191–5196 CrossRef CAS.
- G. Borges, A. Degeneve, W. Mullen and A. Crozier, J. Agric. Food Chem., 2010, 58, 3901–3909 CrossRef CAS.
- J. M. Rouanet, K. Décordé, D. Del Rio, C. Auger, G. Borges, J. P. Cristol, M. E. J. Lean and A. Crozier, Food Chem., 2010, 118, 266–271 Search PubMed.
- T. Sutra, K. Décordé, J. Riss, C. Dallas, J. P. Cristol and J. M. Rouanet, J. Agric. Food Chem., 2007, 55, 4258–4263 Search PubMed.
- K. Decordé, P. L. Teissèdre, T. Sutra, E. Ventura, J. P. Cristol and J. M. Rouanet, Mol. Nutr. Food Res., 2009, 53, 659–666 Search PubMed.
- C. Auger, B. Caporiccio, N. Landrault, P. L. Teissèdre, C. Laurent, G. Cros, P. Besançon and J. M. Rouanet, J. Nutr., 2002, 132, 1207–1213 Search PubMed.
- J. L. Witzum and D. Steinberg, J. Clin. Invest., 1991, 88, 1785–1792 Search PubMed.
- A. Heinloth, K. Heermeier, U. Raff, C. Wanner and J. Galle, J. Am. Soc. Nephrol., 2000, 11, 1819–1825 Search PubMed.
- P. N. Durrington, B. Mackness and M. I. Mackness, Arteriosclerosis, 2001, 21, 473–480 Search PubMed.
- M. Aviram, S. Billecke, R. Sorenson, C. Bisgaier, R. Newton, M. Rosenblat, J. Erogul, C. Hsu, C. Dunlop and B. N. La Du, Arterioscler. Thromb. Vasc. Biol., 1998, 18, 1617–1624 Search PubMed.
- A. D. Watson, J. A. Berliner, S. Y. Hama, B. N. La Du, H. K. Faull, A. M. Fogelman and M. Navab, J. Clin. Invest., 1995, 96, 2882–2891 Search PubMed.
- Durak, H. Ozbek, E. Devrim, N. Karagenç and I. B. Ergüder, Nutr., Metab. Cardiovasc. Dis., 2004, 14, 211–214 Search PubMed.
- B. Mackness, M. I. Mackness, P. N. Durington, S. Arrol, A. E. Evans, D. McMaster, J. Ferrieres, J. B. Ruidavets, N. R. Williams and A. N. Howard, Eur. J. Clin. Invest., 2000, 30, 4–10 Search PubMed.
- N. Ferré, J. Marsillach, J. Camps, B. Mackness, M. Mackness, F. Riu, B. Coll, M. Tous and J. Joven, J. Hepatol., 2006, 45, 51–59 Search PubMed.
- A. Mertens, P. Verhamme, J. K. Bielicki, M. C. Phillips, R. Quarck, W. Verreth, D. Stengel, E. Ninio, M. Navab, B. Mackness, M. Mackness and P. Holvoet, Circulation, 2003, 107, 1640–1646 Search PubMed.
- R. González-Barrio, C. A. Edwards and A. Crozier, Drug Metab. Dispos., 2011 DOI:10.1124/dmd.111.039651.
- M. Larrosa, C. Luceri, E. Vivoli, C. Pagliuca, M. Lodovici, G. Moneti and P. Dolara, Mol. Nutr. Food Res., 2009, 53, 1044–1054 CrossRef CAS.
- E. Verzelloni, C. Pellacani, D. Tagliazucchi, S. Tagliaferri, L. Calani, L. G. Costa, F. Brighenti, G. Borges, A. Crozier, A. Conte and D. Del Rio, Mol. Nutr. Food Res., 2011 DOI:10.1002/mnfr.201000525.
- National Research Council Guide for the Care and the Use of Laboratory Animals; National Institutes of Health Publication no. 85–123 (rev.); U.S. Government Printing Office; Washington, DC. 1985 Search PubMed.
- P. G. Reeves, F. H. Nielsen and G. C. Fahey Jr, J. Nutr., 1993, 123, 1939–1951 CAS.
- L. Jaouad, C. de Guise, H. Berrougui, M. Cloutier, M. Isabelle, T. Fulop, H. Payette and A. Khalil, Atherosclerosis, 2006, 185, 191–200 Search PubMed.
- N. A. Al-Awwadi, C. Araiz, A. Bornet, S. Delbosc, J. P. Cristol, N. Linck, J. Azay, P. L. Teissèdre and G. Cros, J. Agric. Food Chem., 2005, 53, 151–157 CrossRef CAS.
- S. K. Smith, R. I. Krohn, A. K. Mallia, M. D. Provenzano, E. K. Fujimoto, N. M. Goeke, B. J. Olson and D. K. Klenk, Anal. Biochem., 1985, 150, 76–85 CrossRef CAS.
Footnotes |
| † The authors have declared no conflict of interest. |
| ‡ Authors made an equal contribution. |
| This journal is © The Royal Society of Chemistry 2011 |
