Inhibition of angiotensin converting enzyme (ACE) activity by polyphenols from tea (Camellia sinensis) and links to processing method
Junjie
Dong
ab,
Xinqing
Xu
a,
Yuerong
Liang
b,
Richard
Head
c and
Louise
Bennett
*a
aCSIRO Preventative Health Flagship, CSIRO Food and Nutritional Sciences, 671 Sneydes Road, Private Bag 16, Werribee, Victoria 3030, Australia. Fax: +61 3 9731 3390; Tel: +61 3 9731 3200
bZhejiang University, Tea Research Institute, 268 Kaixuan Road, Hangzhou, 310029, PR China
cCSIRO Preventative Health National Research Flagship, PO Box 10041, Adelaide, BC, SA 5000, Australia
First published on 26th May 2011
Abstract
The focus of this study was to investigate Angiotensin Converting Enzyme (ACE) inhibiting activity across 34 teas (Camellia sinensis) produced by 5 different processing methods including green (GT), oolong (OT), white (WT), black (BT) and dark (DT) teas. In vitroACE inhibitory activity was affected by the tea processing method with IC50 values for ACE inhibition: green < oolong < white < black < dark teas. Substrate-dependence of the reaction kinetics was studied for GT and BT polyphenolic size fractions either < or > 3 kDa and also Green Tea Polyphenolic Isolate (GTPI), and revealed that enzyme velocity curves fitted allosteric, not Michaelis–Menten, relationships. Inhibition was weakly dependent on substrate concentration for GT fraction >3 kDa and independent of substrate concentration for all other GT and BT size fractions and GTPI. Furthermore, evidence for direct inactivation of ACE by GTPI was demonstrated. Overall, the results suggest that tea polyphenolics exert a mixed mode of in vitro inhibition of ACE, mostly of a kinetically uncompetitive type. The results are discussed in the context of in vivo and epidemiological evidence for regulation of blood pressure by tea consumption.
1. Introduction
The renin-angiotensin system (RAS) is a key mechanism in the body for the physiological regulation of blood pressure (BP), fluid and electrolyte balance, including dietary salts. In particular, the angiotensin-converting enzyme (ACE), a carboxypeptidase, participates in regulating BP by converting an inactive form of the decapeptide angiotensin to a potent vasopressor octapeptide, angiotensin II. Therapeutic ACE inhibitors represent an important and common class of pharmaceuticals for control of hypertension.1 Additionally, key elements of RAS have been identified in the human gastro-intestinal tract including establishing sub-epithelial expression of ACE and noting the link between treatment with ACE inhibitors for hypertension with lower incidence of esophageal adenocarcenoma.2 Because of the regulation of tissue proliferation and differentiation by angiotensin II,2 the application of therapeutic regulators of RAS to regulation of function of the gastro-intestinal tract is predicted.3 These findings raise the possibility that dietary regulation of ACE may occur at the gut epithelium. A broad range of food components of different chemical classes, including polyphenolics, peptides and polyunsaturated fatty acids, have been shown to lower BP via inhibition of ACE and other mechanisms.4 In particular, polyphenolic-rich extracts from 13 Brazilian plants exhibited a range of in vitro ACE inhibitory activities5 and in vitro ACE-inhibitory activity of a selection of dietary plants was also linked with the polyphenolics.6It has been reported that green tea can inhibit the activity of ACEin vitro,7,8,9 and this raises the possibility that consumption of tea may mimic known synthetic ACE inhibitors. It has been suggested that the in vitro ACE inhibitory activity of tea is probably due to polyphenols; however, the bioactive form of polyphenolics exerting anti-hypertensive effect in vivo has not been confirmed. Wang and Wang10 have attributed BP-lowering and other activities also to tea polysaccharides, as reviewed by Nie and Xie.11
In recent years, tea (Camellia sinensis), the most widely consumed beverage in the world, has been shown to modulate many physiological functions,12 including anti-angiogenic activity, anti-oxidant13 and anti-microbial bioactivities.14 In addition, beneficial effects on arterial and endothelial function, anti-inflammatory, anti-oxidant, anti-thrombotic and lipid-lowering capacities have linked tea strongly with protection against cardiovascular disease.15 However, human epidemiological and interventional studies conducted on animals and humans, have not produced consistent results regarding anti-hypertensive effects of tea in vivo16,13,17 with several biological mechanisms of BP regulation implicated.18–21 Effects observed in a short-term intervention study suggested that habitual moderate strength green, black or oolong tea consumption could reduce the risk of developing hypertension.21 Several epidemiological studies and clinical trials have shown that green tea, and black and oolong teas to a lesser extent, exert a favorable influence on the cardio-vascular system.21–24 Several animal studies have demonstrated that black, oolong and green tea polyphenols, which are potent anti-oxidants, can attenuate BP increase in spontaneously hypertensive rats25–27 and prevent atherosclerosis in Apoprotein E-deficient mice.28 In addition, it has been reported that theanine, at high doses, significantly decreases the BP in spontaneously hypertensive rats but does not alter BP in normal rats.29
The composition of polyphenolics in commercial teas varies with species, season, horticultural conditions and particularly with degree of oxidization or fermentation during the manufacturing process. Teas have been traditionally classified as non-oxidized green tea, partially oxidized white and oolong teas, fully-oxidized black tea and fermented dark (Pu-erh) tea. The unoxidised leaves of green tea contain 30–40% polyphenols and the predominant group of polyphenols are the flavanols, specifically, catechins. There are 8 major catechins in tea based on the absence or presence of a gallyl substituent on the following 4 catechins: (+)-catechin, (−)-epicatechin, (−)-gallocatechin and (−)-epigallo catechin.30Catechins are susceptible to chemical transformations during post-harvest processing, leading to the formation of dimers and polymers of catechins in enzyme-inactivated green and dark teas and to polymeric theaflavins and thearubigins in enzyme-active black and oolong teas.31 In contrast, monomeric catechins are relatively unmodified and therefore more abundant in green tea with significant levels of the catechin: epigallocatechin gallate (EGCG).32
In this study, the effects of processing conditions on ACE inhibitory properties of 34 tea products from five distinct processing methods including, green, oolong, white, black and dark teas, were studied and the results interpreted in the context of processing conditions. Enzyme inhibition kinetics of different size fractions of green and black teas were also studied in order to characterise the mechanism of inhibition. The results assist in understanding the structure to function relationships of respective processed forms of tea, and may inform development of bioactive fractions for subsequent research on in vivo BP regulation by ACE inhibition.
2. Results and discussion
2.1 Characterisation of ACE inhibitory activity of different processed forms of tea
Many products are produced in China from Camellia sinensis, which are classified according to processing method into five main types: non-oxidized green tea, partially oxidized oolong and white teas, totally oxidized black tea and fermented dark tea (Fig. 1). For green tea, fresh tea leaves, polyphenol-oxidase (PPO) enzyme is inactivated by dry heat or steam,33 thereby maintaining polyphenols in their non-oxidised catechin form. In contrast, black tea is produced with extensive oxidation of polyphenols, resulting in the oxidized and polymerized compounds called thearubigins and theaflavins.31 Oolong tea is a partially oxidized product and contains a mixture of monomeric polyphenols and higher molecular weight theaflavins. In ‘fermented’ teas, new compounds are produced with novel bioactivities, e.g., anti-microbial.14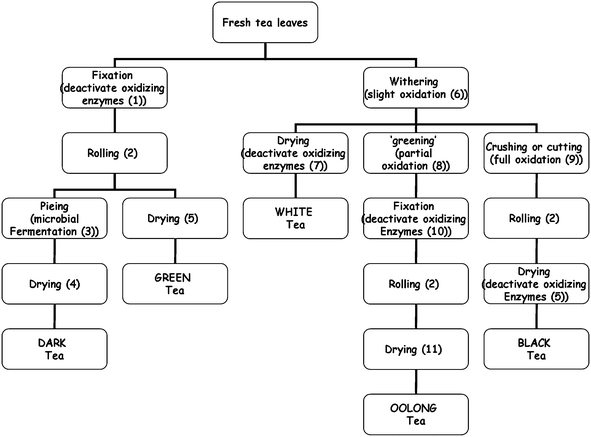 | ||
| Fig. 1 Schematic showing different conditions for green, oolong, white, black and dark tea processing styles practised in China. (1) heating for short time (min) under dry (pan) or wet (steam) heat conditions, at 85 °C; (2) dry heating and rolling at 60–70 °C; (3) activation of endogenous enzymes (e.g., fungi) and incubation at approximately 30 °C under moist conditions; (4) air drying at 40 °C; (5) pan drying at 100 °C; (6) wilting under sun radiation or left in cool breeze, removing ∼25% leaf moisture and slight oxidation; (7) air drying or baking at 40 °C for 10 h; (8) aeration at room temp with agitation (5 cycles of 10 min); (9) chopping and crushing of leaf under ambient conditions for 1 h; (10) pan heating at 100 °C for short time; (11) pan drying at 100 °C under either constant conditions for 8 h or 6 cycles of 1 h. All teas are dried to a total solids of ≥95%. | ||
The IC50 values for ACE inhibition of hot water-extractable solids were determined for the 34 different teas (Table 1) with process-related variation observed both within and between process classes (Fig. 2a). Tea products contained approximately 3.0% of soluble solids which varied within sets of like-processed teas for example, the solubility of the 5 black teas varied by 8% whereas the solubility of the 3 white teas were consistent to within 3% (Table 2).
| Sample code | Class | Commercial name | Camellia sinensis cultivar | Leaf size | Harvest season | Source (Chinese Province) |
|---|---|---|---|---|---|---|
| a GT01-GT10: fixed by dry (pan) heating, at 85 °C. b GT11, GT12: fixed by wet (steam) heating. c OT1–OT5: pan drying at 100 °C for 8 h. d OT6–OT9 oven-baked drying at 100 °C under heating-cooling cycles of ∼6 times 1 h duration. e DT1, DT3, DT5: fermentation under high humidity for 30–40 days at 40–60 °C. f D2, D4: fermentation under ambient humidity for 42 h at 20–30 °C. g tea grade is related to the maturity of the leaf before harvest with grade deteriorating as the leaf ages on the plant. | ||||||
| GT1 a | Green | Bixueyingchun – Grade 1g | Bixueyingchun | Small | Spring | Hubei |
| GT2 a | Green | Bixueyingchun – Grade 2 | Bixueyingchun | Small | Spring | Hubei |
| GT3 a | Green | Tangji - Grade 1 | Jiu keng | Small | Spring | Zhejiang |
| GT4 a | Green | Tangji - Grade 2 | Jiu keng | Small | Spring | Zhejiang |
| GT5 a | Green | Longquanxiangcha – Grade 1 | Jiu keng | Small | Spring | Zhejiang |
| GT6 a | Green | Longquanxiangcha - Grade 2 | Jiu keng | Small | Spring | Zhejiang |
| GT7 a | Green | Swan | Jiu keng | Small | Spring | Zhejiang |
| GT8 a | Green | Zisun | Jiu keng | Small | Spring | Zhejiang |
| GT9 a | Green | Yelangcuipian | Qianmei | Small | Spring | Guizhou |
| GT10 a | Green | Xihulongjing | Jiu keng | Small | Spring | Zhejiang |
| GT11 b | Green | Zhengqing - Grade 1 | Jiu keng | Small | Spring | Zhejiang |
| GT12 b | Green | Zhengqing - Grade 2 | Jiu keng | Small | Spring | Zhejiang |
| OT1 c | Oolong | Jinguanyin | Jin Kuan Yin | Medium | Spring | Fujian |
| OT2 c | Oolong | Jinguanyin | Jin Kuan Yin | Medium | Summer | Fujian |
| OT3 c | Oolong | Jinguanyin | Jin Kuan Yin | Medium | Autumn | Fujian |
| OT4 c | Oolong | Tieguanyin | Ti Kuan Yin | Medium | Spring | Fujian |
| OT5 c | Oolong | Tieguanyin | Ti Kuan Yin | Medium | Summer | Fujian |
| OT6 d | Oolong | Rougui | Cinnamon | Medium | Autumn | Mount Wuyi,Fujian |
| OT7 d | Oolong | Dahongpao | Big Red Robe | Medium | Autumn | Mount Wuyi,Fujian |
| OT8 d | Oolong | Fenghuangdancong-huangzhi | Dān Cōng | Medium | Autumn | Guangdong |
| OT9 d | Oolong | Fenghuangdancong-milan | Dān Cōng | Medium | Autumn | Guangdong |
| WT1 | White | Silver needle with white hair | Da Bai | Medium | Spring | Fujian |
| WT2 | White | White peony - Grade 1 | Da Bai | Medium | Spring | Fujian |
| WT3 | White | White peony - Grade 2 | Da Bai | Medium | Spring | Fujian |
| BT1 | Black | Zhengshanxiaozhong | Meizhan | Small | Summer | Mount Wuyi, Fujian. |
| BT2 | Black | Dianhong - Grade 1 | Fuyun | Large | Summer | Yunnan |
| BT3 | Black | Dianhong - Grade 5 | Fuyun | Large | Summer | Yunnan |
| BT4 | Black | Qihong - Grade 1 | Qihong | Small | Summer | Qimen, Anhui |
| BT5 | Black | Qihong - Grade 5 | Qihong | Small | Summer | Qimen, Anhui |
| DT1 e | Dark | Puer tea (loose leaf) | Fuyun | Large | Spring | Yunnan |
| DT2 f | Dark | Puer sheng tea (3 years old) | Fuyun | Large | Spring | Yunnan |
| DT3 e | Dark | Puer shu tea (3 years old) | Fuyun | Large | Spring | Yunnan |
| DT4 f | Dark | Puer sheng tea (5 years old) | Fuyun | Large | Spring | Yunnan |
| DT5 e | Dark | Puer shu tea (5 years old) | Fuyun | Large | Spring | Yunnan |
| GTPI | Green | Polyphenolic isolate | Hangzhou |
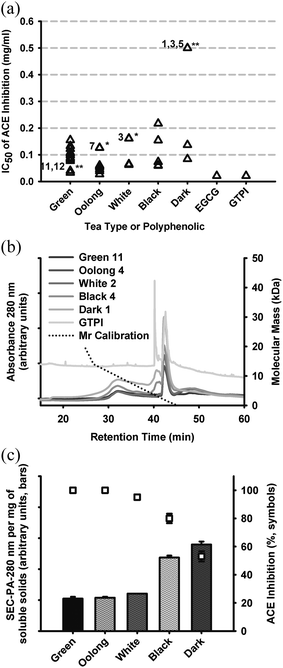 | ||
| Fig. 2 (a) ACE inhibitory activity of hot water-extractable tea solids reported as values of IC50, grouped according to processing style, with statistically different values within each group designated * = P < 0.05 and ** = P < 0.01, also showing values for reference samples: EGCG (0.021 mg ml−1) and GTPI (0.021 mg ml−1). (b) HPSEC profiles monitored at 280 nm, of representative tea products identified as per Table 1, also showing the relationship of retention time to molecular mass using standards over the range 75 Da to 66 kDa. (c) correlation between HPSEC profile total peak area at constant total soluble solids (10 mg ml−1) and percentage ACE inhibition determined for respective samples. | ||
| Tea type | Samples represented in average | Proportion of soluble solids (%) |
|---|---|---|
| Green | GT01–GT12 | 3.23 ± 0.29 |
| Oolong | OT01–OT09 | 3.18 ± 0.29 |
| White | WT01–WT03 | 2.77 ± 0.15 |
| Black | BT01–BT05 | 2.86 ± 0.43 |
| Dark | DT01–DT05 | 3.18 ± 0.33 |
The dried solids from hot water extractions of different teas exhibited IC50 values ranging from 0.02 to 0.5 mg ml−1 with highest (apparent) activity observed for EGCG and GTPI, both at 0.021 mg ml−1. For green teas, IC50s ranged from 0.03 to 0.16 mg ml−1 with GT11 and GT12 grouped together having lowest IC50 (P < 0.001). The low IC50 of GT11 and GT12 appeared to be related to the use of steam to ‘fix’ (inactivate) oxidative enzymes. All other green teas, fixed with dry heat exhibited relatively higher values of IC50 (Fig. 1).
For oolong teas, IC50 values ranged from 0.02 to 0.13 mg ml−1 with OT7 an outlier (P < 0.05) with highest IC50. In this case, IC50 values were not sorted according to differentiated conditions of drying, i.e., constant heat versus heat-cool cycling (Table 1) and the tea of lowest ACE inhibitory activity, OT7, was a single representative of Big Red Robe cultivar (Table 1).
For white teas, IC50 values ranged from 0.06 to 0.17 mg ml−1 with WT3 an outlier (P < 0.05) with highest IC50, possibly related to leaf maturity or grade. The greatest range of bioactivity was observed for black teas with IC50s ranging from 0.06 to 0.22 mg ml−1. BT1 and BT2 appeared to outlie the BT dataset (NS) with loss of activity also possibly related to leaf maturity or grade.
For dark teas, the IC50 values ranged from 0.08 to >0.5 mg ml−1 with D1, D3 and D5 outliers (P < 0.001) having highest IC50 values. The distinguishing processing condition for dark tea is ‘pie-fermentation’ driven by endogenous fungi on the leaves.34DT1, DT3 and DT5 were wet-stored in this step by covering with a wet cloth at around 50 °C for 30–50 days in order to hasten fermentation, while DT2 and DT4 were fermented at room temperature and ambient moisture, which produces a lower extent of fermentation. DT2 and DT4 were atypical of DT processing and exhibited similar ACE inhibitory activity to other teas. The results suggested that extensive fermentation reduced the influence of polyphenolics involved with ACE inhibition, with lowest IC50 for ACE inhibition observed for DT2 and DT4.
Hot-water extracted solutes across a set of representative teas from each type were compared by size-exclusion chromatography showing that tea polyphenolics ranged from the lowest calibration point of 75 Da to 20 kDa (Fig. 2b) and varied regarding the ratio of low to higher mass range phenolics. The size range of GTPI was <5 kDa. The highest proportions of ‘polymeric’ phenolics, eluting between 30 and 40 min were associated with black and dark teas with lowest proportions present in green, oolong and white teas (Fig. 2b). A species eluting at ∼42 min of low abundance in green tea was apparently concentrated (or possibly chemically converted) by the process used for isolation of GTPI.
The ACE inhibitory activity of the same sample series, standardized for solids (Fig. 2c), indicated that, except for GTPI, enzyme inhibition was inversely correlated to total peak area at 280 nm. That is, ACE inhibition activity was attenuated in the black and dark teas, which produced the darkest-coloured solution per total soluble solids. The GTPI product exhibited both high absorptivity at 280 nm and ACE inhibition activity (not shown). The lightest-colored teas represented processing methods involving the least oxidation and without fermentation (Fig. 1). In contrast, the black and dark teas were produced by full oxidation (black) or fermentation without oxidation (dark).
The chemical nature of ACE inhibitory factors present in green and black teas were further investigated by determining the effects of treatment with exogenous enzymes: Mannanase (containing broad glucanase activity including: high galactomannanase and beta-mannanase and some 1,4-beta glucanase, xylanase and pectinase activity), and polyphenol oxidase (PPO). Treatment with Mannanase did not affect ACE inhibition activity of green tea but produced enzyme concentration and incubation time-dependent changes in activity for black tea, particularly at 0.1% Mannanase (Fig. 3a). This suggested that glycan residues of polyphenolics present in black tea were involved with ACE enzyme inhibition, by interaction with either the enzyme or substrate. The effect of PPO on ACE inhibitory activity of green and black teas was negligible at 0.1 mg ml−1 (not shown) but produced a small increase, at 1.0 mg ml−1GT and BT (P < 0.05, Fig. 3b). Enzyme action during processing on glycan and oxidisable groups could account for modulation of ACE inhibition activity in tea polyphenolics.
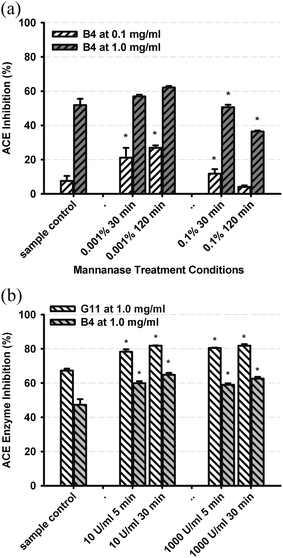 | ||
| Fig. 3 Effect on ACE inhibition activity of pre-treatment of (a) BT4 with Mannanase, at each of 2 levels (0.001%, 0.1%) and incubation times (30, 120 min), and (b) GT11 and BT4 with PPO at each of 2 levels (10 and 1000 U ml−1) and incubation times (5, 30 min), showing results after correction of enzyme and reagent controls. Values represent mean and standard deviation of triplicate determinations (* = P < 0.05 versus control). | ||
In summary, the highest ACE inhibitory activity was associated with green, oolong and white teas and lowest activity was associated with oxidized and fermented black and dark teas, respectively. Other properties of teas described in Table 1 could not be directly correlated with bioactivity but results suggested that there is broad compositional variation as a function of processing conditions affecting ACE inhibition activity. For example, the presence of moisture during fixation for green teas (increasing ACE inhibition activity) and during fermentation for dark teas (decreasing ACE inhibition activity, Fig. 1), seemed to chemically modify polyphenolics towards opposing ACE inhibitory activities indicating that the sequence and specific conditions of processing produced variable effects on polyphenolic chemistry and would require systematic investigation. Notwithstanding the unknown relationship between in vitro and in vivo activity, there appears to be potential for further optimisation of BP influence as a function of tea type.
2.2 Mixed mechanism mode inhibition of ACE by GTPI
The percentage of inhibition of ACE by GTPI at constant concentrations of enzyme and substrate was observed to increase as a function of reaction time (Fig. 4a), suggesting non-ideal enzyme kinetics. Furthermore, time-dependent inhibition of ACE activity, as reflected by a lowering in production of HA was observed in the presence of GTPI (Fig. 4b). The effect of GTPI on enzyme, substrate and product levels showed that levels of HA in either the presence of HA or HHL, and absence of ACE, were constant (Fig. 4b). In the presence of ACE and HHL under standard conditions, HA levels decreased towards zero, as a function of GTPI, indicating complete conversion of starting levels of HHL to HA (Fig. 4b). The progressive increase in ACE inhibition as a function of concentration of GTPI under either standard conditions (filled squares) or after pre-incubation of ACE with GTPI for 30 min (unfilled squares), were significantly different (P < 0.05) with pre-incubation lowering the apparent ACE activity (i.e, increasing the extent of inhibition) by approximately 15%. This suggested that GTPI was directly inactivating ACE and contributing to the total inhibition. The parallel rate of HA production with (filled squares) or without (unfilled squares) pre-incubation suggested that the mechanism of inhibition operative during the pre-incubation phase by GTPI was the same as that under standard assay conditions. That is, for GTPI, it appeared that enzyme inhibition occurred by irreversible inactivation of ACE. This direct inactivation of ACE by GTPI may also be applicable to tea polyphenolics in other processed forms of tea.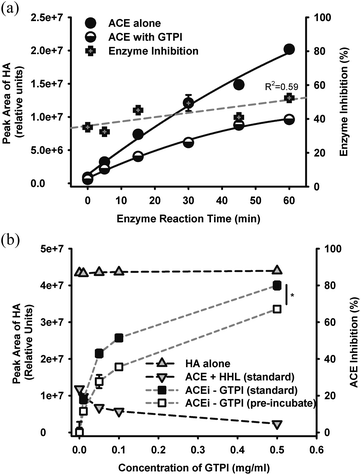 | ||
| Fig. 4 (a) ACE-mediated production of product HA from cleavage of the substrate HHL as a function of time, in the presence of 0.1 mg ml−1GTPI, showing upward trend in enzyme inhibition as a function of reaction time (R2 = 0.59). Standard assay conditions utilized a reaction time of 30 min. (b) Effect of added GTPI on the detectability of HA (shaded grey) or calculated inhibition of ACE (shaded black or white), under selected combinations of reagents, as follows: ‘HA alone’ contained 50 μl GTPI, 100 μl HA, 50 μL buffer, 30 min incubation before 100 μL HCl; ‘ACE + HHL (standard) ’ contained 50 μl GTPI, 50 μL ACE, 100 μl HHL, 30 min incubation before 100 μL HCl; ‘ACEi-GTPI (standard)’ contained 50 μl GTPI, 100 μl HHL, 50 μl ACE, 30 min incubation before 100 μl HCl and ACEi-GTPI (pre-incubated) contained 50 μl GTPI, 50 μl ACE pre-incubated for 30 min, then 100 μl HHL, 30 min further incubation before 100 μL HCl. | ||
The kinetics of ACE inhibition by GTPI was also studied. Inhibition kinetics plotted as enzyme velocity versus substrate concentration could not be analysed by the Michaelis-Menton equation, exhibiting a sigmoidal relationship and therefore required application of an allosteric enzyme model (Fig. 5a), also suggesting that irreversible chemical de-activation of the enzyme might be occurring. While it was possible to force the data to a Michaelis-Menton relationship, and achieve apparent mixed kinetic relationship by Lineweaver–Burk plotting, the data was clearly sigmoidal for all products studied, as exemplified for GTPI (Fig. 5a). The following analysis has therefore assumed an allosteric model, where K prime is the concentration of substrate at 50% of the maximum rate for a sigmoidal kinetic relationship. For GTPI, K prime was virtually independent of GTPI concentration (Fig. 5b), indicating that the mode of inhibition was substrate-independent.
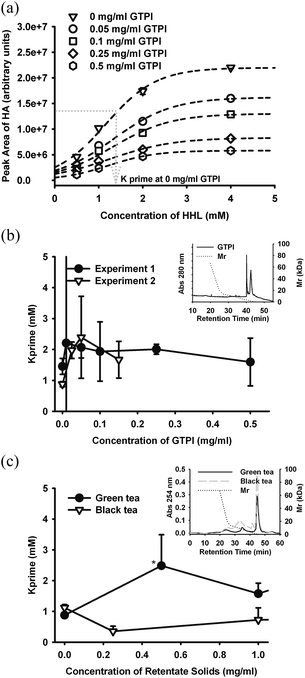 | ||
| Fig. 5 Relationships between K prime and concentration of tea-derived ACE inhibitors, after fitting to an allosteric (sigmoidal) reaction kinetic model showing (a) raw data for GTPI and the method of determining K prime, the concentration of substrate at half the maximum reaction velocity. Data points correspond to experiment 1 in (b), and are the mean of duplicate determinations and error bars are within the symbol dimensions. (b) K prime versus concentration of GTPI showing mean and coefficient of variation of duplicate determinations, for 2 independent experiments, and for (c) size fraction of GT11 and BT4 < 3kDa. Differences between GT and BT data points are only significant at 0.5 mg ml−1 (P < 0.05). Inserts show HPSEC profiles corresponding to tested samples. | ||
Green tea (GT11) and black tea (BT4) were processed to recover size fractions > and < 3 kDa and these products were also subjected to inhibition kinetic analysis (Fig. 5c). For, GT and BT products <3 kDa, K prime was independent of the concentration of tea solids <3 kDa (not shown). For GT and BT products >3 kDa, K prime was independent of the concentration of tea product for BT but exhibited transient concentration-dependence relationship to K prime for GT (Fig. 5c). These results suggest that the mechanism of ACE inhibition by both fractions of BT was viaenzyme inactivation and not substrate-dependent kinetics (i.e, was uncompetitive). In contrast, although GTPI inhibition of ACE was also independent of substrate, there was evidence for components of GT (>3 kDa, i.e, eluting <40 mins in Fig. 2b) that exhibited substrate-dependent enzyme inhibition. Thus, reaction kinetics analysis of ACE inhibition by tea polyphenolics resolved irreversible enzyme inactivation from ‘ideal’ (reversible) inhibition activity and revealed that only a minor proportion of polyphenolics in either GT or BT exerted ‘ideal’ enzyme inhibition. ‘Ideal’ substrate-dependent inhibition was only evident over a narrow concentration range (<0.6 mg ml−1) of the GT fraction >3 kDa. These results are supported by evidence that in vitro ACE inhibitory activity of purified tea epicatechins depended strongly on extent of polymerization with epicatechin pentamers and hexamers exhibiting lower IC50 and stronger substrate-dependent Mikaelis-Menton inhibition kinetics compared with monomeric, dimeric and trimeric epicatechins.8,9
In vitro ACE inhibitory activity was characterized for a series of purified flavan-3-ols from Chinese tea.35 The ACE inhibitory efficiency was dependent on structure with IC50 values ranging from 26.6 (epigallocatechin-3-O-methyl gallate) to 195.9 μM (gallocatechin). The reported IC50 value for EGCG of 37.4 μM35 compared favourably with that reported in this study of (0.021 mg ml−1, 46 μM). For the tea catechins, ACE inhibition was not affected by added BSA (i.e, proteins did not cause direct inactivation) however, some tea catechins also inhibited trypsin and chymotrypsin (i.e, non-specific) but did not inactivate ACE by the mechanism of Zn2+ complexation.35 The inhibition kinetics for catechin 3-O-gallate was non-competitive by Lineweaver–Burk plot, which predicted binding of catechin to both substrate and ACE in a reversible manner. This was in contrast to the present study of size-selected mixtures of BT and GT where substrate-dependent enzyme inhibition was only observed for polymeric GT fraction >3 kDa. The necessity to fit the raw data to a sigmoidal kinetic model inferred that tea phenolics were involved with irreversible interactions with ACE that inactivated the enzyme, as was shown specifically for GTPI (Fig. 5b). Epigallocatechin 3-O-methyl gallate, which inhibited ACE, trypsin and chymotrypsin, was also very effective at lowering BP in Spontaneous Hypertensive rats after intravenous administration of angiotensin I35 indicating that non-specific inhibition behavior in vitro did not preclude in vivo efficacy.
2.3 Tea and protection against cardiovascular disease
Prospective epidemiological studies have produced inconsistent trends from; substantial risk reduction for CVD to, lack of protection, which was attributed to other confounding factors associated with tea consumption.23,15 Neither BT nor GT have been shown to lower cholesterol in humans according to epidemiological studies although animal studies involving specific catechins administered at relatively elevated levels compared with typical consumption patterns, were able to show cholesterol lowering in aortic tissues but not plasma.24 Conflicting evidence between in vitro and in vivo studies have been interpreted to reflect the discrepancy between the inherently lower bioavailable proportion of orally-consumed tea polyphenolics compared with dosing under in vitro conditions. This was the case for effects of GT and BT on hemostatic proteins where platelet aggregation was suppressed in vitro but not significantly in human studies.23Tea consumption has been linked with protection against cardiovascular disease (CVD) through several mechanisms apart from inhibition of ACE.23 An important protective function is thought to be related to the anti-oxidant capacity of tea polyphenolics and capacity for inhibition of LDL oxidation,24 and the related inflammatory cascade involved with atherosclerosis. There is some evidence for specific anti-inflammatory activity of teas by regulation of prostaglandin production and also by immune cell stimulation of anti-inflammatory mediators. BP lowering by black and green teas were shown in the stroke-prone spontaneously hypertensive rat, through anti-oxidant-mediated mechanisms although ACE-regulatory factors were not specifically measured.25 The lowering of BP in SH rats by EGCG was attributed to stimulation of NO production with improvement in insulin sensitivity and raised plasma adiponectin levels, both of which are protective against metabolic syndrome.26 While, the regulation of ACE was not addressed, Igarashi et al.36 showed that a GT catechin extract (monomers) produced moderate BP lowering over a 10 week feeding study in a Type 2 diabetic Goto-Kakizaki rat model.
The current study showed differentiated in vitro ACE inhibitory activity for different types of processed tea, as related to relative abundance of un-oxidised catechins;35catechin polymers8 and oxidized products.9 The current study has also suggested that in vitro inhibition of ACE for GT was of a mixed mechanism involving a minor substrate-dependent pathway and predominantly direct enzyme inactivation (i.e, un-competitive). For BT, the mechanism was exclusively via inactivation. In addition to the association of tea polyphenolics with non-ACE mechanisms of BP lowering, i.e, viaNO-mediated vasodilation,25 recent studies with normotensive20 and mildly hypertensive subjects19 have shown BP lowering that correlated with ACE inhibition. Thus, it appears that both mechanisms of BP lowering by tea polyphenolics might be operative in vivo.
A prospective epidemiological study provided evidence for significant risk reduction in developing hypertension associated with consumption of green or oolong teas, that was positively correlated with both daily intake volume and length of time.21 Other mechanisms of BP lowering by tea in humans have been via improvement of endothelium-regulated mechanisms of vasodilation involving NO-dependent, vascular smooth muscle and endothelium-based receptors, where positive effects were detected following a short intervention study with black tea.18 A biomarker of BT intake was also positively correlated with BP lowering in older women supporting that tea consumption was protective against hypertension.22 An intervention study comparing monomeric catechin extracts from Benifuuki and Yabukita green teas, produced slight benefits after 8 weeks in normo-tensive humans, that was attributed to ACE inhibitory activity in vivo.19 Furthermore, single dose studies comparing GT, BT and Rooibos tea demonstrated significant inhibition of ACE after 30 min or 60 min for GT and Rooibos teas respectively, but not BT, that was ACE genotype-dependent. However, no significant changes in NO production were evident in the same cohorts.20 The lack of significant in vitro ACE inhibitory activity and moderate in vitro NO stimulation activity demonstrated for Rooibos tea,7 in contrast with the previous in vivo results, suggest that in vivo bio-efficacy of catechins can be significantly differentiated from in vitro activity. In the current study, ACE enzyme inhibition kinetics of monomeric catechins in GTPI was apparently irreversible while Liu et al.35 demonstrated non-competitive behaviour for catechin 3-O-gallate by Mikaelis-Menten modelling.
Pharmaceutical ACE inhibitors are able to exert allosteric enzyme inhibition due to mixed mechanisms. In addition to their direct inhibitory action on ACE and the kininase that inactivates bradykinin (B), ACE inhibitors have been reported to also directly act as agonists of the B1 receptor and to activate the B2 receptor as a heterodimeric complex of ACE together with inhibitor.37 The suppression of angiotensin II in combination with stimulation of NO production, produce a synergistic BP lowering effect.37 Based on the current studies, it is likely that most tea products, containing heterogeneous mixtures of monomeric and polymeric phenolics, might exert a mixed mode of ACE inhibition in vitro. While a mixed mode of inhibition might be advantageous and has been exploited in pharmaceutical ACE inhibitors, the type of inhibition, i.e, irreversible or substrate-dependent, required for in vivo efficacy by tea polyphenolics, is not yet established. The current inconsistencies in epidemiological evidence and discrepancies between animal and human intervention effects might support the hypothesis that abundance of active species vary across tea types and for some teas, may be inadequate to exert significant protection by normal consumption patterns.
This study has specifically focused on in vitro properties of tea polyphenolics with attention to the effects of processing on relative levels of inhibition of ACE and subsequently on the mechanism of inhibition inferred from analysis of the inhibition reaction kinetics. The in vitro data are correlated with and relevant to, the reported relative levels of bioactivity of green and black tea polyphenolics, with superior in vivo activity usually observed for green tea cf. black tea. This is supported by generally lower IC50 values of minimally processed (e.g. green) teas versus heat and enzyme-modified (e.g. black) teas. The study is intended to form a basis for informed process-mediated differentiation of tea polyphenolics for subsequent in vivo evaluation based initially on relative levels of in vitro bioactivity.
Bio-availability of tea polyphenolics is generally low, with a peak circulating level of 0.2 μM following ingestion of one dose of 400 μmol of green tea flavan-3-ols,38 and ranging from 1.3 to 5.0 μM for single doses of selected catechins.39 However, the recent elaboration of RAS system components functioning in the gastro-intestinal (GI) tract casts a new perspective surrounding the opportunity of tea (and other dietary) phenolics to exert a potential physiological influence. If dietary factors can exert an effect in the GI tract, then the use of in vitro assays to optimise for bioactivity becomes a valid approach that also circumvents the unknown bioactivity of tea polyphenolic metabolites.
3. Experimental
3.1 Materials
A selection of 34 tea products varying with respect to cultivar, leaf size, harvest season and tea grade, were sourced from markets local to Hangzhou, as produced in provinces across the Peoples Republic of China (Table 1). Tea grade is related to the maturity of the leaf before harvest with grade deteriorating as the leaf ages on the plant. The sample ID was assigned according to tea group (GT, green tea; OT, oolong tea; WT, white tea; BT, black tea; DT, dark tea) and sample number on the basis of the order of collection. The key points of differentiation of the 12 green teas were leaf size and fixation by steam (GT11 and GT12) versus pan dry heating. Key points of differentiation of the 9 oolong teas included leaf size, harvest season and drying-related oxidation (∼ 4–50% for OT1–OT5 and ∼60–70% for OT6–OT9). White teas were mainly differentiated with respect to grade as were black teas. Dark (Puerh) teas were differentiated according to fermentation under either high moisture (DT1, mDT3, DT5) or ambient conditions (DT2, DT4), (Fig. 1). Commercial green tea polyphenolic isolate (GTPI, Orient Tea Development Co., Ltd, Hangzhou, China), containing 60–80% catechins, was used as a reference extract.Angiotensin converting enzyme (ACE) extracted from rabbit lung, hippury-L-histidyl-L-leucine (HHL), hippuric acid (HA), trifluoroacetic acid (TFA), epi-gallocatechin gallate (EGCG), ZnCl2 and polyphenol oxidase (PPO) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Water was filtered through a MQ system (Millipore, Bedford, MA, USA). Mannanase (from Aspergillus niger) was obtained from Enzyme Solutions (Croydon South, Victoria, Australia).
3.2 Sample preparation and extraction
Ground tea samples (500 mg) were typically extracted with freshly boiled MQ water (50 ml) for 10 min. The extract was centrifuged at 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g at 20 °C for 15 min, before dilution of the supernatant to final nominal concentrations: 10, 5, 2.5, 1, 0.5 mg ml−1, for determination of the IC50 for ACE inhibition. Nominal concentrations were subsequently converted to actual concentration based on the proportion of soluble solids determined for individual tea types. The value of IC50, defined as the concentration of sample that inhibited 50% of ACE activity, was determined from sample concentration response relationship and interpolating regression of two-parameter hyperbolic decay equations analysis (GraphPad Prism 5, GraphPad Software, Inc. CA, USA).
000 × g at 20 °C for 15 min, before dilution of the supernatant to final nominal concentrations: 10, 5, 2.5, 1, 0.5 mg ml−1, for determination of the IC50 for ACE inhibition. Nominal concentrations were subsequently converted to actual concentration based on the proportion of soluble solids determined for individual tea types. The value of IC50, defined as the concentration of sample that inhibited 50% of ACE activity, was determined from sample concentration response relationship and interpolating regression of two-parameter hyperbolic decay equations analysis (GraphPad Prism 5, GraphPad Software, Inc. CA, USA).
3.3 ACE inhibition assay
The ACE inhibition assay was adapted from the original method of Sharma et al.40 using the substrate HHL, but modified for HPLC detection of the reaction product HA, based on the method of Actis-Goretta et al.,9 and applied previously by ourselves.41 A further modification was to include Zn2+ in the assay which was shown to be necessary to optimize enzyme activity by Liu et al.42 and recognized the need to ensure that Zn2+ was not limiting enzyme activity and was therefore not affected by potential introduction of additional Zn2+ possibly present in tea samples.43 The reaction was carried at 37 °C for either 30 or 10 min for IC50 or enzyme kinetics analysis, respectively. The substrate, HHL, and enzyme reaction product, HA were detected at 228 nm. ACE inhibitory activity (%) was expressed as per eqn (1):| ACE Inhibitory Activity (%) = (PA0 − PAi) × 100/PA0 | (1) |
3.4 Effects of selected enzymes on the ACE inhibitory activity
Soluble solids from green tea and black tea were treated with Mannanase and PPO, in order to determine whether ACE inhibitory activities were sensitive to enzymatic hydrolysis of sugars or oxidation of polyphenolics, respectively. Dried supernatant samples of GT11 and BT4 were made up to 5 mg ml−1 in 50 mM potassium phosphate buffer (pH 3.5). Mannanase enzyme was added at each of 2 levels (0.001%, 0.1%) and incubation times (30, 120 min), and PPO at each of 2 levels (10 and 1000 U ml−1) and incubation times (5, 30 min), before inactivating the enzyme by heating to 90 °C for 10 min. Samples were then freeze dried and reconstituted to constant volume for ACE inhibition testing. Reagent, sample and enzyme-only controls were also prepared and tested.3.5 Size-based fractionation of soluble tea solids
Black (BT4, Table 1) and green teas (GT11, Table 1) were ground using a coffee grinder (Breville, NSW, Australia) before dispersion in hot water at 12–15% solids (w/v) and boiled for 10 min. After cooling to room temperature, dispersions were clarified by filter bag (100 μm, Sefar filtration, NSW, Australia). The filtrates were collected and centrifuged for 30 min at 4 °C (Sorvall Instruments, Minnesota, USA) at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g). Supernatants from green and black teas were fractionated using a 3 kDa spiral membrane (S1Y3, Millipore, Billerica, MA, USA) with both retentates and permeates recovered and freeze dried. Total soluble solids recovered from black and green teas were 7.6 and 13.4% respectively, with (1.9% and 5.6%) and (8.2% and 5.1%) distributed between permeate and retentate fractions for black and green teas respectively.
000 × g). Supernatants from green and black teas were fractionated using a 3 kDa spiral membrane (S1Y3, Millipore, Billerica, MA, USA) with both retentates and permeates recovered and freeze dried. Total soluble solids recovered from black and green teas were 7.6 and 13.4% respectively, with (1.9% and 5.6%) and (8.2% and 5.1%) distributed between permeate and retentate fractions for black and green teas respectively.
3.6 Size exclusion chromatography
Dry tea solids were dissolved in 10% ethanol at 10 mg ml−1, sonicated (Unisonics, NSW, Australia) for 20 min and filtered through 0.8/0.2 μm syringe filters (Pall, New York, USA). The high performance size exclusion chromatography (HPSEC) method utilized a Waters HPLC system (Massachusetts, USA) with a BioSep-SEC-S 2000 column (7.8 × 300 mm, Phenomenex, Uppsala, Sweden), and UV detection at 254 or 280 nm. Sample injection was 20 μl and the mobile phase was 0.05 M phosphate buffer (pH 6.8) and program separation as follows: 0.3 ml min−1 for 40 min, 0.8–1.0 ml min−1 for 20 min and re-equilibrate at 0.3 ml min−1 for 5 min. Calibration was performed using a series of 6 standard molecules and proteins over the molecular mass range of 75 Da to 66 kDa.3.7 Determination of ACE inhibition kinetics
Reaction mixtures contained HHL substrate over the concentration range 0.5 to 4 mM and the reaction rate was linear at the timepoint of measuring reaction velocity, under the standard enzyme inhibition assay conditions. The relationships between reaction velocity, as measured by peak area of HA, and substrate concentration did not follow the classical Michaelis-Menton Equation, so KM values (i.e, concentration of substrate at 50% of maximum reaction velocity) were determined according to an allosteric (sigmoidal) model. This sigmoidal behaviour implied co-operative subunits within the enzyme and differential interactions with the sample. Allosteric enzyme kinetics can also reflect the occurrence of irreversible covalent modifications to enzyme. Sigmoidal substrate-velocity curves were fitted by eqn (2) (GraphPad Prism 5).| Velocity = Vmax × [substrate]h/(Kprime + [substrate]h) | (2) |
3.8 Data analysis
All analyses were carried out in at least triplicate independent replicates with mean and standard deviations shown as error bars. Statistical analysis was conducted using Sigma Plot for Windows, Version 11 (Systat Software, Inc. CA, USA).4. Conclusion
Values of IC50 for in vitro inhibition of ACE were strongly dependent on tea type with variation both within and between groupings related to complex effects of processing. In general, ACE inhibitory activity was inversely related to 280 nm absorptivity of extractable polyphenolics. We have shown that ACE was inhibited by GTPI through irreversible, substrate-independent processes, and also that a mixed mode of inhibition including irreversible and substrate-dependent (competitive or non-competitive) mechanisms may apply to GT as distinct from irreversible inactivation of ACE by BT. It is necessary for these results to be correlated with in vivo activity in order to confirm if the mechanism of inhibition is important for exerting in vivo activity and indeed, if tea polyphenolics are actually involved with the regulation of BP by ACE inhibition or by another mechanism. The findings may also explain why epidemiological and dietary intervention studies involving tea consumption seem to require long term consumption before effects are detectable, possibly reflecting the low abundance of species present in tea polyphenolic extracts that are actually active in vivo. This study can therefore provide a rationale for developing tea products with differentiated mechanisms of ACE inhibition, and to explore their usefulness for influencing in vivo physiological regulation of ACE including cardiovascular and gastro-intestinal responses.5. Acknowledgements
Junjie Dong wishes to acknowledge support for this work from the CSIRO Preventative Health Flagship, Australia. The assistance of Sunanda Sudharmarajan and Katherine Robinson with HPLC analysis and membrane processing is gratefully acknowledged.6. References
- B. S. Heran, M. M. Y. Wong, I. K. Heran and J. M. Wright, Cochrane Database Syst Rev., 2008, 344 Search PubMed.
- A. Casselbrant, A. Edebo, P. Hallersund, E. Spak, H. F. Helander, C. Jonson and L. Fandriks, Am. J. Physiol.: Gastrointest. Liver Physiol., 2009, 297, G1019–G1027 Search PubMed.
- L. Fandriks, J. Renin-Angiotensin-Aldosterone Syst., 2010, 11, 43–48 Search PubMed.
- Z. Y. Chen, C. Peng, R. Jiao, Y. M. Wong, N. Yang and Y. Huang, J. Agric. Food Chem., 2009, 57, 4485–4499 Search PubMed.
- C. P. Serra, S. F. Cortes, J. A. Lombardi, A. B. de Oliveira and F. C. Braga, Phytomedicine, 2005, 12, 424–432 Search PubMed.
- Y. I. Kwon, E. Apostolidis, Y. C. Kim and K. Shetty, J. Med. Food, 2007, 10, 266–275 Search PubMed.
- I. A. L. Persson, M. Josefsson, K. Persson and R. G. G. Andersson, J. Pharm. Pharmacol., 2006, 58, 1139–1144 Search PubMed.
- L. Actis-Goretta, J. I. Ottaviani, C. L. Keen and C. G. Fraga, FEBS Lett., 2003, 555, 597–600 Search PubMed.
- L. Actis-Goretta, J. I. Ottaviani and C. G. Fraga, J. Agric. Food Chem., 2006, 54, 229–234 Search PubMed.
- D. G. Wang and S. R. Wang, Chinese Traditional and Herbal Drugs, 1991, 2, 4–5 Search PubMed.
- S. P. Nie and M. Y. Xie, Food Hydrocolloids, 2011, 25, 144–149 Search PubMed.
- Y. X. Zhu, H. Huang and Y. Y. Tu, Int. J. Food Sci. Technol., 2006, 41, 333–340 Search PubMed.
- C. J. Dufresne and E. R. Farnworth, J. Nutr. Biochem., 2001, 12, 404–421 Search PubMed.
- H. Z. Mo, Y. Zhu and Z. M. Chen, Trends Food Sci. Technol., 2008, 19, 124–130 Search PubMed.
- V. Stangl, M. Lorenz and K. Stangl, Mol. Nutr. Food Res., 2006, 50, 218–228 CrossRef CAS.
- J. M. Hodgson, I. B. Puddey, V. Burke, L. J. Beilin and N. Jordan, J. Hypertens., 1999, 17, 457–463 Search PubMed.
- D. L. McKay and J. B. Blumberg, J. Am. Coll. Nutr., 2002, 21, 1–13 CAS.
- S. J. Duffy, J. F. Keaney, M. Holbrook, N. Gokce, P. L. Swerdloff, B. Frei and J. A. Vita, Circulation, 2001, 104, 151–156 Search PubMed.
- I. Kurita, M. Maeda-Yamamoto, H. Tachibana and M. Kamei, J. Agric. Food Chem., 2010, 58, 1903–1908 Search PubMed.
- I. A. L. Persson, K. Persson, S. Hagg and R. G. G. Andersson, Public Health Nutr., 2010, 13, 730–737 Search PubMed.
- Y. C. Yang, F. H. Lu, J. S. Wu, C. H. Wu and C. J. Chang, Arch. Intern. Med., 2004, 164, 1534–1540 Search PubMed.
- J. M. Hodgson, A. Devine, I. B. Puddey, S. Y. Chan, L. J. Beilin and R. L. Prince, J. Nutr., 2003, 133, 2883–2886 Search PubMed.
- J. M. Hodgson, Clin. Exp. Pharmacol. Physiol., 2006, 33, 838–841 CrossRef CAS.
- L. B. M. Tijburg, T. Mattern, J. D. Folts, U. M. Weisgerber and M. B. Katan, Crit. Rev. Food Sci. Nutr., 1997, 37, 771–785 Search PubMed.
- H. Negishi, J. W. Xu, K. Ikeda, M. Njelekela, Y. Nara and Y. Yamori, J. Nutr., 2004, 134, 38–42 CAS.
- M. A. Potenza, F. L. Marasciulo, M. Tarquinio, E. Tiravanti, G. Colantuono, A. Federici, J. A. Kim, M. J. Quon and M. Montagnani, Am. J. Physiol.: Endocrinol. Metab., 2007, 292, E1378–E1387 Search PubMed.
- M. Tanida, N. Tsuruoka, J. Shen, Y. Kiso and K. Nagai, Metab.-Clin. Exp., 2008, 57, 526–534 Search PubMed.
- Y. Miura, T. Chiba, I. Tomita, H. Koizumi, S. Miura, K. Umegaki, Y. Hara, M. Ikeda and T. Tomita, J. Nutr., 2001, 131, 27–32 Search PubMed.
- H. Yokogoshi, Y. Kato, Y. M. Sagesaka, T. Takiharamatsuura, T. Kakuda and N. Takeuchi, Biosci., Biotechnol., Biochem., 1995, 59, 615–618 Search PubMed.
- J. J. Dalluge and B. C. Nelson, J. Chromatogr., A, 2000, 881, 411–424 CrossRef CAS.
- T. Tanaka, Y. Matsuo and I. Kouno, Int. J. Mol. Sci., 2010, 11, 14–40 Search PubMed.
- T. O. Cheng, Int. J. Cardiol., 2006, 108, 301–308 Search PubMed.
- V. Sharma, A. Gulati and S. D. Ravindranath, Food Chem., 2005, 93, 141–148 Search PubMed.
- M. Abe, N. Takaoka, Y. Idemoto, C. Takagi, T. Imai and K. Nakasaki, Food Microbiol., 2008, 124, 199–203 Search PubMed.
- J. C. Liu, F. L. Hsu, J. C. Tsai, P. Chan, J. Y. H. Liu, G. N. Thomas, B. Tomlinson, M. Y. Lo and J. Y. Lin, Life Sci., 2003, 73, 1543–1555 Search PubMed.
- K. Igarashi, K. Honma, O. Yoshinari, F. Nanjo and Y. Hara, J. Nutr. Sci. Vitaminol., 2007, 53, 496–500 Search PubMed.
- E. G. Erdos, F. L. Tan and R. A. Skidgel, Hypertension, 2010, 55, 214–220 Search PubMed.
- D. Del Rio, L. Calani, C. Cordero, S. Salvatore, N. Pellegrini and F. Brighenti, Nutrition, 2010, 26, 1110–1116 Search PubMed.
- J. V. Higdon and B. Frei, Crit. Rev. Food Sci. Nutr., 2003, 43, 89–143 CrossRef CAS.
- D. W. Cushman and H. S. Cheung, Biochem. Pharmacol., 1971, 20, 1637 Search PubMed.
- X. Xu, T. K. Singh and A. J. Hillier, Aust. J. Dairy Technol., 2006, 61, 228–228 Search PubMed.
- B. Holmquist, P. Bunning and J. F. Riordan, Anal. Biochem., 1979, 95, 540–548 CAS.
- T. Tsushida and T. Takeo, J. Sci. Food Agric., 1977, 28, 255–258 Search PubMed.
| This journal is © The Royal Society of Chemistry 2011 |
