Role of reverse micelles on lipid oxidation in bulk oils: impact of phospholipids on antioxidant activity of α-tocopherol and Trolox
Bingcan
Chen
,
Ashley
Han
,
Michaël
Laguerre
,
David Julian
McClements
and
Eric Andrew
Decker
*
Department of Food Science, University of Massachusetts, Amherst, MA 01003, USA. E-mail: edecker@foodsci.umass.edu; Fax: +1 (413) 545-1262; Tel: +1 (413) 545-1026
First published on 2nd June 2011
Abstract
Phospholipids self-assemble in bulk oils to form structures such as reverse micelles that can alter the microenvironment where chemical degradation reactions occur, such as lipid oxidation. In this study, we examined the influence of phospholipid reverse micelles on the activity of non-polar (α-tocopherol) and polar (Trolox) antioxidants in stripped soybean oil (SSO). Reverse micelles were formed by adding 1000 μM 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) to SSO. The addition of DOPC reverse micelles had a prooxidant effect, shortening the lag phase of SSO at 55 °C. DOPC improved the activity of low α-tocopherol or Trolox concentrations (10 μM) but decreased the activity of high concentrations (100 μM). Hydrophilic Trolox had better antioxidant activity than hydrophobic α-tocopherol. Fluorescence steady state and lifetime decay studies suggests that differences in the antioxidant activity of Trolox and α-tocopherol could be due to differences in their physical location in DOPC reverse micelles. These results will improve our understanding and control of lipid oxidation in bulk oils.
Introduction
Lipid oxidation is one of the main factors limiting the shelf life of bulk oils, since it adversely affects flavor and quality, and potentially produces toxic reaction products.1 Preventing or inhibiting the oxidation of bulk oils is therefore of great importance to consumers and the food industry. A variety of mechanisms have been proposed to be responsible for the oxidation of bulk oils during processing and storage, with photosensitized oxidation, metal-promoted and autoxidation being the most well-known. Some factors that impact the oxidative stability of bulk oils include: oil extraction and processing conditions; light exposure; temperature; fatty-acid composition; antioxidant composition; oxygen levels; and the presence of minor components.2 Manipulation of these factors can be used to retard lipid oxidation in edible oils.One of the most effective ways of inhibiting lipid oxidation in bulk oils is to incorporate antioxidants.3 Depending on their mechanism of action, antioxidants can be classified as either “primary” or “secondary” antioxidants.4 Tocopherols are the most common primary antioxidant present in many vegetable oils, which may originate naturally from the extracted oil itself or may be manually added after oil refining.5 However, tocopherols may not be the most effective antioxidants in bulk oil systems. Indeed, research has shown that hydrophilic antioxidants (Trolox or ascorbic acid) possess better antioxidant activity than their hydrophobic analogues (tocopherol and ascorbyl palmitate) in some bulk oil systems.6 The greater tendency for hydrophilic antioxidants to accumulate at the air–water interface where oxidation may be expected to begin is one of the mechanisms proposed to account for their better antioxidant activity in bulk oils.6 However, other mechanisms have also been proposed to account for the ability of hydrophilic antioxidants to act as better antioxidants than their hydrophobic analogs in bulk oils since air is more hydrophobic than oil and thus there is no driving force to concentrate hydrophilic antioxidants at the oil-air interface.4,7 Studies have shown that the addition of phospholipids to bulk oils increased the antioxidant activity of tocopherol.7 It was postulated that the phospholipids formed microstructures, known as association colloids within the bulk oil, which caused the tocopherol molecules to accumulate in the phospholipid microstructures where lipid oxidation primarily occurred. Other researchers have also demonstrated the ability of phospholipids to act as antioxidants in various kinds of bulk oils.8,9 Several mechanisms were proposed to account for the antioxidant activity of the phospholipids, including their ability to chelate metals, decompose lipid hydroperoxides, and scavenge free radicals. Nevertheless, there is still a poor understanding of the importance and contribution of the combination of phospholipids and tocopherols to the oxidative stability of bulk oils.
Recently, we characterized the formation of reverse micelles in bulk oils, and their impact on lipid oxidation.10 These reverse micelles were formed by adding phospholipid (1,2-dioleoyl-sn-glycero-3-phosphocholine, DOPC) into stripped soybean oil (SSO) containing water levels similar to that found in commercial refined oils. Using small angle X-ray scattering, this study showed that the combination of DOPC and water resulted in the formation of reverse micelles in bulk oil. Alternately, when phosphatidylcholine with short chain fatty acyl residues (1,2-dibutyryl-sn-glycero-3- phosphocholine, DC4PC) was added to the same system, no reverse micelles were formed. The lipid oxidation chemistry of these two systems was different with DOPC reverse micelles demonstrating a prooxidative effect while DC4PC had no effect on oxidation rates. This study suggests that the combination of phospholipids and water can form physical structures in bulk oils and these structures can impact lipid oxidation chemistry.
In the present study, we examined if reverse micelles in bulk oil produced by phosphatidylcholine (i.e., DOPC) were able to impact the activity of free radical scavenging antioxidants. The antioxidants tested included alpha-tocopherol (non-polar) and Trolox (polar), which are chemical analogs. In addition, the system had equal molar concentrations of phospholipids with one system containing reverse micelles (DOPC) and the other containing no measurable reverse micelles (DC4PC). We also measured how the tocopherol analogs impacted the structure of the reverse micelles with the aim of better understanding the location and properties of antioxidants in bulk oils. By better understanding how physical structure in bulk oils impact the activity of antioxidants such as tocopherols, it might be possible to design systems to improve the activity of these important “natural” antioxidants.
Materials and methods
Materials
1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) and 1,2-dibutyryl-sn-glycero-3- phosphocholine (DC4PC) were acquired from Avanti Polar Lipids, Inc (Alabaster, AL). N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)-1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine, triethylammonium salt (NBD-PE, Cat.No. N-360) was acquired from Invitrogen. Soybean oil was purchased from a local store and stored at 4 °C. Silicic acid, activated charcoal, hexane, alpha-tocopherol, Trolox were purchased from Sigma-Aldrich Co. (St. Louis, MO). All other reagents were of HPLC grade or purer distilled and deionized water was used as needed.Preparation of stripped soybean oil
Stripped soybean oil has been prepared according to the method of Boon and coworkers.11 In short, silicic acid (100 g) was washed three times with a total of 3 L of distilled water and activated at 110 °C for 20 h. The activated silicic acid (22.5 g) and fine charcoal (5.625 g) were suspended in 100 and 70 mL of n-hexane, respectively. A chromatographic column (3.0 cm internal diameter × 35 cm height) was then packed sequentially with 22.5 g of silicic acid followed by 5.625 g of activated charcoal and then another 22.5 g of silicic acid. Thirty grams of soybean oil was dissolved in 30 mL of hexane and passed through the column by eluting with 270 mL of n-hexane. To retard lipid oxidation during stripping, the collected soybean oil was held in an ice container, covered with aluminum foil. The solvent in the stripped oils was removed with a vacuum rotary evaporator (RE 111 Buchi, Flawil, Switzerland) at 37 °C, and the remaining trace solvent was removed by flushing with nitrogen for at least one hour. The colorless stripped oil was kept at −80 °C for subsequent studies. Removal of minor components was verified by spotting sample on a silica gel G thin layer chromatography (TLC) plate and developed in a tank with hexane/diethyl ether/acetic acid (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v/v) and were detected by iodine vapor.12 Water content of the stripped oils was determined by Karl Fisher.13
1 v/v/v) and were detected by iodine vapor.12 Water content of the stripped oils was determined by Karl Fisher.13
Formation of DOPC reverse micelles in stripped soybean oil
The formation of DOPC reverse micelles was done using our previous procedure.10 Briefly, DOPC (1000 μM) in chloroform was pipetted into an empty beaker and then chloroform was removed by flushing with nitrogen. The appropriate amount of stripped soybean oil was then added followed by double distilled water to a final amount of ∼ 200 ppm. The sample in beaker was magnetically stirred at the speed of 1000 rpm in a 20 °C incubator room for a 24 h. Antioxidants were dissolved in ethanol, mixed with stripped soybean oil and stirred for at least 12 h to obtain the homogenous samples. In the lipid oxidation studies, a mixture of 75% of medium chain TAG and 25% SSO were used due to the high amount of samples needed. Samples for the oxidation studies were aliquoted into GC vials (1 mL/vial) stored at 55 °C in the dark. For fluorescence, the NBD-PE probe was mixed into stripped soybean oil at the same time as DOPC and water.Measurement of oxidation parameters
Lipid hydroperoxides were measured as the primary oxidation products using a method adapted from Shanta and Decker.14 Accurate weighted oil sample was added to a mixture of methanol/butanol (2.8 mL, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v:v) followed by addition of 15 μL of 3.94 M thiocyanate and 15 μL of 0.072 M Fe2+. The solution was vortexed, and after 20 min the absorbance was measured at 510 nm using a Genesys 20 spectrophotometer (ThermoSpectronic, Waltham, MA). The concentration of hydroperoxides was calculated from a cumene hydroperoxide standard curve.
1, v:v) followed by addition of 15 μL of 3.94 M thiocyanate and 15 μL of 0.072 M Fe2+. The solution was vortexed, and after 20 min the absorbance was measured at 510 nm using a Genesys 20 spectrophotometer (ThermoSpectronic, Waltham, MA). The concentration of hydroperoxides was calculated from a cumene hydroperoxide standard curve.
Hexanal was monitored using a GC-17A Shimadzu gas chromatograph equipped with an AOC-5000 autosampler (Shimadzu, Kyoto, Japan).15 Sample (1 mL) in 10 mL glass vials capped with aluminum caps with PTFE/silicone septa were heated at 55 °C for 15min in an autosampler heating block before measurement. A 50/30 μm DVB/Carboxen/PDMS solid-phase microextraction (SPME) fiber needle from Supelco® was injected into the vial for 2 min to absorb volatiles and then was transferred to the injector port (250 °C) for 3 min. The injection port was operated in split mode, and the split ratio was set at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. Volatiles were separated on a Supleco 30 m × 0.32 mm Equity DB-1 column with a 1 μm film thickness at 65 °C for 10 min. The carrier gas was helium at 15.0 mL/min. A flame ionization detector was used at a temperature of 250 °C. Hexanal concentrations were determined from peak areas using a standard curve prepared from authentic relative standard.
5. Volatiles were separated on a Supleco 30 m × 0.32 mm Equity DB-1 column with a 1 μm film thickness at 65 °C for 10 min. The carrier gas was helium at 15.0 mL/min. A flame ionization detector was used at a temperature of 250 °C. Hexanal concentrations were determined from peak areas using a standard curve prepared from authentic relative standard.
Small angle X-ray scattering (SAXS) study of bulk oil with DOPC and antioxidants
SAXS measurements were performed on the oil samples using a Molecular Metrology SAXS instrument at the W.M. Keck Nanostructures Laboratory at the University of Massachusetts-Amherst. The instrument generates X-rays with a wavelength of λ 1.54 Å and utilizes a 2-dimensional multi-wire detector with a sample-to-detector distance of 1.5 m. Samples were inserted into the 1 mm outer diameter quartz capillary (Hampton Research, USA) which were then sealed by Duco® Cement, and these were enclosed in an airtight sample holder. This assembly was then put in the X-ray beam path, and data were collected on the samples for 3 h.Fluorescence measurement of bulk oil with DOPC and antioxidants
Front-face fluorescence with surface active probes was used to verify the impact of antioxidants in the association colloids. The surface active fluorescent probe used was NBD-PE which is phospholipid analog with the fluorescent functional groups on the head group. Steady-state emission measurements were recorded with a PTI spectrofluorimeter with the sample holder thermostated at 22 °C. To avoid any reflection of the excitation beam by the cell window and by the underlying liquid surface of the sample into the emission monochromator, samples are stored in triangular suprasil cuvette. The emission was observed at 90° to the incident beam, i.e., 22.5° with respect to the illuminated cell surface. Spectral bandwidths of 2.0 and 2.0 nm for the excitation and the emission slits, respectively, were employed for excitation at 468 nm. The integration time in both was 1 s, and the wavelength increment during spectrum scanning was 1 nm. The intensity of the spectra was determined as the emission signal intensity (counts per second) measured by means of a photomultiplier. In order to avoid self quenching of probes, the concentrations of NBD-PE were selected as 9.5 μM (1/100 of DOPC).The fluorescence decay of NBD-PE in the systems was carried out at 22 °C using a PTI Laserstrobe fluorescence lifetime instrument with a PTI GL-3300 nitrogen laser and a GL-302 tunable dye laser with C-500 laser dye, exciting the oil samples at 500 nm. Each data point on a lifetime decay curve represents five laser flashes, and each decays represents 100 of these data points spaced out over the collection time interval. The instrument response function (IRF) was determined by measuring light scatter from a solution of Ludox. Data were analyzed using the commercial PTI software. Fluorescence decays were fitted to sums of two and three exponentials, and the average lifetime was calculated.
Statistical analysis
Duplicate experiments have been performed with freshly prepared samples. All data shown represents the mean values ± standard deviation of triplicate measurements. Statistical analysis of lipid oxidation kinetics was performed using a one-way analysis of variance. A significance level of p<0.05 between groups was accepted as being statistically difference. In all cases, comparisons of the means of the individual groups were performed using Duncan's multiple range tests. All calculations were performed using SPSS17 (http://www.spss.com; SPSS Inc., Chicago, IL).Results
Impact of phospholipids on oxidative stability of SSO
Commercial soybean oil is a highly complex liquid system containing a number of different components that could potentially alter the rate and extent of lipid oxidation such as tocopherols, chlorophyll, phospholipids, fatty acids, and water.4 Consequently, stripped soybean oil (SSO) was used as a model oil in this study to reduce any effects associated with these minor components. Previous research using small angle X-ray diffraction showed that the combination of DOPC and water resulted in the formation of reverse micelles in bulk oil while DC4PC did not form structures.10 Initially, we examined the influence of phospholipid type (DOPC and DC4PC at 1000 μM) on the formation of lipid hydroperoxides and headspace hexanal in SSO during storage at 55 °C (Fig. 1A, B). In the absence of added phospholipids, the lag phase for both lipid hydroperoxides and headspace hexanal lasted 2 days. Addition of DC4PC had little impact on the lipid oxidation profile, but the addition of DOPC reduced the lag-period by 1 day, confirming that it acted as a prooxidant.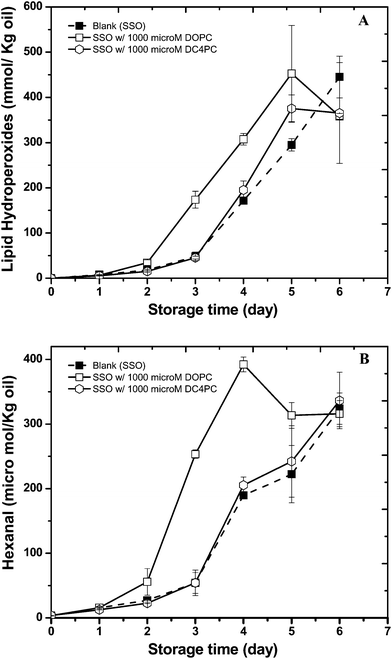 | ||
| Fig. 1 Formation of lipid hydroperoxides (A) and hexanal (B) in stripped soybean oil containing 1000 μM of 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) or 1,2-dibutyryl-sn-glycero-3- phosphocholine (DC4PC) during storage at 55 °C. Some of the error bars are within data points. | ||
Previous studies of the oxidative stability of bulk oils containing phospholipids suggest that phosphatidylethanolamine (PE) improves their oxidative stability.16,17 On the other hand, studies on the impact of phosphatidylcholine (PC) have indicated seemingly contradictory results. Prooxidant, antioxidant, and no effects of PC in bulk oil oxidation have been reported in the literature.18,19 In agreement with our study, a prooxidant activity of DOPC was reported by Takenaka, et al.19 in bonito oil, which was attributed to the unsaturated hydrocarbon chain of the PC used. In our study we used phosphatidylcholine with oleic acid side chains. DOPC was chosen because of its ease of handling and dispersion into bulk oil due to its low melting point and high oxidative stability. In general, the oxidative stability of oleic acid is 10–20 times greater than the linolenic and linolenic acids found in SSO and thus the fatty acyl residues in the added DOPC would not be expected to decrease the oxidative stability of the oil. In addition, Le Grandios, et al.20 showed that oleyl and linolenyl residues in PC are more oxidatively stable than the same residues in triacylglycerols (TAG) again suggesting that the oleyl residues in the DOPC added to the SSO would not be responsible for the observed increase in oxidation rates. These data suggest that the prooxidant activity of DOPC in comparison to DC4PC is not due to its unsaturated fatty acids.
It is possible that the impact of the phospholipids on the oxidation stability could be due to the polar head groups. However, since both phospholipids tested had the same head group at equal molar concentrations this is not likely. One major difference between DOPC and DC4PC in SSO is their ability to form physical structure with DOPC forming reverse micelles and DC4PC forming cylindrical structures.10 In addition, this study showed that DOPC could form physical structures at much lower concentrations than DC4PC due to its lower critical micelle concentration. Overall, these data suggested that the prooxidant activity of DOPC could be related to its ability to form reverse micelles.
The impact of phospholipids on the oxidative stability of SSO in the presence of α-tocopherol
Previous studies of the influence of antioxidant polarity on lipid oxidation concluded that polar antioxidants inhibit lipid oxidation in bulk oil systems more effectively than non-polar antioxidants.6,21,22 A number of studies have previously examined the effects of combinations of phospholipids and antioxidants on lipid oxidation in bulk oils, such as α-tocopherol, flavonoids, etc.8,23,24 Some of these studies used lecithin with a high proportion of PE as the phospholipid source, some of them used commercial oils with unknown intrinsic antioxidant levels, and some used model systems like triolein, methyl linoleate, or methyl laurate which may not accurately reflect commercial bulk oil compositions.24 For this reason, we used a model system that consisted of stripped soybean oil to mimic commercial oil triacylglycerol compositions and well-defined phospholipids and antioxidants to provide a more fundamental understanding of the complex physicochemical phenomenon involved.The formation of lipid hydroperoxides (LH) and headspace hexanal in SSO containing different levels of phospholipid (0 and 1000 μM DOPC) and the non-polar antioxidant α-tocopherol (0, 10 and 100 μM) was measured during incubation at 55 °C (Fig. 2A and B). As discussed earlier, in the absence of phospholipid and antioxidants the lag phase of lipid hydroperoxides (LH) and hexanal formation for the control was 3 days for the SSO. The incorporation of 1000 μM DOPC reduced the lag phase of LH and hexanal formation to 2 days, again indicating that this phospholipid was prooxidative. In the absence of DOPC, the duration of the lag phase increased with increasing α-tocopherol concentration compared to the control. The addition of 10 and 100 μM α-tocopherol increased the lag phase for LH to 9 and 27 days, respectively and headspace hexanal formation to 7 and 27 days, respectively. When DOPC was present, the impact of α-tocopherol on lipid oxidation was more complex (Fig. 2A and 2B). DOPC enhanced the antioxidant activity of 10 μM α-tocopherol by extending the lag phase of LH formation from 9 to 11 days (Fig. 2A), and hexanal formation from 7 to 11 days (Fig. 2B). The opposite trends were observed when 100 μM α-tocopherol and DOPC were added to the SSO. In this case, the lag time was shorter (less oxidatively stable) in the presence of DOPC than in its absence (Fig. 2A, B). For example, DOPC decreased the lag phase of LH formation from 27 to 23 days (Fig. 2A) and hexanal formation from 27 to 20 days (Fig. 2B) in the presence of 100 μM α-tocopherol.
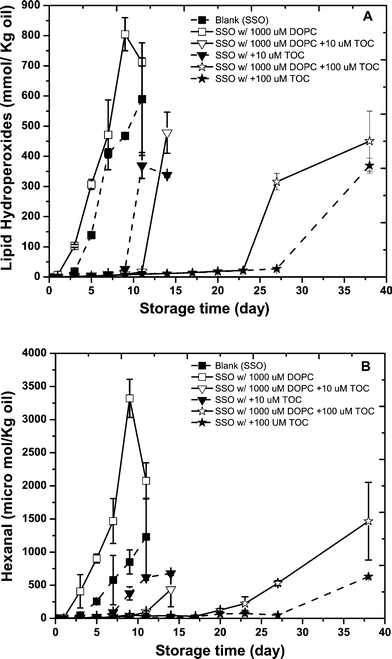 | ||
| Fig. 2 Formation of lipid hydroperoxides (A) and hexanal (B) in stripped soybean oil containing 1000 μM of 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) with or with 10 or100 μM α-tocopherol during storage at 55 °C. Some of the error bars are within data points. | ||
The impact of DC4PC on the antioxidant activity of α-tocopherol was also determined (Fig. 3A and B). As observed previously, DC4PC did not impact with lipid hydroperoxide or hexanal formation rates compared to the control. α-Tocopherol again decreased oxidation rates but in this case DC4PC decreased the effectiveness or α-tocopherol at both 10 and 100 μM unlike DOPC which enhances antioxidant activity only at 10 μM.
 | ||
| Fig. 3 Formation of lipid hydroperoxides (A) and hexanal (B) in stripped soybean oil containing 1000 μM of 1,2-dibutyryl-sn-glycero-3- phosphocholine (DC4PC) with or with 10 or100 μM α-tocopherol during storage at 55 °C. Some of the error bars are within data points. | ||
The impact of phospholipids on the oxidative stability of SSO in the presence of Trolox
The impact of different concentrations of Trolox (10 and 100 μM), the polar analog of α-tocopherol, on the oxidative stability of SSO with or without 1000 μM DOPC was also investigated. Again, the formation of LH and hexanal were determined during storage at 55 °C (Fig. 4 A and B). Trolox at 10 μM increased the lag phase for both lipid hydroperoxides and headspace hexanal formation from 3 (control) to 14 days. When 10 μM Trolox was added to the SSO in combination with 1000 μM DOPC the lag phase for lipid hydroperoxides increased from 14 to17 days while headspace hexanal formation was similar in the presence and absence of DOPC. Increasing the concentration of Trolox to 100 μM further decreased lipid oxidation rates with no appreciable increase in lipid hydroperoxides or hexanal after 37 days of storage (again more effective than α-tocopherol). However, like α-tocopherol, the presence of 1000 μM of DOPC decreased the antioxidant activity of 100 μM Trolox with the lag phase of LH and hexanal formation decreasing to 27 and 20 days, respectively.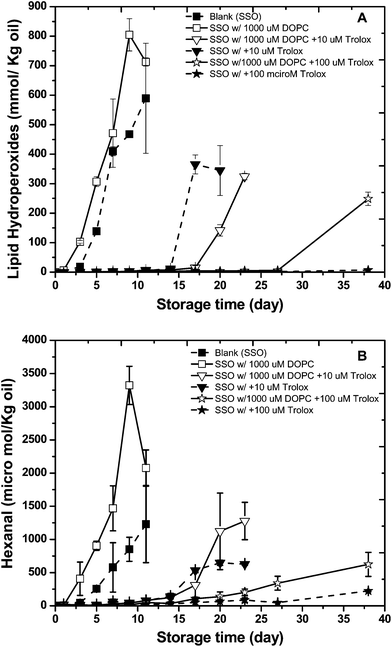 | ||
| Fig. 4 Formation of lipid hydroperoxides (A) and hexanal (B) in stripped soybean oil containing 1000 μM of 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) with or with 10 or 100 μM Trolox during storage at 55 °C. Some of the error bars are within data points. | ||
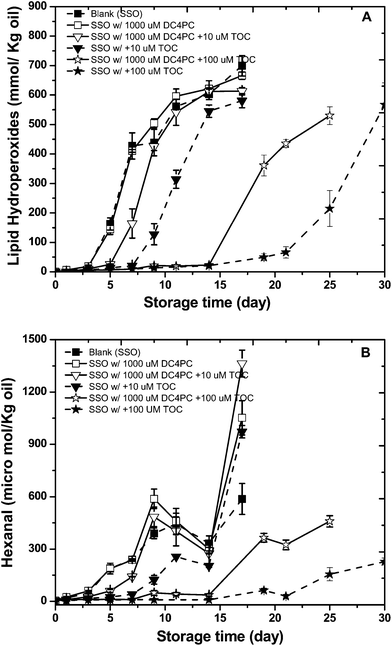
In the presence of DC4PC, the antioxidant activity of Trolox decreased (Fig. 5A and B). At a concentration of 10 μM Trolox, DC4PC decreased the lag phase for hydroperoxide formation from 9 to 7 days and hexanal formation from 9 to 5 days. At a concentration of 100 μM Trolox, DC4PC decreased the lag phase for hydroperoxide and hexanal formation to 25 days (no LH or hexanal formation was observed for Trolox alone).
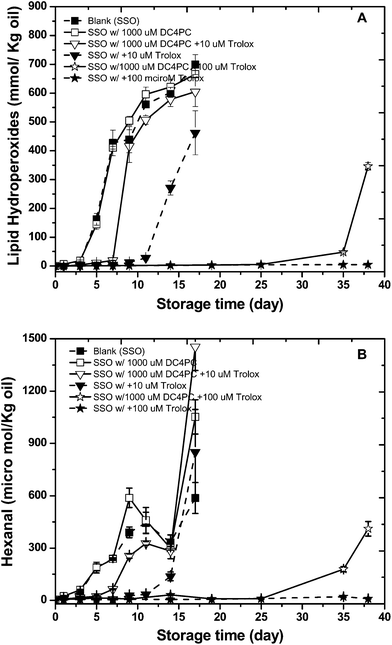 | ||
| Fig. 5 Formation of lipid hydroperoxides (A) and hexanal (B) in stripped soybean oil containing 1000 μM of 1,2-dibutyryl-sn-glycero-3- phosphocholine (DC4PC) with or with 10 or100 μM Trolox during storage at 55 °C. Some of the error bars are within data points. | ||
Overall, both α-tocopherol and Trolox inhibited lipid hydroperoxide and hexanal formation with increasing concentrations from 10 to 100 μM increasing antioxidant activity. The more polar Trolox was more effective than α-tocopherol which is similar to trends reported by other researchers.6
The impact of antioxidants on the properties of DOPC reverse micelles in SSO
Aqueous self-assembly of amphiphilic surfactants to form association colloids are recognized to be driven primarily by hydrophobic interactions.25 In non aqueous systems, uncertain effects could also drive the self-assembly of amphiphiles into association colloids and this is referred to as reverse self-assembly.26 Our previous research illustrated 1000 μM of amphiphilic DOPC plus 200 ppm water resulted in the formation of reverse micelles, a type of association colloid. Here, the physical structures of the DOPC/water reverse micelles containing different concentrations of antioxidants were measured using small angle X-ray scattering (SAXS) and the fluorescence steady state and lifetime decay of the surface active fluorescent probe, NBD-PE. These studies were not carried out with DC4PC as this phospholipid does not form association colloids at the concentrations used in this study.SAXS is a unique technique which has been widely used to distinguish structure transitions in reverse micelles. In our previous study, SAXS was able to determine that DOPC and water formed reverse micelles and that the size and shape of the reverse micelles changed with varying water concentrations. The same technique was employed in this study to determine if α-tocopherol and Trolox had any impact on the structure of DOPC reverse. SAXS patterns revealed that the scattering patterns of DOPC reverse micelles were not altered by either α-tocopherol or Trolox (data not shown) indicating that the antioxidants did not have a major impact on the size and shape of the reverse micelles. The only difference of SAXS between the samples was scattering intensity; however, this difference did not increase with increasing antioxidants concentration. Others reported the phospholipids aliphatic chain is the main factor that impacts the scattering intensity of phospholipids structures.27 Therefore, the intensity differences caused by the antioxidants could be due to their association with the phospholipid acyl chains thus causing attenuation of the X-ray scattering.
Fluorophores have been reported to provide valuable insights into microenvironmental changes in reverse micelles.28 Previously, we selected NBD-PE, a phospholipid analog grafted with a fluorophore on the phosphate head groups to study the interfacial properties of DOPC reverse micelle systems. NBD-PE emission intensity increases in a polar environment. The lowest recorded emission of NBD-PE was observed in the SSO control. The addition of DOPC and water in SSO increased the emission to 8.5 × 105 counts, 30% higher than in SSO alone. Neither α-tocopherol nor Trolox caused a shift in the fluorescence emission peak wavelength (data not shown). The addition of 10 μM α-tocopherol decreased the emission intensity of NBD-PE in the DOPC reverse micelles but further increases in α-tocopherol did not further decrease NBD-PE fluorescence (Fig. 6). Conversely, a concentration dependent decrease in NBD-PE fluorescence occurred in the presence of Trolox.
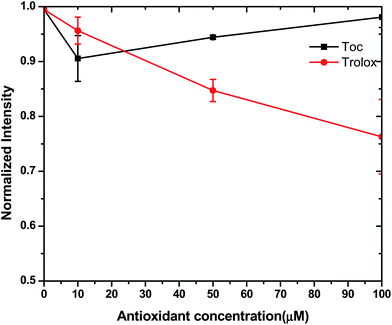 | ||
| Fig. 6 The normalized fluorescence intensity of NBD-PE at stripped soybean oil containing 1000 μM of 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) with varying concentrations (0–100μM) of α-tocopherol and Trolox. | ||
The low NBD-PE emission in the SSO control is likely due to the water concentrations (< 60 ppm in pure SSO). When DOPC and water were added to the SSO, NBD-PE was incorporated into the reverse micelles thus placing it in a more polar environment thus increasing its emission intensity. The ability of increasing concentrations of the water soluble Trolox to decrease fluorescence intensity suggests to that Trolox can interact with water in the reverse micelle thus decreasing NBD-PE-water interactions and thus fluorescence intensity. Additionally, the polar Trolox molecules could directly interact with NBE-PE thus decreasing fluorescence intensity by decreasing the ability of NBD-PE to become excited. Overall, this data suggests that both the NBD-PE probe and Trolox are incorporated into the DOPC reverse micelles.
Information about the properties of the antioxidants in the reverse micelles can also be gained by measuring the lifetime decay of NBD-PE. Overall, neither α-tocopherol not Trolox had a major impact on the fluorescence lifetime of NBE-PE (Fig. 7A and B). However, there was a slight trend of Trolox decreasing the lifetime of NBE-PE in DOPC reverse micelles. This again suggests that both the NBD-PE probe and Trolox are incorporated into the DOPC reverse micelles.
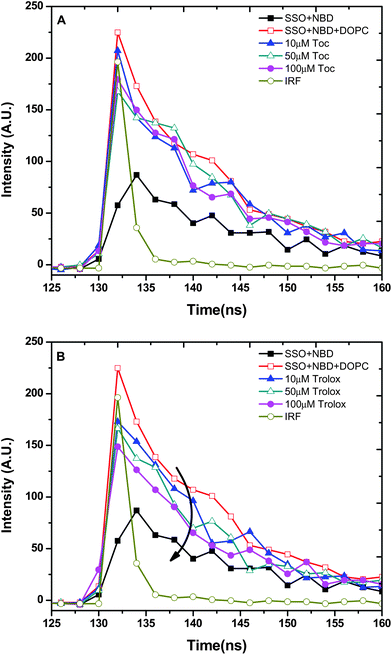 | ||
| Fig. 7 The fluorescence lifetime decay of NBD-PE at stripped soybean oil containing 1000 μM of 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) with varying concentrations (0–100μM) of α-tocopherol (A) and Trolox (B) concentrations. IRF is the instrument response function. | ||
Discussion
The main motivation of investigating the impact of DOPC based reverse micelles in stripped soybean oil on the physicochemical properties of antioxidants was to better understand the underlying phenomena of lipid and antioxidant interactions in bulk oil. The parameters evaluated were phospholipids with the same polar head groups and different fatty acyl chains (DC4PC and DOPC) that would result in formation of different types of physical structures. Our previous study showed that DOPC but not DC4PC formed reverse micelles in SSO.10 In addition, DOPC reverse micelles accelerated lipid oxidation while DC4PC, which does not form physical structure had no impact on lipid oxidation rates. This data suggest that lipid oxidation in bulk oils is not only influenced by the traditional chemical factors, such as lipid compositions, transition metals and antioxidants, but is also related to the existence of physical structures. Since one of the most effective techniques employed for retarding the lipid oxidation is the addition of free radical scavengers, this study was conducted as an extension of the previous study to determine how physical structure impact the activity of antioxidant in bulk oils. In this study, α-tocopherol and Trolox were chosen as hydrophobic and hydrophilic antioxidants that have similar free radical scavenging functional groups (e.g. same chromanol ring) with different hydrocarbon tails.29DOPC by itself promoted lipid oxidation while DC4PC had no effect. This would suggest that the physical structure formed by DOPC increased lipid oxidation rates since both DOPC and DC4PC have the same polar head groups and thus would impact the chemistry of lipid oxidation in a similar manner. This is supported by the previous observation that DOPC below its critical micelle concentration, where no reverse micelles are formed, does not promote lipid oxidation.10 The prooxidative effect of DOPC suggest that reverse micelles facilitate lipid oxidation.
One would expect the prooxidant activity of DOPC reverse micelles to decrease the activity of the antioxidants. However, at low antioxidant concentrations, DOPC increased the activity of both Trolox and α-tocopherol. Koga and Terao7 have postulated that physical structures formed by phospholipids can increase the antioxidant activity of tocopherols by allowing them to concentration at the oil–water interface where lipid oxidation is most prevalent. However, increasing α-tocopherol and Trolox concentrations to 100 μM in the presence of a constant DOPC concentrations resulted in a reduction in the effectiveness of the antioxidant. Antioxidants such as tocopherols in bulk oils have been shown to lose effectiveness as their concentrations increase and this could help to explain this observation. However, loss of effectiveness with increasing concentrations is not typically seen at the α-tocopherol and Trolox concentrations used in this study.30 A simple explanation for this observation is not available but it could be due to differences in the number and/or type of association colloids found in this simple model system compared to commercial oils that would contain a much greater variety of endogenous surface active molecules. Another possible explanation is that the DOPC reverse micelles used in this study would produce a negatively charged interface that could attract prooxidative transition metals. Partitioning of high concentrations of α-tocopherol and Trolox into the same physical location as the transition metals could allow the antioxidants to reduce the metals into their more prooxidative state and thus increase lipid oxidation rates which would be seen as a decrease in the effectiveness of the antioxidants. This pathway could be further increased by the accumulation of lipid hydroperoxides, the substrate for metal promoted lipid oxidation, into these same structures since they have been reported to partition into reverse micelle structures in oils and act as the co-surfactant.31 The presence of DC4PC only decreased the activity of α-tocopherol and Trolox indicating that it was not able to enhance the activity of the antioxidants.
Trolox was a more effective antioxidant in the presence of the DOPC micelles than α-tocopherol which is in agreement with previous research.6 The fluorescence of NBD-PE was affected to a much greater extent by Trolox than α-tocopherol suggesting that Trolox was partitioning into the DOPC reverse micelles differently than α-tocopherol.
This could be due to Trolox's higher water solubility which would allow it to partition into the water phase while α-tocopherol has virtually no water solubility and thus at best would align at the oil–water interface. Differences in the physical location of Trolox and α-tocopherol could alter the overall impact on lipid oxidation by influencing their antioxidant (free radical scavenging) or prooxidant (reduction of transition metals) activity.
This study showed that the antioxidant activity of both Trolox and α-tocopherol were influenced by physical structures in bulk oils. Work such as this can provide important data on how physical structures in bulk oils impact the activity of antioxidants. Since few new antioxidants are available for food applications, this information might provide information on how to alter the physical structures in bulk oils such that we can utilize the available food antioxidants more effectively.
Acknowledgements
This material is based upon work supported by United States Department of Agriculture (project No. 2007-02650).References
- D. B. Min and J. M. Boff, Food lipid chemistry, nutrition, and biotechnology, 2002, 2, 335–363 Search PubMed.
- E. Choe and D. B. Min, Crit. Rev. Food Sci. Nutr., 2006, 5, 169–186 Search PubMed.
- E. A. Decker, K. Warner, M. P. Richards and F. Shahidi, J. Agric. Food Chem., 2005, 53, 4303–4310 Search PubMed.
- W. Chaiyasit, R. J. Elias, D. J. McClements and E. A. Decker, Crit. Rev. Food Sci. Nutr., 2007, 47, 299–317 Search PubMed.
- E. N. Frankel, Food Chem., 1996, 57, 51–55 Search PubMed.
- S. W. Huang, A. Hopia, K. Schwarz, E. N. Frankel and J. B. German, J. Agric. Food Chem., 1996, 44, 444–452 CrossRef CAS.
- T. Koga and J. Terao, J. Agric. Food Chem., 1995, 43, 1450–1454 CrossRef CAS.
- D. H. Hildebrand, J. Terao and M. Kito, J. Am. Oil Chem. Soc., 1984, 61, 552–555 CAS.
- B. J. F. Hudson and M. Ghavami, Lebensmittel-ú & Technologie, 1984, 17, 191–194 Search PubMed.
- B. Chen, A. Han, D. J. McClements and E. A. Decker, J. Agric. Food Chem, 2010, 58, 11993–11999 Search PubMed.
- C. S. Boon, Z. Xu, X. Yue, D. J. McClements, J. Weiss and E. A. Decker, J. Agric. Food Chem., 2008, 56, 1408–1414 Search PubMed.
- Y. H. Wang, Q. Y. Mai, X. L. Qin, B. Yang, Z. L. Wang and H. T. Chen, J. Agric. Food Chem., 2009, 58, 642–649 Search PubMed.
- A. Felgner, R. Schlink, P. Kirschenbüler, B. Faas and H. D. Isengard, Food Chem., 2008, 106, 1379–1384 Search PubMed.
- N. C. Shantha and E. A. Decker, J. AOAC Int., 1994, 77, 421–424 CAS.
- T. Waraho, V. Cardenia, M. T. Rodriguez-Estrada, D. J. McClements and E. A. Decker, J. Agric. Food Chem., 2009, 57, 7112–7117 Search PubMed.
- C. V. Nwosu, L. C. Boyd and B. Sheldon, J. Am. Oil Chem. Soc., 1997, 74, 293–297 Search PubMed.
- H. Saito and K. Ishihara, J. Am. Oil Chem. Soc., 1997, 74, 1531–1536 Search PubMed.
- N. M. Bandarra, R. M. Campos, I. Batista, M. L. Nunes and J. M. Empis, J. Am. Oil Chem. Soc., 1999, 76, 905–913 Search PubMed.
- A. Takenaka, M. Hosokawa and K. Miyashita, J. Oleo Sci., 2007, 56, 511–516 Search PubMed.
- J. Le Grandois, E. Marchioni, M. Zhao, F. Giuffrida, S. Ennahar and F. Bindler, J. Agric. Food Chem., 2010, 58, 2973–2979 Search PubMed.
- E. N. Frankel, S. W. Huang, J. Kanner and J. B. German, J. Agric. Food Chem., 1994, 42, 1054–1059 CrossRef CAS.
- W. L. Porter, E. D. Black and A. M. Drolet, J. Agric. Food Chem., 1989, 37, 615–624 CrossRef CAS.
- L. I. Mazaletskaya, N. I. Sheludchenko and L. N. Shishkina, Appl. Biochem. Microbiol., 2010, 46, 135–139 Search PubMed.
- M. F. Ramadan, LWT–Food Sci. Technol., 2008, 41, 581–587 Search PubMed.
- S. Svenson, Curr. Opin. Colloid Interface Sci., 2004, 9, 201–212 CrossRef CAS.
- R. Nagarajan, Curr. Opin. Colloid Interface Sci., 1996, 1, 391–401 CrossRef CAS.
- M. Fanun, J. Dispersion Sci. Technol., 2009, 30, 115–123 Search PubMed.
- A. Chattopadhyay, S. Mukherjee and H. Raghuraman, J. Phys. Chem. B, 2002, 106, 13002–13009 CrossRef CAS.
- W. Chaiyasit, D. J. McClements and E. A. Decker, J. Agric. Food Chem., 2005, 53, 4982–4988 Search PubMed.
- J. C. Evans, D. R. Kodali and P. B. Addis, J. Am. Oil Chem. Soc., 2002, 79, 47–51 Search PubMed.
- V. D. Kortenska, N. V. Yanishlieva, O. T. Kasaikina, I. R. Totzeva, M. I. Boneva and I. F. Russina, Eur. J. Lipid Sci. Technol., 2002, 104, 513–519 Search PubMed.
| This journal is © The Royal Society of Chemistry 2011 |
