Insights into the putative catechin and epicatechin transport across blood-brain barrier
Ana
Faria
*ab,
Diogo
Pestana
a,
Diana
Teixeira
a,
Pierre-Olivier
Couraud
cd,
Ignacio
Romero
e,
Babette
Weksler
c,
Victor
de Freitas
b,
Nuno
Mateus
b and
Conceição
Calhau
a
aDepartment of Biochemistry (U38-FCT), Faculty of Medicine, University of Porto, Al. Prof. Hernâni Monteiro, 4200-319, Porto, Portugal. E-mail: anafaria@med.up.pt; Fax: +351 22 551 36 24; Tel: +351 22 551 36 24
bChemistry Investigation Centre (CIQ), Department of Chemistry, Faculty of Sciences, University of Porto, 4169-007, Porto, Portugal
cInstitut Cochin, Université Paris Descartes, CNRS (UMR 8104), Paris, France
dInstitut National de la Santé et de la Recherche Médicale (INSERM) U1016, Paris, France
eDepartment of Biological Sciences, The Open University, Walton Hall, Milton Keynes, United Kingdom
First published on 17th November 2010
Abstract
The identification of mechanisms associated with phenolic neuroprotection is delayed due to a lack of information regarding the ability of phenolic compounds to enter the central nervous system (CNS). The aim of this work was to evaluate the transmembrane transport of catechin and epicatechin across blood-brain barrier (BBB). Two BBB cell lines, RBE-4 cells (immortalized cell line of rat capillary cerebral endothelial cells) and hCMEC/D3 (immortalized human cerebral microvessel endothelial cell line), were used. HPLC-DAD/MS was used to detect these compounds and their metabolites in the studied samples. The metabolites of the tested flavan-3-ols were synthesized to be used as standards. Catechin and epicatechin could cross both cells in a time-dependent manner. This transport was stereoselective (epicatechin ≫ catechin), involving one or more stereoselective entities. Additionally, these cells were capable of metabolizing these compounds, particularly by conjugation with glucuronic acid, since this metabolite was detected in the basolateral media. Several studies suggest that blood levels of catechin and epicatechin are far below the levels used in this study and that these compounds appeared mainly as methyl, sulfate and glucuronide metabolites. Nevertheless, the information obtained by this study is valuable for the new insights about flavan-3-ols transport. In conclusion: (i) catechin and epicatechin are capable of crossing the BBB; (ii) a stereoselective process was involved in the passage of these compounds across BBB cells; (iii) these endothelial cells have enzymes capable of metabolizing these compounds.
Introduction
The concept that polyphenols are neuroprotective is developing into a consensus. Oxidative stress and the consequent damage of brain macromolecules is a key process in neurodegenerative diseases. The emerging hypothesis on the mechanism of neuroprotection by polyphenols is that they act by a combination of protection of neuronal cells from oxidative stress through induction of antioxidant defences, modulation of signalling cascades, apoptotic processes and/or by the synthesis/degradation of the amyloid β peptide.1In general, flavan-3-ols are widely distributed and highly present in human diet. Catechin is abundant in broad beans, red grapes, apricots and strawberries. Epicatechin concentration is especially high in apples, blackberries, broad beans, grapes, pears and chocolate. After consumption of food or beverages with high concentrations of these compounds, they are detected in plasma resulting in access to the entire organism.2 Some epidemiological and dietary intervention studies in humans and animals indicate that flavonoid consumption is important in neuronal health.3 The role of flavonoids in the modulation of neurodegeneration, especially age-related cognitive and motor decline, and in protection against oxidative stress, cerebral ischemia/reperfusion injuries and other brain pathologies is a hot investigation topic nowadays. Flavonoid protection is often related to their great antioxidant capacity, but other mechanisms may also be involved. These mechanisms will depend, among other factors, on the ability of compounds to enter the brain. The compounds must first cross the BBB, which ultimately regulates the composition of extracellular fluid in the central nervous system (CNS) by tightly controlling molecular traffic and buffering against changes in the systemic circulation.
It is, however, not at all clear whether most of these compounds reach the brain in sufficient concentrations and in a biologically active form to have any beneficial effects. Although numerous studies have reported flavonoid-mediated neuroprotection, there is little information about the interaction of flavonoid or their metabolites with the blood-brain barrier (BBB). The BBB is an important key target in discussion of polyphenol disposal, especially in what concerns the CNS and its local effects. It is now well established that the cerebral endothelium is the main cellular element responsible for the BBB in mammals.4 Features that distinguish brain endothelium from other organs include complex tight junctions, a low density of pinocytotic vesicles, and the presence of specific transporters including efflux carriers. These properties make the BBB a regulatory interface that selectively limits passage of most small polar molecules and macromolecules from the cerebrovascular circulation to the brain, exerting tight control over transendothelial molecular traffic and contributing to regulation of brain extracellular fluid composition. Therefore, hydrophilic compounds cannot cross the BBB in the absence of specific mechanisms such as membrane transporters or endocytosis. Although the importance of the BBB is well recognized and, consequently of polyphenols transport across it, there is a paucity of information concerning the mechanisms of transport, especially when compared with other organs such as the intestine. In addition to difficulties on the detection of flavanols, especially in what concerns the detection of their metabolites, a good BBB in vitro model is also an important issue that makes the progress of the knowledge in this subject difficult. However, there are sufficient results that reinforce the interest in using BBB cell lines in the transport studies.5–7
The aim of this work was to evaluate the transmembrane transport of flavan-3-ols (catechin and epicatechin) across BBB. Since these compounds could be metabolised in the BBB, probably resulting in a detoxification process, the corresponding flavan-3-ol metabolites were also measured. The experiments were performed using an immortalized cell line of rat capillary cerebral endothelial cells (RBE4 cells) and an immortalized human cerebral microvessel endothelial cell line (hCMEC/D3) that was recently developed and used as a model of the human blood-brain barrier (hBBB). The RBE4 cell line was obtained by transfection of rat brain microvessel endothelial cells with a plasmid containing the E1A adenovirus gene.8 These cells display a non-transformed endothelial phenotype expressing the brain microvessel-associated enzymes γ-glutamyl-transpeptidase, alkaline phosphatase,9P-glycoprotein10 and the GLUT1 isoform responsible for glucose transport.11hCMEC/D3 cells between passage 25 and 35 are most often applied in research, remained phenotypically nontransformed, and cells maintained many characteristics of human brain endothelial cells.12 Thus, RBE4 cells and hCMEC/D3 appear to constitute two interesting models to study the transmembrane polyphenols transport across BBB, without interference from other tissue elements.
Materials and methods
Reagents
(+)-Catechin, (−)-epicatechin, Minimum Essential Medium, Ham's F10, neomycine, penicillin G, amphotericin B, streptomycin, HEPES, trypsin-EDTA, collagen type I from rat tail, dithiothreitol, saccharic acid-1,4-lactone, S-(5′-adenosyl)-L-methionine iodide (SAM), uridine 5′-diphosphoglucuronic acid triammonium salt (UDPGA) (Sigma-Aldrich®, Madrid, Spain); fetal bovine serum, basic fibroblast growth factor, HBSS (Gibco, Barcelona, Spain); EBM-2 medium, VEGF, IGF-1, EGF, basic FGF, hydrocortisone, ascorbate, gentamycin were from Clonetics (Cambrex BioScience, Wokingham, UK).Cell and culture conditions
The RBE4 cell line was kindly supplied by Dr Francoise Roux (INSERM U. 26, Hôpital Fernand Widal, Paris, France). RBE4 clone was maintained in a humidified atmosphere of 5% CO2-95% air at 37 °C. These cells (passages 64–70) were grown in Minimum Essential Medium/Ham's F10 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) supplemented with 300 μg ml−1neomycine, 10% fetal bovine serum (FBS), 1 ng ml−1 basic fibroblast growth factor, 100 U ml−1penicillin G, 0.25 mg ml−1 amphotericin B, 100 mg ml−1streptomycin, and 25 mM HEPES. The cell medium was changed every 48 h, and the cells reached confluence after 6–7 days of culture. For subculturing, the cells were dissociated with 0.25% trypsin-EDTA, diluted 1
1) supplemented with 300 μg ml−1neomycine, 10% fetal bovine serum (FBS), 1 ng ml−1 basic fibroblast growth factor, 100 U ml−1penicillin G, 0.25 mg ml−1 amphotericin B, 100 mg ml−1streptomycin, and 25 mM HEPES. The cell medium was changed every 48 h, and the cells reached confluence after 6–7 days of culture. For subculturing, the cells were dissociated with 0.25% trypsin-EDTA, diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and subcultured in Petri dishes with a 21 cm2growth area (Corning Costar, Badhoevedorp, The Netherlands).
5 and subcultured in Petri dishes with a 21 cm2growth area (Corning Costar, Badhoevedorp, The Netherlands).
The hCMEC/D3 cell line was kindly supplied by Dr Pierre-Olivier Couraud (INSERM U. 567, Université René Descartes, Paris, France). Cells were maintained in a humidified atmosphere of 5% CO2-95% air at 37 °C, between passages 26–30. Cells were grown in EBM-2 medium supplemented with VEGF, IGF-1, EGF, basic FGF, hydrocortisone, ascorbate, gentamycin and 2.5% fetal bovine serum (FBS) as recommended by the manufacturer, 100 U ml−1penicillin G, 0.25 mg ml−1 amphotericin B and 100 mg ml−1streptomycin. The cell medium was changed every 48 h, and the cells reached confluence after 5–6 days of culture. For subculturing, the cells were dissociated with 0.25% trypsin-EDTA, diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and subcultured in Petri dishes collagen-coated with a 21 cm2growth area (Corning Costar®, Badhoevedorp, The Netherlands).
5 and subcultured in Petri dishes collagen-coated with a 21 cm2growth area (Corning Costar®, Badhoevedorp, The Netherlands).
For the experiments, both cells were seeded on transwell inserts (collagen-coated polytetrafluoroethylene membrane, 0.4 μm pore size, 12 mm diameter, Corning Costar®). Inserts were placed in 12 well plates. All experiments were performed 9–10 days after initial seeding.
Transport studies
Transepithelial electrical resistance (TEER) of cells grown in the transwell was measured using an epithelial voltohmmeter fitted with planar electrodes (EVOM; World Precision Instruments, Stevenage, UK). Experiments were conducted only in cell monolayers that showed a TEER > 100 Ω cm−2. Medium was removed and cells were washed with HBSS medium with 1.0 mM MgCl2 and 0.25 mM CaCl2, pH 7.4. Flavonoid solution in HBSS with 0.1% of FBS was added to the apical side of the cells and the same medium free of polyphenols was added to the basolateral compartment. Transepithelial transport was followed as a function of time, at 37 °C. Samples were taken from the basolateral side and replaced by fresh medium. The samples were frozen until HPLC analysis.HPLC analysis
Catechin and epicatechin were analysed by HPLC (Elite Lachrom system (L-2130)) on a 150 × 4.6 mm i.d. reversed-phase C18 column (Merck, Darmstadt, Germany); detection was carried out using a diode array detector (L-2455). The solvents were A: H2O/HCOOH (9.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1), and B: CH3CN. The program initiated with 93%A and 7%B for 4 min and a gradient of 7–25%B during 46 min at a flow rate of 0.5 mL min−1. The column was washed with 100% B for 10 min and then stabilized at the initial conditions for another 10 min.
0.1), and B: CH3CN. The program initiated with 93%A and 7%B for 4 min and a gradient of 7–25%B during 46 min at a flow rate of 0.5 mL min−1. The column was washed with 100% B for 10 min and then stabilized at the initial conditions for another 10 min.
For LC-MS analyses a liquid chromatograph (Hewlett-Packard 1100 series) equipped with a Thermo Finnigan (Hypersil Gold®) reversed-phase column (150 mm × 4.6 mm, 5 μm, C18) thermostatted at 25 °C was used. The samples were analyzed using the same solvents, gradients, injection volume and flow rate referred above for HPLC analysis. The mass detector was a Finnigan LCQ DECA XP MAX (Finnigan Corp., San Jose, CA) quadrupole ion trap equipped with atmospheric pressure ionization (API) source, using electrospray ionization (ESI) interface. The vaporizer and the capillary voltages were 5 kV and 4 V, respectively. The capillary temperature was set at 325 °C. Nitrogen was used as both sheath and auxiliary gas at flow rates of 80 and 30, respectively (in arbitrary units). Spectra were recorded in positive ion mode between m/z 120 and 1500. The mass spectrometer was programmed to do a series of three scans: a full mass, a zoom scan of the most intense ion in the first scan, and a MS-MS of the most intense ion using relative collision energy of 30 and 60.
Synthesis of flavan-3-ol metabolites
The methylation of substrates was conducted in 500 μl of a mixture containing 1 mM of DTT, 1.2 mM of MgCl2, 100 μM of catechin or epicatechin, 200 μM of SAM, 34 mg ml−1 of rat hepatic cytosolic protein in Tris-HCl 10 mM, pH 7.4. This mixture was incubated at 37 °C for 30 min, after which the reaction was stopped with 500 μl of ice-cold methanol with 1% ascorbic acid. This was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 2 min, and the supernatant was analyzed by HPLC-DAD/MS using the conditions described above.
000 rpm for 2 min, and the supernatant was analyzed by HPLC-DAD/MS using the conditions described above.
For glucuronidation, the reaction mixture consisted of 2 mg ml−1 of rat liver microsomal protein, 1 mM of UDPGA, 0.15 mM of ascorbic acid, 2 mM of MgCl2, 1 mM of saccharic acid-1,4-lactone and 500 μM of catechin or epicatechin in Tris-HCl 40 mM, pH 7.4, in a final volume of 500 μl. The reaction was stopped with 500 μl of ice-cold methanol with 1% ascorbic acid. After centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 2 min, the supernatant was analyzed by HPLC-DAD/MS, as described above
000 rpm for 2 min, the supernatant was analyzed by HPLC-DAD/MS, as described above
Statistical analysis
Values are expressed as the arithmetic mean ± SEM. Statistical significance of the difference between various groups was evaluated by two-way analysis of variance (ANOVA) followed by the Bonferroni test. Differences were considered significant when p < 0.05.Results
Transport efficiency AP → BL: characterization in two cell lines
The transport of two of the most common flavan-3-ols, (+)-catechin and (−)-epicatechin (Fig. 1) was evaluated on cells cultured on semi-permeable supports. The transepithelial electrical resistance (TEER) of cells was measured in the beginning and only inserts with TEER >100 Ω cm−2 were used. After 18 h the TEER was measured again and only results of inserts with TEER >100 Ω cm−2 were considered. Also, 14C-sorbitol permeability was controlled, and no differences were found between the beginning and the end of the experiment, in the presence or absence of tested compounds, in both cell lines used in this study (RBE4 and hCMEC/D3). | ||
| Fig. 1 Chemical structures of: (A) (+)-catechin; (B) (−)-epicatechin. | ||
The transport of 30 μM of catechin and epicatechin (Fig. 1), across RBE4 cells was evaluated and both flavanols effectively crossed this barrier in a time-dependent manner. The percentage of transport efficiency of epicatechin was significantly higher than of catechin (Fig. 2). The transport was followed by HPLC and confirmed by retention times and photodiode array spectra. After 18 h, both flavanols tested showed clear transport (15.4 ± 0.7 and 27.5 ± 0.8% of initial concentration for catechin and epicatechin, respectively).
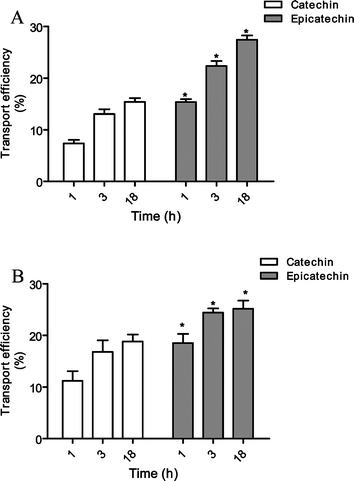 | ||
| Fig. 2 Transport efficiency of 30 μM catechin and epicatechin through (A) RBE4 cells and (B) hCMEC/D3 cells (apical → basolateral). Results are presented as transport efficiency (%) (mean ± SEM). Transport efficiency percentages were calculated based on (compound concentrations at the basolateral side overtime)/(compound concentrations at the apical side at the zero hours) × 100. * Significantly different from catechin (p < 0.05). | ||
The same experiments were conducted in hCMEC/D3 cells, an immortalized human brain endothelial cell line. This cell line retains most of the morphological and functional characteristic of brain endothelial cells, even without co-culture with glial cells, constituting a reliable in vitro model of the human BBB.13 The results obtained are presented in Fig. 2. Catechin and epicatechin were efficiently transported through this cell line to the basolateral side. The transport was also time-dependent similar to what was observed in RBE4. In fact, there were no significant differences between the transport efficiency patterns in these two cell lines.
After 18 h of incubation with catechin or epicatechin, basolateral media was analysed by HPLC-LC-MS. From this analysis, it was possible to identify not only the peak of catechin ([M − H]+ 291 m/z) but also a peak with [M − H]+ 467 m/z. This data match with the metabolite of catechin conjugated with glucuronic acid, by comparison of both mass and retention times of synthesized standards. Therefore, these RBE4 cells were able to metabolize catechin through UGT pathway. A peak with the same pseudo-molecular ion was also detected in the sample of incubation with epicatechin, which was confirmed as being the conjugated of epicatechin with glucuronic acid (Table 1).
| Compound | t R/min | m/z ([M − H]+) |
|---|---|---|
| (+)-Catechin | 15.5 | 291 |
| (+)-Catechin glucuronide | 9.1 | 467 |
| (−)-Epicatechin | 22.7 | 291 |
| (−)-Epicatechin glucuronide | 17.3 | 467 |
Analogous peaks were identified after the incubation of the compounds in the hCMEC/D3 cells, demonstrating that these cells were similarly able to metabolize those flavan-3-ols.
Transport efficiency AP → BL: pharmacological characterization
The transport of epicatechin and catechin was evaluated in the presence of several compounds, which interfere with several cellular mechanisms, in an attempt to elucidate the mechanisms behind flavanol transport across BBB. Results are shown in Table 2 and 3.| (−)-Epicatechin | (+)-Catechin | |||||
|---|---|---|---|---|---|---|
| Time/h | 1 | 3 | 18 | 1 | 3 | 18 |
| a Significantly different from epicatechin or catechin alone (p < 0.05). | ||||||
| 15.4 ± 0.6 | 22.4 ± 1.0 | 27.5 ± 0.8 | 7.4 ± 0.7 | 13.1 ± 0.9 | 15.4 ± 0.7 | |
| β-Estradiol | 15.9 ± 0.3 | 21.0 ± 0.4 | 36.0 ± 3.8a | 7.9 ± 1.2 | 11.3 ± 1.3 | 23.5 ± 1.3a |
| Progesterone | 16.6 ± 1.3 | 33.4 ± 3.1a | 49.0 ± 4.1a | 8.1 ± 4.0 | 9.5 ± 2.3 | 21.6 ± 2.3a |
| Dinitrophenol | 10.7 ± 1.1 | 25.5 ± 2.8 | 26.1 ± 4.1 | 8.1 ± 1.0 | 13.4 ± 2.2 | 19.5 ± 2.5 |
| Phloridzin | 12.7 ± 1.4 | 21.3 ± 0.4 | 26.4 ± 3.3 | 13.5 ± 3.6a | 18.1 ± 3.1 | 21.7 ± 2.1 |
| BHC | 15.5 ± 0.0 | 23.2 ± 6.5 | 24.8 ± 1.3 | 8.2 ± 0.2 | 12.3 ± 0.1 | 21.3 ± 2.5 |
| Cimetidine | 21.1 ± 2.6a | 29.7 ± 2.6a | 42.5 ± 6.3a | 10.5 ± 2.0 | 19.5 ± 3.3a | 28.3 ± 5.3a |
| L-Carnitine | 12.5 ± 1.2 | 18.8 ± 0.5 | 18.6 ± 1.2a | 7.5 ± 1.0 | 14.5 ± 0.9 | 15.8 ± 1.5 |
| Probenecid | 14.2 ± 0.3 | 19.4 ± 1.6 | 25.0 ± 0.4 | 9.1 ± 0.7 | 15.8 ± 0.4 | 19.6 ± 1.2 |
| Rhodamine 123 | 17.0 ± 1.2 | 28.1 ± 2.8 | 29.0 ± 2.1 | 10.0 ± 0.5 | 17.6 ± 1.4 | 19.5 ± 2.7 |
| Cyclosporine A | 15.0 ± 0.1 | 23.1 ± 1.3 | 37.1 ± 1.9a | 7.3 ± 0.7 | 14.1 ± 0.5 | 19.3 ± 1.6 |
| (−)-Epicatechin | (+)-Catechin | |||||
|---|---|---|---|---|---|---|
| Time/h | 1 | 3 | 18 | 1 | 3 | 18 |
| a Significantly different from epicatechin or catechin alone (p < 0.05). | ||||||
| 17.9 ± 1.5 | 24.2 ± 0.7 | 25.4 ± 1.2 | 11.9 ± 1.4 | 14.3 ± 1.5 | 17.8 ± 1.3 | |
| β-Estradiol | 15.1 ± 1.0 | 23.5 ± 2.3 | 27.9 ± 0.8 | 12.1 ± 1.5 | 16.1 ± 1.2 | 18.5 ± 1.2 |
| Progesterone | 14.5 ± 4.6 | 18.4 ± 5.0 | 28.9 ± 0.6 | 11.5 ± 2.8 | 15.7 ± 0.2 | 18.3 ± 0.7 |
| Dinitrophenol | 19.0 ± 7.1 | 26.6 ± 0.2 | 24.9 ± 0.8 | 10.2 ± 0.4 | 11.8 ± 0.9 | 15.9 ± 3.4 |
| Phloridzin | 17.7 ± 2.7 | 24.8 ± 1.0 | 29.0 ± 3.7 | 13.5 ± 1.6 | 16.4 ± 1.0 | 16.2 ± 2.0 |
| BHC | 19.5 ± 2.1 | 25.1 ± 2.1 | 27.6 ± 1.0 | 9.2 ± 0.0 | 18.6 ± 3.6 | 21.1 ± 0.5 |
| Cimetidine | 33.8 ± 8.4a | 37.3 ± 9.8 | 45.6 ± 23.1a | 25.6 ± 1.8a | 38.4 ± 5.1a | 50.9 ± 8.7a |
| L-Carnitine | 11.7 ± 3.2 | 17.6 ± 4.8 | 15.7 ± 3.7 | 9.4 ± 3.2 | 16.0 ± 1.1 | 15.7 ± 2.2 |
| Probenecid | 18.0 ± 1.1 | 21.7 ± 1.8 | 19.8 ± 0.6 | 17.3 ± 3.5 | 19.6 ± 4.7 | 20.1 ± 2.7 |
| Rhodamine 123 | 19.1 ± 0.0 | 22.4 ± 0.7 | 25.6 ± 0.6 | 12.3 ± 1.6 | 10.8 ± 2.2 | 14.8 ± 0.1 |
| Cyclosporine A | 24.3 ± 4.4 | 29.0 ± 3.7 | 27.9 ± 3.3 | 16.1 ± 4.3 | 17.4 ± 5.8 | 20.1 ± 3.6 |
Dinitrophenol, which inhibits ATP production, phloridzin, an inhibitor of the sodium-dependent glucose transporter, BHC (2-aminobicyclo(2,2,1)-heptane-2-carboxylic acid), a selective inhibitor of the L-type amino acid transporters, and probenecid and rhodamine 123, inhibitors of some efflux transporters, did not have any significant effect on epicatechin and catechin transport. β-Estradiol increased the flavonols transport after 18 h in RBE4 cells.
Cimetidine and L-carnitine are substrates of OAT3 and OCTN1/2 respectively. The presence of cimetidine enhanced epicatechin and catechin appearance on the basolateral side in both cell lines. L-Carnitine decreased epicatechin transport after 18 h in RBE4. In the hCMEC/D3 cell line, L-carnitine exerts the same trend over epicatechin transport.
Cyclosporine A, an inhibitor of the efflux transport P-glycoprotein, was able to statistically increase epicatechin's transport, but not catechin, after 18 h in RBE4 cells.
Discussion
In this study, the passage of two of the most common flavan-3-ol monomers, (+)-catechin and (−)-epicatechin, in two BBB cell lines was investigated. Previous results have shown that catechin could be transported across RBE4, which is a rat cell line.14 Thus, it is of most importance to perform transport studies in a human cell line, based in the differences that can exist between these two species. The similarity of the behavior of the two cell lines used (RBE4 and hCMEC/D3) confirms, and reinforces, the generality of the observations. The detection of both tested compounds in the basolateral side confirms the passage of these compounds through the cells. Moreover, the detection of these compounds conjugated with glucuronic acid, as detected by LC-MS, corroborate the passage of both catechin and epicatechin across the cell monolayer. The presence of these metabolites in the basolateral media also indicates that both cell lines, RBE4 and hCMEC/D3, have the enzymes necessary for this reaction, namely the UDP-glucuronyltransferases. Biotransformation may be an essential process to make these compounds more water-soluble, and, in this way, allow their subsequent elimination from the body or, in contrast, allow an easier xenobiotic transport and delivery around the body. The physiological role of conjugated-flavonoid is discussible, but the fact is that they may appear in the extracellular fluid in the CNS, exerting their biological effects. In fact, some reports have suggested that flavonoid metabolites possess more biological activity than the intact form.15–20Nevertheless, reports concerning biological activity of flavonoid metabolites are scarce, probably because the synthesis in large amounts and the purification of these compounds is considerably difficult and these metabolites are not commercial available. On the other hand, it is difficult to know if an observed effect is mediated by flavonoid or its metabolites just because each cell has enzymatic machinery allowing conjugation but also deconjugation reactions.
One was expecting transport efficiency of catechin and epicatechin not to have great differences between them since they are stereoisomers. The difference in their behavior was a very surprising result: these compounds differ only in the orientation of the hydroxyl group in the position 3 (Fig. 1), which led us to assume the involvement of a stereoselective process during the passage of these compounds across BBB cells. Thus, this process may occur (i) in the uptake, (ii) in the efflux, or (iii) in a cellular process involving stereoselective entities like enzymes.
Dinitrophenol was tested because of its capacity to inhibit ATP production. Since no differences in flavan-3-ol transport were found in the presence or absence of this compound, one could conclude that the transport of flavanols does not require energy. BHC (2-aminobicyclo(2,2,1)-heptane-2-carboxylic acid) is a selective inhibitor of the L-type amino acid transporters which are responsible for the transport of several amino acids, namely phenylalanine. This amino acid is a precursor of polyphenols synthesis, so if flavonoid maintained some chemical resemblance with this molecule they could use this transporter to enter the cell. Such was not confirmed, since there were no differences on catechin or epicatechin transport in the presence of this inhibitor.
Phloridzin, as an inhibitor of the sodium-dependent glucose transporter (SGLT1), which has been suggested to transport some flavonoids,21 was tested. The results obtained in RBE4 showed that only catechin transport is significantly affected, which is in agreement with a stereospecific effect.
Cimetidine and L-carnitine are substrates of OAT3 and OCTN1/2 respectively, and therefore, inhibitors of these transporters respectively. Cimetidine was, unexpectedly, able to significantly increase the transport of both compounds in both cell lines. On the other hand, L-carnitine, an organic cation transporter OCTN ligand (OCTN1 and OCTN2) was able to significantly inhibit this transport in RBE4 cells. On hCMEC/D3, L-carnitine had the same tendency in epicatechin's transport. Curious is the fact that epicatechin has no resemblance with L-carnitine or with the substrates usually transporter by OCTN1/2.
Efflux transporters, particularly P-glycoprotein, have been detected at the apical membranes of epithelial cells in the excretory organs such as intestine, liver, kidney and endothelial cells in the BBB. They may limit the bioavailability and brain distribution and facilitate both biliary excretion and renal elimination of its substrate drugs. Efflux transporters are essential components of the BBB, controlling the entry of xenobiotics into the brain. The interaction between flavonoid and efflux transporters is documented but this relationship is not yet clear. Nevertheless, besides flavonoid interaction with efflux transporters, some reports have suggested them as substrates of these transporters.17,18
Since MRP1, BCRP and P-glycoprotein are expressed in the BBB, particularly in these both cell lines,22 some specific inhibitors of these transporters were used: probenecid, rhodamine 123 and cyclosporine A, respectively, in order to investigate if by inhibiting them, efflux of flavan-3-ols was also inhibited. The increase in epicatechin transport across the BBB, but not in catechin, when simultaneously incubated with cyclosporine A, a P-glycoprotein inhibitor, showed the possibility of these compounds having different affinities for this transporter. Furthermore, it suggests the involvement of P-glycoprotein on epicatechin efflux, but not in catechin.
Alkaline phosphatase (ALP) is an ecto-phosphatase, dephosphorylating extracellular substrates or cell-surface proteins. It has been suggested an association between ALP and regulation of transport systems.5,6 The hypothesis of an influence of ALP on flavan-3-ols transport was considered. Epicatechin transport in the presence of progesterone (an ALP activator) was significantly increased after 3 and 18 h, as well as catechin transport after 18 h of incubation. This suggests a possible involvement of an entity (transporter or enzyme) that has its activity modulated by ALP. Since it has been reported that ALP activity may contribute to the modulation of P-glycoprotein, and that progesterone also could modulate directly P-glycoprotein, the involvement of this transporter in the flavan-3-ols transport across BBB is reinforced. Progesterone, as a female hormone, seems to be an important endogenous factor for these flavan-3-ol’s effects on the central neural system.
Several studies suggest that blood levels of catechin and epicatechin are far below the levels used in this study and that these compounds appeared mainly as methyl, sulfate and glucuronide metabolites. Nevertheless, the information obtained by this study is valuable for the new insights about flavan-3-ol transport.
Despite this, the following advances were made: (i) the studied flavan-3-ols are capable of crossing the BBB, possibly reaching the central nervous system, which correlates with some neuroprotective effects already described in the literature for this class of flavonoids; (ii) the involvement of a stereoselective process during the passage of these compounds across BBB cells; (iii) the synthesis of flavanol metabolites allowed detection of these compounds in the studied samples; (iv) these endothelial cells have enzymes capable of metabolizing these compounds, and, particularly, conjugating them with glucuronic acid.
Abbreviations
| BBB | Blood-brain barrier |
| CNS | Central nervous system |
| RBE4 | Rat brain endothelial cell |
| hCMEC/D3 | Human brain endothelial cell |
Acknowledgements
This work was supported by FCT (Fundação para a Ciência e Tecnologia) (POCI, FEDER, Programa Comunitário de Apoio) for three Ph.D. studentship grants (SFRH/BD/28160/2006, SFRH/BD/46640/2008 and SFRH/BD/64691/2009) and one research grant (PTDC/QUI/65501/2006).References
- D. E. Stevenson and R. D. Hurst, Cell. Mol. Life Sci., 2007, 64, 2900–2916 CrossRef CAS.
- C. Manach, G. Williamson, C. Morand, A. Scalbert and C. Remesy, Am. J. Clin. Nutr., 2005, 81, 230S–242S CAS.
- K. A. Youdim and J. A. Joseph, Free Radical Biol. Med., 2001, 30, 583–594 CrossRef CAS.
- H. F. Cserr and M. Bundgaard, Am. J. Physiol., 1984, 246, R277–288 CAS.
- C. Calhau, F. Martel, S. Pinheiro-Silva, H. Pinheiro, P. Soares-da-Silva, C. Hipolito-Reis and I. Azevedo, J. Cell. Biochem., 2002, 84, 389–400 CrossRef CAS.
- C. Calhau, F. Martel, P. Soares-da-Silva, C. Hipolito-Reis and I. Azevedo, Naunyn-Schmiedebergs Arch. Pharmacol., 2002, 365, 349–356 CrossRef CAS.
- F. Martel, C. Calhau, P. Soares-da-Silva and I. Azevedo, Naunyn-Schmiedebergs Arch. Pharmacol., 2001, 363, 1–10 CrossRef CAS.
- F. Roux, O. Durieu-Trautmann, N. Chaverot, M. Claire, P. Mailly, J. M. Bourre, A. D. Strosberg and P. O. Couraud, J. Cell. Physiol., 1994, 159, 101–113 CrossRef CAS.
- B. el Hafny, J. M. Bourre and F. Roux, J. Cell. Physiol., 1996, 167, 451–460 CrossRef CAS.
- D. J. Begley, D. Lechardeur, Z. D. Chen, C. Rollinson, M. Bardoul, F. Roux, D. Scherman and N. J. Abbott, J. Neurochem., 2002, 67, 988–995 CrossRef.
- A. Regina, F. Roux and P. A. Revest, Biochim. Biophys. Acta, Gen. Subj., 1997, 1335, 135–143 CrossRef CAS.
- H. Mkrtchyan, S. Scheler, I. Klein, A. Fahr, P. O. Couraud, I. A. Romero, B. Weksler and T. Liehr, Cytogenet. Genome Res., 2009 Search PubMed.
- B. B. Weksler, E. A. Subileau, N. Perriere, P. Charneau, K. Holloway, M. Leveque, H. Tricoire-Leignel, A. Nicotra, S. Bourdoulous, P. Turowski, D. K. Male, F. Roux, J. Greenwood, I. A. Romero and P. O. Couraud, FASEB J., 2005, 19, 1872–1874 CAS.
- A. Faria, D. Pestana, D. Teixeira, J. Azevedo, V. De Freitas, N. Mateus and C. Calhau, Cell. Mol. Biol. Lett., 2010, 15, 234–241 Search PubMed.
- F. Catterall, L. J. King, M. N. Clifford and C. Ioannides, Xenobiotica, 2003, 33, 743–753 CrossRef CAS.
- C. Starp, B. Alteheld and P. Stehle, Ann. Nutr. Metab., 2006, 50, 59–65 CrossRef CAS.
- J. B. Vaidyanathan and T. Walle, Pharm. Res., 2001, 18, 1420–1425 CrossRef CAS.
- L. Zhang, G. Lin, B. Kovacs, M. Jani, P. Krajcsi and Z. Zuo, Eur. J. Pharm. Sci., 2007, 31, 221–231 CrossRef CAS.
- T. Koga and M. Meydani, Am. J. Clin. Nutr., 2001, 73, 941–948 CAS.
- M. Natsume, N. Osakabe, A. Yasuda, T. Osawa and J. Terao, J. Clin. Biochem. Nutr., 2008, 42, 50–53 Search PubMed.
- R. A. Walgren, J. T. Lin, R. K. Kinne and T. Walle, J. Pharmacol. Exp. Ther., 2000, 294, 837–843 CAS.
- K. A. Youdim, B. Shukitt-Hale and J. A. Joseph, Free Radical Biol. Med., 2004, 37, 1683–1693 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
