Anti-obesity and anti-diabetic effects of ethanol extract of Artemisia princeps in C57BL/6 mice fed a high-fat diet
Norio
Yamamoto
*a,
Yuki
Kanemoto
b,
Manabu
Ueda
b,
Kengo
Kawasaki
a,
Itsuko
Fukuda
c and
Hitoshi
Ashida
bc
aFood Science Research Center, House Wellness Foods Corporation, 3-20 Imoji, Itami, Hyogo 664-0011, Japan. E-mail: Yamamoto_Norio@house-wf.co.jp; Fax: +81 72 778 0892; Tel: +81 72 778 1127
bDepartment of Agrobioscience, Graduate School of Agricultural Science, Kobe University, 1-1 Rokkodai-Cho, Nada-ku, Kobe, Hyogo 657-8501, Japan
cResearch Center for Food Safety and Security, Graduate School of Agricultural Science, Kobe University, 1-1 Rokkodai-Cho, Nada-ku, Kobe, Hyogo 657-8501, Japan
First published on 10th November 2010
Abstract
Artemisia princeps is commonly used as a food ingredient and in traditional Asian medicine. In this study, we examined the effects of long-term administration of an ethanol extract of A. princeps (APE) on body weight, white adipose tissue, blood glucose, insulin, plasma and hepatic lipids, and adipocytokines in C57BL/6 mice fed a high-fat diet. Daily feeding of a 1% APE diet for 14 weeks normalized elevated body weight, white adipose tissue, and plasma glucose and insulin levels, and delayed impaired glucose tolerance in mice a fed high-fat diet. These events were not observed in mice fed a control diet containing 1% APE. Liver triglyceride and cholesterol levels were similar in mice fed a 1% APE-diet and those fed a control diet. In the high-fat diet groups, APE inhibited hepatic fatty acid synthase (FAS) and suppressed the elevation of plasma leptin, but had no effect on adiponectin levels. These findings suggest that the regulation of leptin secretion by APE may inhibit FAS activity with subsequent suppression of triglyceride accumulation in the liver and adipose tissues. Inhibition of lipid accumulation can, in turn, lead to improvements in impaired glucose tolerance.
Introduction
Obesity is a metabolic disorder resulting from an imbalance between energy uptake and expenditure. Dietary fat is considered to be one of the most important factors in the pathophysiology of obesity, and shows an almost linear relationship with body weight and glucose tolerance.1 C57BL/6 mice are commonly used for obesity research, because they are lean when fed a low-fat diet, but show obese characteristics, including increased body-fat mass, hyperglycemia and hyperinsulinemia, when fed a high-fat (HF) diet.2,3An HF diet may induce hepatic triglyceride accumulation as a result of the import of excess amounts of fatty acids into the liver.3,4 Hepatic triglyceride accumulation has been directly linked to systemic insulin resistance.5,6 The hepatic triglyceride level increases when the rate of fatty acid input exceeds that of their output. The triglyceride level in hepatocytes thus represents complex interactions among the uptake of fatty acids, their derivation from non-esterified fatty acids (NEFA), de novofatty acid synthesis, fatty acid oxidation, and fatty acid export as very low-density lipoprotein (VLDL)-triglyceride.7
The genus Artemisia (Asteraceae) includes approximately 250 species of mostly perennial plants distributed in the northern hemisphere. They have a range of uses, including in medicines, foods, and spices, and as ornaments. Several Artemisia species have been reported to help prevent hyperglycemia and inflammation.8–10A. princeps (Japanese mugwort, or yomogi) is the best known Artemisia species in Japan, where it comprises a fundamental ingredient of the Japanese confection, kusa-mochi. This plant has been also used in traditional Asian medicine for the treatment of inflammation, diarrhea, and many circulatory disorders. Recent studies have shown it to have anti-atherosclerotic, anti-oxidant, and anti-inflammatory effects.11,12 The present study investigated the effects of an ethanol extract of A. princeps (APE) on obesity and hyperglycemia in C57BL/6 mice fed an HF diet, and analyzed the obesity factors and hepatic enzyme activities involved in fatty acid oxidation and synthesis.
Experimental
Plant material
Dried powder of the aerial part of A. princeps (Kazuzaki yomogi), which had been cultivated as a food ingredient in a properly managed field, was obtained from Uenochu (Osaka, Japan). The powder (1200 g) was soaked in ethanol (18 L) at 4 °C for 3 days. The obtained fluid was filtered through a cotton cloth and GA-200 glass-fiber filter paper (Advantec Toyo, Tokyo, Japan), stirred with 1% (w/v) activated charcoal for 2 h, and filtered again using GA-200 glass-fiber filter. The filtrate was concentrated and the remaining paste (15.6 g, 1.3%) was mixed with three times its weight of cellulose powder (Ceolus ST, Asahi Kasei Chemical, Tokyo, Japan) until uniformly dispersed.HPLC analysis of polyphenols
Polyphenol profiles of the APE were analyzed using an HPLC system, as previously described.13 Briefly, the ethanol extract was filtered through a Millex-LG 0.2 μm membrane filter (Millipore, Bedford, MA), then analyzed using a Hitachi HPLC series D-7000 (Tokyo, Japan) equipped with Hitachi model D-7000 chromatography data station software and diode array detection system D7450 to monitor all wavelengths from 200–600 nm. Chromatographic separation was carried out on a Capcell pak C18 UG120 (250 mm × 4.0 mm internal diameter, S-5, 5 μm, Shiseido Co., Ltd., Tokyo, Japan) by a stepwise elution with 50 mM sodium phosphate (pH 3.3) containing 10% (v/v) methanol (solution A) and 70% methanol (solution B). The gradient program was as follows: 0–15 min ratio of solution A to solution B 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (v/v), 15–45 min 70
0 (v/v), 15–45 min 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, 45–65 min 65
30, 45–65 min 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35, 65–70 min 60
35, 65–70 min 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, 70–85 min 50
40, 70–85 min 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, 85–110 min 0
50, 85–110 min 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100. The flow rate was 1 mL min−1, the injection volume was 10 μl, and the oven temperature was 35 °C. Phenolic compounds were quantified on the basis of their absorbance at 250 or 320 nm, relative to external standards or to previously generated calibration curves in the system library.
100. The flow rate was 1 mL min−1, the injection volume was 10 μl, and the oven temperature was 35 °C. Phenolic compounds were quantified on the basis of their absorbance at 250 or 320 nm, relative to external standards or to previously generated calibration curves in the system library.
Animal treatment
Male C57BL/6 mice aged 4 weeks (Japan SLC, Shizuoka, Japan) were maintained at 22 ± 3 °C under an automatic lighting schedule (0900–2100 h light). Food and water available ad libitum. On the day after the 7-days acclimatization, they were randomly divided into eight groups (n = 5), and fed with control diet (C) or HF diets containing 30% w/w lard for 14 weeks. The compositions of the diets and their energy contents are listed in Table 1. Body weight and food intake were measured weekly. At the end of the experiment, the tissues were collected, and frozen immediately using liquid nitrogen and kept at −80 °C until use. All animal treatments were approved by the Institutional Animal Care and Use Committee (Permission number 19-5-32) and carried out according to the Guidelines of Animal Experimentation of Kobe University Animal Experimentation Regulations.| Control diet | HF diet | |||||||
|---|---|---|---|---|---|---|---|---|
| 0% APE | 0.2% APE | 0.5% APE | 1.0% APE | 0% APE | 0.2% APE | 0.5% APE | 1.0% APE | |
| a APE-containing diets were prepared by adding APE-containing cellulose powder to the control or HF diet. b APE, ethanol extract of A. princeps. c The energy was calculated by counting out the energy of APE. | ||||||||
| APE b (%) | — | 1.19 | 0.48 | 0.96 | — | 1.19 | 0.48 | 0.96 |
| Cellulose (%) | 8.66 | 8.47 | 8.18 | 7.70 | 8.66 | 8.47 | 8.18 | 7.70 |
| Lard (%) | — | — | — | — | 28.85 | 28.85 | 28.85 | 28.85 |
| Cornstarch (%) | 44.81 | 44.81 | 44.81 | 44.81 | 15.96 | 15.96 | 15.96 | 15.96 |
| Casein (%) | 13.46 | 13.46 | 13.46 | 13.46 | 13.46 | 13.46 | 13.46 | 13.46 |
| L-Cystine (%) | 0.19 | 0.19 | 0.19 | 0.19 | 0.19 | 0.19 | 0.19 | 0.19 |
| Dextrin (%) | 14.90 | 14.90 | 14.90 | 14.90 | 14.90 | 14.90 | 14.90 | 14.90 |
| Sucrose (%) | 9.62 | 9.62 | 9.62 | 9.62 | 9.62 | 9.62 | 9.62 | 9.62 |
| Soybean oil (%) | 3.85 | 3.85 | 3.85 | 3.85 | 3.85 | 3.85 | 3.85 | 3.85 |
| Mineral mix (AIN-93M) (%) | 3.37 | 3.37 | 3.37 | 3.37 | 3.37 | 3.37 | 3.37 | 3.37 |
| Vitamin mix (AIN-93) (%) | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 |
| Choline bitartrate (%) | 0.29 | 0.29 | 0.29 | 0.29 | 0.29 | 0.29 | 0.29 | 0.29 |
| Tertiary butyl hydroxy quinone (%) | 0.0008 | 0.0008 | 0.0008 | 0.0008 | 0.0008 | 0.0008 | 0.0008 | 0.0008 |
| Total (%) | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Energyc (MJ per 100 g diet) | 1400 | 1400 | 1400 | 1400 | 2084 | 2084 | 2084 | 2084 |
Oral glucose tolerance test
An oral glucose test (OGTT) was performed using an oral dose of glucose (2 g kg−1 body weight) at week 12. Animals were food restricted for 12 h prior to the OGTT. Blood samples were collected from a tail vein into heparinized tubes at the indicated times after the glucose load. Plasma glucose concentration was measured using a commercial assay kit (Glucose CII-test, Wako Pure Chemical, Osaka, Japan).Measurement of plasma parameters related to glucose and lipid metabolism and adipocytokines
After 14 weeks, the mice were fasted for 12 h and sacrificed by collecting blood via cardiac puncture using a heparinized syringe, under anesthesia with sodium pentobarbital. The plasma was stored at −80 °C until use. Plasma triacylglycerol, total cholesterol, free fatty acid, and glucose were measured using appropriate commercial assay kits (Triglyceride-E test, Cholesterol-E test, NEFA-C test, and Glucose CII-test, Wako Pure Chemical). Plasma insulin, adiponectin, and leptin were measured by assay kits, according to the manufacturer's directions (mouse insulin ELISA kit, Shibayagi, Gunma, Japan; mouse/rat adiponectin ELISA kit, Otsuka Pharmaceutical, Tokyo, Japan; and mouse leptin ELISA kit, Morinaga, Yokohama, Japan, respectively.) The index of the homeostasis model assessment of insulin resistance (HOMA-IR) was calculated using the relationships between the plasma glucose and insulin levels, according to the following formula:14| HOMA-IR = fasting glucose (mg per 100 mL) × fasting insulin (μU per mL)/405. |
Measurement of hepatic lipid levels
Approximately 100 mg of liver was homogenized with 0.35 mL of distilled water, and the homogenate was extracted three times with 0.7 mL of chloroform–methanol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) solution. The chloroform layer was collected by centrifugation at 1800 g for 10 min, and washed with a 1/4 volume of 0.88% (w/v) KCl. The obtained chloroform layer was evaporated, and measured the weight of the residue as total lipids. The residue was dissolved in isopropanol containing 10% (v/v) Triton-X, and triglyceride and cholesterol levels were measured using commercial kits, as described above.
1, v/v) solution. The chloroform layer was collected by centrifugation at 1800 g for 10 min, and washed with a 1/4 volume of 0.88% (w/v) KCl. The obtained chloroform layer was evaporated, and measured the weight of the residue as total lipids. The residue was dissolved in isopropanol containing 10% (v/v) Triton-X, and triglyceride and cholesterol levels were measured using commercial kits, as described above.
Hepatic enzyme activities related to lipid metabolism
Approximately 300 mg of liver was homogenized in 2 mL of 3 mM Tris buffer (pH 7.4) containing 1 mM ethylenediaminetetraacetic acid (EDTA), 0.25 M sucrose, 1 mM dithiothreitol (DTT) and protease inhibitors (25 μM calpain inhibitor I (ALLN), 100 μM phenylmethylsulfonylfluoride, 5 μg mL−1 leupeptin, and 10 μM (L-3-trans-carboxyoxirane-2-carbonyl)-L-leucylagmatine hemihydrate (E-64)) with 10 strokes of a motor-driven Teflon pestle in a 5 mL glass vessel. An aliquot of the homogenate was stored at −80 °C as crude homogenate fraction until enzymatic analysis. The remaining homogenate was centrifuged at 9000 g at 4 °C for 15 min and the supernatant was stored at −80 °C as an S9 fraction until enzymatic analysis. Fatty acid synthase (FAS) was determined spectrophotometrically, as described previously.15,16Acyl-CoA oxidase (ACO) was determined using a previously described method.17Carnitine palmitoyltransferase (CPT) was determined using 96-well plates, according to a previously described method.18Statistical analysis
Data are expressed as mean ± SE. Statistical analysis (ANOVA) was carried out using the Tukey-Kramer multiple comparison test. Differences were considered significant when P values were <0.05.Results
Polyphenol analysis
The polyphenol profile of APE was analyzed using a HPLC system constructed for simultaneous determination of polyphenols in food stuffs (Table 2). Comparison with the library in the HPLC system identified the presence of chlorogenic acid (19.0 mg g−1), protocatechuic acid (38.2 mg g−1), and trace of luteolin in APE. 3,5-Di-O-caffeoylquinic acid (278.1 mg g−1), which was one of the major phenolic components, was analyzed by comparison with a standard chromatogram. A previous study reported that one of the flavones, eupatilin (5,7-dihydroxy-3,4,6-trimethoxyflavone), is an active compound in A. princeps.19 We checked the amount of eupatilin in our APE as compared with standard compound obtained from PhytoLab (Vestenbergsgreuth, Germany), and confirmed that our APE did not contain eupatilin.| Namea | Concentration in APE/mg g−1 | Retention time of peak/min |
|---|---|---|
| a The phenolic content was determined using an HPLC system constructed for the simultaneous determination of polyphenols in foods.13 b These values were calculated using previously generated calibration curves available in the system library. c This value was calculated using a curve generated from an external standard. | ||
| Protocatechuic acid | 19.0b | 9.7 |
| Chlorogenic acid | 38.2b | 13.7 |
| 3,5-Di-O-caffeoylquinic acid | 278.1c | 31.6 |
| Luteolin | traceb | 80.0 |
Effects of APE on body weight and adipose tissue weight
At the end of 14 week HF diet feeding, body weights were significantly higher than that of those in the control diet group. However, the body weights of APE-supplemented groups (HF-0.2%APE, HF-0.5%APE and HF-1.0%APE) were 8.6%, 20.4%, and 20.4% lower compared with the HF-0%APE group (Table 3). Both 0.5% and 1.0% APE supplementation significantly decreased HF diet-induced body weight gain from week 11 (Fig. 1). There were no significant differences in body weights among the control diet groups. The weights of all the white adipose tissues were significantly increased in the HF-0%APE group, compared with the C-0%APE group (Table 3). APE suppressed the HF diet-induced increase in adipose tissue weight. Supplementation of the HF diet with 1.0% APE significantly reduced the weights of almost all white adipose tissues, except for epididymal adipose tissue, compared with the HF-0%APE group. The weights of mesenteric adipose tissue of APE-supplemented groups (HF-0.2%APE, HF-0.5%APE, and HF-1.0%APE) were 29.3%, 49.7%, and 62.1% lower compared with the HF-0%APE group. APE supplementation tended to reduce body weight and adipose tissue weights in the control diet groups, but the differences were not significant. These results suggest that dietary APE has the potential to counteract obesity.| Control diet | High-fat diet | |||||||
|---|---|---|---|---|---|---|---|---|
| 0% APE | 0.2% APE | 0.5% APE | 1.0% APE | 0% APE | 0.2% APE | 0.5% APE | 1.0% APE | |
| a Mice were fed with control or a high-fat diets containing APE for 14 weeks. At the end of experiment, body weight, tissue weights, and plasma lipid levels were measured after fasting for 12 h. Values are the mean ± SE (n = 5). Values without common letters in a row indicate significant differences (P < 0.05) by the Tukey-Kramer multiple comparison test. | ||||||||
| Total energy intake (MJ per mouse) | 4,895 | 4,519 | 4,397 | 4,071 | 5,690 | 5,803 | 5,456 | 5,941 |
| Body weight/g | 28.2 ± 0.9a | 28.3 ± 0.9a | 28.0 ± 1.1a | 27.1 ± 1.2a | 44.2 ± 0.7b | 40.4 ± 1.2b | 35.2 ± 1.2c | 35.2 ± 1.1c |
| Tissue weight (g per 100 g body weight) | ||||||||
| Liver | 3.72 ± 0.12ab | 3.79 ± 0.11ab | 3.72 ± 0.19ab | 3.78 ± 0.05ab | 4.01 ± 0.12a | 3.32 ± 0.27b | 3.32 ± 0.06ab | 3.47 ± 0.09ab |
| White adipose tissue | ||||||||
| Total | 8.10 ± 1.53ab | 8.08 ± 1.38ab | 7.26 ± 1.16a | 6.64 ± 0.79a | 23.1 ± 0.71c | 21.8 ± 1.69c | 19.3 ± 2.13cd | 14.0 ± 1.33bd |
| Epididymal | 1.94 ± 0.31a | 2.20 ± 0.35a | 1.96 ± 0.2a | 2.09 ± 0.50a | 4.80 ± 0.37b | 5.47 ± 0.36b | 5.17 ± 0.51b | 4.11 ± 0.30b |
| Mesenteric | 0.90 ± 0.10ab | 0.97 ± 0.14ab | 0.77 ± 0.14a | 0.84 ± 0.25a | 3.62 ± 0.30c | 2.56 ± 0.31d | 1.82 ± 0.29bd | 1.37 ± 0.13ab |
| Retroperitoneal | 1.35 ± 0.27ab | 1.36 ± 0.23ab | 1.10 ± 0.21a | 1.19 ± 0.32ab | 3.80 ± 0.28c | 3.33 ± 0.28cd | 3.26 ± 0.37cd | 2.41 ± 0.21bd |
| Subcutaneous | 3.91 ± 0.90ab | 3.55 ± 0.69ab | 3.43 ± 0.57ab | 2.53 ± 0.34a | 10.9 ± 0.31c | 10.5 ± 0.97c | 9.02 ± 1.07cd | 6.09 ± 0.76bd |
| Brown adipose tissue | 0.53 ± 0.03abc | 0.63 ± 0.06abcd | 0.47 ± 0.05a | 0.58 ± 0.12abcd | 0.94 ± 013ed | 1.07 ± 0.11e | 0.83 ± 0.06bce | 0.66 ± 0.05acd |
| Plasma lipid levels | ||||||||
| Total cholesterol/mg dL−1 | 105 ± 3 a | 105 ± 4a | 109 ± 10a | 115 ± 8ab | 180 ± 6c | 144 ± 9b | 144 ± 10b | 122 ± 3ab |
| NEFA/ meq L−1 | 0.91 ± 0.05a | 0.70 ± 0.06abc | 0.74 ± 0.07abc | 0.79 ± 0.12ab | 0.63 ± 0.04abc | 0.65 ± 0.05abc | 0.49 ± 0.02c | 0.53 ± 0.04bc |
| Triglyceride/mg dL−1 | 134 ± 5a | 120 ± 4ab | 116 ± 5b | 119 ± 1ab | 107 ± 1b | 110 ± 2b | 113 ± 3b | 108 ± 2b |
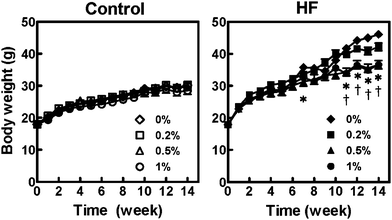 | ||
| Fig. 1 Changes in body weight of mice fed with control and high-fat (HF) diets containing an ethanol extract of A. princeps for 14 weeks. Closed symbols represent the HF-diet groups and open ones represent the control-diet groups. Values are the means ± SE (n = 5). Significant differences between the 0% group and 1.0% (*) or 2.0% (†) groups are indicated (P < 0.05 by the Tukey-Kramer multiple comparison test). | ||
Preventive effect of APE on HF diet-induced hyperglycemia
An OGTT was performed after feeding the mice with APE for 12 weeks (Fig. 2A). Plasma glucose levels in the HF-0 group remained high between 15 and 120 min, but supplementation of the HF-diet with APE (HF-0.5%APE and HF-1.0%APE) significantly improved glucose tolerance compared with the HF-0 group. There were no significant differences in OGTT results throughout the experiment in the control diet groups. The area under the curve (AUC) in the HF-0%APE group was 37% higher than that in the C-0%APE group (P < 0.05), while that in the HF-0.5%APE group was 32% lower than that in the HF-0%APE (P < 0.05) (Fig. 2B).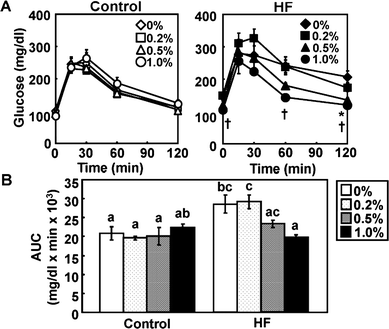 | ||
| Fig. 2 Oral glucose tolerance test (OGTT) in mice fed control and high-fat (HF) diets containing an ethanol extract of A. princeps (APE) at week 12. (A) Fasting plasma glucose levels after oral glucose administration (2.0 g kg−1 body weight). Closed symbols represent the HF-diet groups and open ones represent the control-diet groups. Values are the mean ± SE (n = 6). Significant differences between 0% group and 1.0% (*) or 2.0% (†) group are indicated (P < 0.05 by the Tukey-Kramer multiple comparison test). (B) Area under the curve (AUC) from the values of (A). Values are the mean ± SE (n = 5). The same letters indicate no significant differences according to the Tukey-Kramer multiple comparison test. P < 0.05 was considered significant. | ||
The plasma glucose level at the end of the experiment was significantly higher in the HF-0 group, compared with the C-0%APE group (Fig. 3A). Supplementation of the HF diet with APE reduced plasma glucose levels in a dose-dependent manner. The plasma insulin level was also higher in the HF-0%APE group than in the C-0%APE group (Fig. 3B). Insulin levels in the HF-0.5%APE and HF-1.0%APE groups were normalized, and equivalent to that in the control diet group. Neither glucose nor insulin levels changed in the control diet groups. HOMA-IR is a good predictor of total insulin sensitivity, and was significantly higher in the HF-0 than in the C-0%APE group (Fig. 3C). Supplementation of the HF diet with 0.5% and 1.0% APE significantly attenuated the HF diet-induced increase in HOMA-IR.
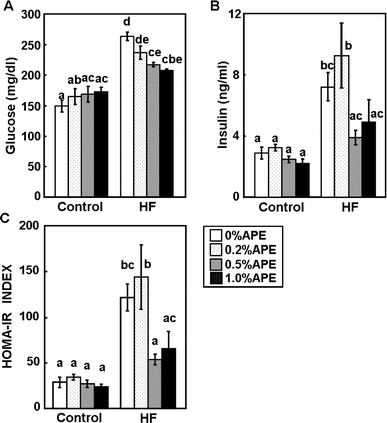 | ||
| Fig. 3 Effect of an ethanol extract of A. princeps (APE) on plasma glucose and insulin levels in mice fed control and high-fat (HF) diets for 14 weeks. Plasma levels of glucose (A) and insulin (B) were measured, and the homeostasis model assessment of insulin resistance index (HOMA-IR) was calculated (C). Values are the mean ± SE (n = 4). The same letters represent no significant differences according to the Tukey-Kramer multiple comparison test. P < 0.05 was considered significant. | ||
APE suppressed hyperglycemia and insulin resistance, as described above, and α-glucosidase activities in the small intestine were measured to clarify the mechanisms behind the antihyperglycemic effect. Both maltase and sucrase activates tended to decrease in the HF-diet groups, compared with the control groups, though the differences were not significant (data not shown). APE did not inhibit maltase or sucrase activities in the small intestine.
Effect of APE on lipid metabolism
Intake of a HF diet induces fatty liver and hepatic lipid accumulation, which are involved in systemic insulin resistance.5,6 The hepatic total lipid level was significantly higher in the HF-0%APE group than in the C-0%APE group (Fig. 4). APE suppressed the HF diet-induced hepatic lipid accumulation in a dose-dependent manner, and the hepatic total lipid level was significantly lower in HF-1.0%APE group than in the HF-0%APE group. The hepatic triglyceride level in the HF-0%APE group was also higher than that in the C-0%APE group, and this higher value was significantly lowered by supplementation with 1.0% APE (Fig. 4). The hepatic cholesterol level was significantly higher in the HF-0 group than in the C-0 group, and this was also significantly lowered by supplementation with 1.0% APE (Fig. 4). These results suggest that APE has the ability to prevent fatty liver induced by an HF diet.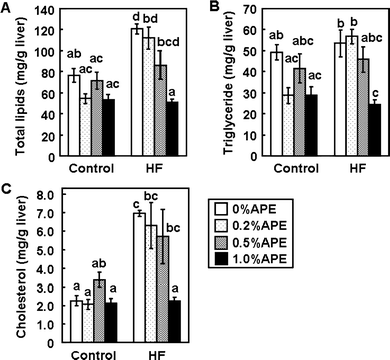 | ||
| Fig. 4 Effects of an ethanol extract of A. princeps (APE) on hepatic lipid levels in mice fed control and high-fat (HF) diets for 14 weeks. Total lipid (A), triglyceride (B) and cholesterol (C) levels were measured. Values are the mean ± SE (n = 5). The same letters represent no significant differences according to the Tukey-Kramer multiple comparison test. P < 0.05 was considered significant. | ||
APE suppressed hepatic lipid levels, and plasma lipid levels were therefore also measured. The total plasma cholesterol level was significantly higher in the HF-0%APE group, compared with the C-0%APE group (Table 3), but was significantly lower in the HF-0.2%APE, HF-0.5%APE, and HF-1.0%APE groups, compared with the HF-0%APE group. The cholesterol level in the HF-1.0%APE group was similar to that in the control diet groups. Plasma NEFA and triglyceride levels in the HF diet groups were lower than those in the control diet groups (Table 3). APE tended to decrease NEFA levels in both the control and HF-diet groups.
The activities of the hepatic enzymes related to lipid metabolism were also measured (Fig. 5). CPT, ACO and FAS activities were lower in the HF-diet groups than in the control groups. CPT and ACO are responsible for β-oxidation in mitochondria and peroxisomes, respectively,20APE did not affect CPT or ACO activities in either control or HF-diet groups. FAS is a key enzyme that catalyzes fatty acid biosynthesis,21,22APE significantly reduced FAS activity in the HF-diet groups in a dose-dependent manner.
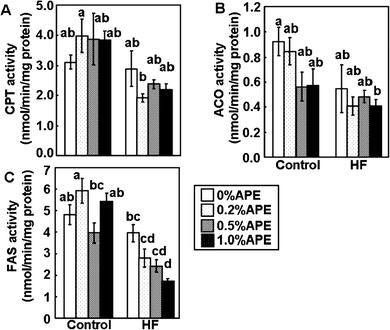 | ||
| Fig. 5 Effects of an ethanol extract of A. princeps (APE) on the activities of hepatic enzymes related to lipid metabolism in mice fed control and high-fat (HF) diets for 14 weeks. Carnitine palmitoyltransferase (CPT) (A), acyl-CoA oxidase (ACO) (B), and fatty acid synthase (FAS) (C) activities in the liver were measured. Values are the mean ± SE (n = 5). The same letters represent no significant differences according to the Tukey-Kramer multiple comparison test. P < 0.05 was considered significant. | ||
Effect of APE on adipocytokines
White adipose tissue is a major endocrine tissue that releases various adipocytokines into the bloodstream. Because leptin and adiponectin are major adipocytokines associated with maintaining glucose homeostasis,23 we measured the plasma levels of these adipocytokines (Fig. 6). The plasma leptin level was 13.5-fold greater in the HF-0%APE than in the C-0%APE group, and APE significantly and dose-dependently reduced HF diet-induced leptin levels. Plasma leptin levels were 34.7%, 51.4%, and 73.3% lower in the HF-0.2%APE, HF-0.5%APE, and HF-1.0%APE groups, respectively, compared with the HF-0%APE group. There were no significant differences in leptin levels among the control diet groups. In contrast, plasma adiponectin levels were similar in all groups in this study, despite the fact that circulating adiponectin levels are reportedly reduced in obese states.23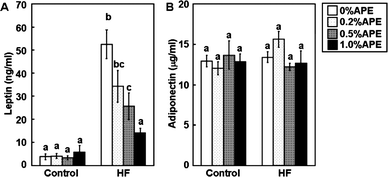 | ||
| Fig. 6 Effect of an ethanol extract of A. princeps (APE) on plasma leptin and adiponectin levels in mice fed control and high-fat (HF) diets for 14 weeks. Plasma leptin (A) and adiponectin (B) were measured. Values are the mean ± SE (n = 5). The same letters represent no significant differences according to the Tukey-Kramer multiple comparison test. P < 0.05 was considered significant. | ||
Discussion
The genus Artemisia comprises numerous diverse species, many of which are used as medical plants to alleviate human conditions including hyperglycemia and diabetes.8–10 This study investigated the use of an ethanol extract of A. princeps as a potential dietary supplement for the management of hyperglycemia and obesity in HF diet-induced obese C57BL/6 mice. A regular HF-diet is generally accepted to cause obesity, and prevention of obesity and fat accumulation is important for the promotion of human health. APE suppressed fat accumulation without reducing food intake, suggesting that dietary APE has the potential to counteract obesity.Obesity is strongly associated with insulin resistance, and improved insulin resistance is important in preventing the development of type 2 diabetes. The results of this study suggest that dietary APE can prevent HF diet-induced insulin resistance and hyperglycemia. Inhibition of carbohydrate-hydrolyzing enzymes in the small intestine represents an effective method of preventing and treating hyperglycemia.24 Synthetic α-glucosidase inhibitors such as acarbose and miglitol are widely for treating type 2 diabetes patients.18 These inhibitors block the action of the α-glucosidase enzymes in the small intestine, thereby delaying glucose absorption.24 Certain plant extracts have been reported to inhibit α-glucosidase activities.25,26 Although HPLC analysis identified chlorogenic acid and 3,5-di-O-caffeoylquinic acid, both compounds with reported α-glucosidase inhibitory activities,27 as components of APE in this study, APE had no effect on α-glucosidase activities in this study. The suppression of hyperglycemia and insulin resistance by APE is therefore to the result of α-glucosidase inhibition.
Previously, an anti-diabetic effect of Korean A. princeps in type-2 diabetic mice was reported.19,28 The active constituent is thought to be a flavones, eupatilin, which had a functional anti-diabetic effect by enhancing hepatic and plasma glucose metabolism.19 Hence, we analyzed the amount of eupatilin in our APE by HPLC analysis, and confirmed that our APE did not contain eupatilin. The A. princeps used in the previous studies were variants cultivated in Korea, and may have had a different composition of phytochemicals than Japanese A. princeps.
APE significantly suppressed the accumulation of white adipose tissue, including visceral adipose tissue. Visceral adipose tissue is an important predictor of insulin resistance, hyperglycemia and other metabolic risk factors.29,30 Increased adipose tissue weights are accompanied by the induction of inflammatory cytokines involved in insulin resistance,31–33 and inhibition of fat accumulation by APE may therefore also contribute to its prevention of hyperglycemia.
APE normalized liver weight and hepatic lipid content in the HF-diet groups, suggesting that it could prevent HF diet-induced fatty liver. Visceral adipose tissue has recently been correlated with intrahepatic triglyceride content, and an increase in intrahepatic triglycerides is associated with the metabolic abnormalities.5,6,34 The prevention of fatty liver by APE may thus also contribute to the prevention of hyperglycemia. The activities of hepatic enzymes related to lipid metabolism were measured, to clarify the mechanisms whereby APE prevented hepatic lipid accumulation. APE inhibited FAS activity in mice fed an HF-diet. FAS catalyzes the final step in fatty acid synthesis, and is believed to be a determinant of the capacity for de novofatty acid synthesis.22APE thus inhibited fatty acid synthesis through inhibition of FAS, a rate-limiting enzyme in fatty acid synthesis, resulting in a decrease in hepatic lipid content. FAS activities were higher in the control diet groups than in the HF-diet groups. Lipogenic enzyme activities are reduced by fasting or by intake of an HF-diet, and increased by intake of a carbohydrate-rich diet or by re-feeding.35,36 Inhibition of FAS by APE could therefore help to prevent HF diet-induced fatty liver.
Jung and Kang et al. reported that ethanol extracts of A. princeps improved glucose and insulin tolerance via enhancing hepatic and plasma glucose metabolism28 and reduced FAS in diabetic animals, db/db mice.37 Inhibition of hepatic activity of glucose-6-phosphatase, a rate-limiting enzyme of gluconeogenesis may partly contribute to the anti-hyperglycemic effect of APE. Furthermore their groups reported that eupatilin isolated from their APE played the role of an antidiabetic functional component in A. princeps by enhancing hepatic and plasma glucose metabolism as well as by increasing insulin secretion in type 2 diabetic mice.19 We observed the anti-diabetic and anti-obese effects in the absence of eupatilin in our APE. The regulation of FAS activity by APE might be the one with another compound that is not eupatilin. Artemisia plant contain various phytochemicals, such as β-sitosterol,38 scopoletin,38sesquiterpenoid lactones39,40 and number of volatile chemicals.41 Some individual components may have synergistic effects, while some particular components may have strong independent effects. The previous observation in the db/db mice, a genetic model of diabetes and the present observation in the environmentally-induced diabetic model is sure to make the effect of A. princeps certain regardless of whether the active ingredient is eupatilin.
The down-regulation of FAS activity by APE may, in turn, result from prevention of hyperleptinemia. Lipogenesis has recently been shown to be controlled by leptinviasignal transducer and activator of transcription 3-independent central mechanisms.42 Furthermore, intraperitoneal leptin administration in C57BL/6 mice was able to directly suppress the expression of FAS in the liver and white adipose tissue, accompanied by reduced liver triglyceride levels.43 Prevention of leptin secretion by APE may therefore contribute to the inhibition of FAS activity and triglyceride accumulation in the liver.
The hepatic triglyceride content is determined by the balance between fatty acid input (e.g., by de novofatty acid synthesis) and output (e.g., by oxidation and export of VLDL-triglycerides). Measurements of hepatic CPT and ACO activities indicated that APE supplementation had no effect on the activities of these enzymes. The oxidation of intrahepatocellular fatty acids occurs mainly in mitochondria, and to a much lesser extent in peroxisomes and microsomes. CPT regulates the transport of fatty acids from the cytoplasm to the mitochondrial matrix across the membrane,20 while ACO is the initial enzyme in the peroxisomal β-oxidation system.44,45 The results in this study suggest that APE does not affect β-oxidation in either the mitochondria or peroxisomes.
Han et al. reported the antiatherosclerotic and anti-inflammatory activities in LDLR(−/−) mice.11 Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance and endothelial dysfunction is an early event in atherosclerosis, and large-vessel atherosclerotic disease is the major cause of morbidity and mortality in diabetes. Not only metabolizing glucose and lipid but also these functions might have contributed effectively.
The dosage of APE in mice fed a HF-diet containing 1.0% of APE becomes about 100 mg per person per day at 60 kg in weight in humans according to conversion based on body surface area. However, it is not appropriate to apply dosage from a present result in the study of mice to the dosage to the human. Further study will be needed to clarify its effect on the health of human.
Conclusion
We investigated the effects of APE on obesity and hyperglycemia in C57BL/6 mice fed an HF diet. Dietary APE prevented body weight gain, fat accumulation, and hyperglycemia in mice fed an HF diet. APE supplementation suppressed hyperleptinemia, which may prevent hepatic lipid accumulation through inhibition of FAS activity in the liver. This inhibition of hepatic lipid accumulation will thus contribute to the prevention of hyperglycemia. The results also suggested that A. princeps might be an excellent natural food additive because of its antiobesity and antidiabetic properties, and that it could be useful for the development of a more potent and selective agent.Acknowledgements
Part of this work was supported by the program Special Coordination Funds for Promoting Science and Technology, Creation of Innovation Centers for Advanced Interdisciplinary Research Areas (Innovative Bioproduction Kobe), MEXT, Japan.References
- M. Takahashi, S. Ikemoto and O. Ezaki, J. Nutr. Science Vitaminol., 1999, 45, 583–593 Search PubMed.
- S. Nishikawa, A. Yasoshima, K. Doi, H. Nakayama and K. Uetsuka, Exp. Anim., 2007, 56, 263–272 Search PubMed.
- R. S. Surwit, M. N. Feinglos, J. Rodin, A. Sutherland, A. E. Petro, E. C. Opara, C. M. Kuhn and M. Rebuffe-Scrive, Metab., Clin. Exp., 1995, 44, 645–651 CrossRef CAS.
- G. F. Lewis, A. Carpentier, K. Adeli and A. Giacca, Endocr. Rev., 2002, 23, 201–229 CrossRef CAS.
- K. M. Korenblat, E. Fabbrini, B. S. Mohammed and S. Klein, Gastroenterology, 2008, 134, 1369–1375 CrossRef CAS.
- J. H. Hwang, D. T. Stein, N. Barzilai, M. H. Cui, J. Tonelli, P. Kishore and M. Hawkins, Am. J. Physiol., 2007, 293, E1663–1669 CAS.
- H. N. Ginsberg, Y. L. Zhang and A. Hernandez-Ono, Arch. Med. Res., 2005, 36, 232–240 CrossRef CAS.
- D. M. Ribnicky, A. Poulev, M. Watford, W. T. Cefalu and I. Raskin, Phytomedicine, 2006, 13, 550–557 CrossRef CAS.
- X. H. Xing, Z. M. Zhang, X. Z. Hu, R. Q. Wu and C. Xu, J. Ethnopharmacol., 2009, 125, 410–416 CrossRef CAS.
- Y. Sun, Y. H. Li, X. X. Wu, W. Zheng, Z. H. Guo, Y. Li, T. Chen, Z. C. Hua and Q. Xu, Int. J. Mol. Med., 2006, 17, 957–962 Search PubMed.
- J. M. Han, M. J. Kim, S. H. Baek, S. An, Y. Y. Jin, H. G. Chung, N. I. Baek, M. S. Choi, K. T. Lee and T. S. Jeong, J. Agric. Food Chem., 2009, 57, 1267–1274 CrossRef CAS.
- M. J. Kim, J. M. Han, Y. Y. Jin, N. I. Baek, M. H. Bang, H. G. Chung, M. S. Choi, K. T. Lee, D. E. Sok and T. S. Jeong, Arch. Pharmacal Res., 2008, 31, 429–437 Search PubMed.
- H. Sakakibara, Y. Honda, S. Nakagawa, H. Ashida and K. Kanazawa, J. Agric. Food Chem., 2003, 51, 571–581 CrossRef CAS.
- K. K. Trout, C. Homko and N. C. Tkacs, Biol. Res. Nursing, 2007, 8, 305–318 Search PubMed.
- C. M. Nepokroeff, M. R. Lakshmanan and J. W. Porter, Methods Enzymol., 1975, 35, 37–44 Search PubMed.
- D. S. Kelley, G. J. Nelson and J. E. Hunt, Biochem. J., 1986, 235, 87–90 CAS.
- T. Osumi and T. Hashimoto, Biochem. Biophys. Res. Commun., 1978, 83, 479–485 CrossRef CAS.
- M. A. Markwell, E. J. McGroarty, L. L. Bieber and N. E. Tolbert, J. Biol. Chem., 1973, 248, 3426–3432 CAS.
- Y. J. Kang, U. J. Jung, M. K. Lee, H. J. Kim, S. M. Jeon, Y. B. Park, H. G. Chung, N. I. Baek, K. T. Lee, T. S. Jeong and M. S. Choi, Diabetes Res. Clin. Pract., 2008, 82, 25–32 CrossRef CAS.
- J. P. Bonnefont, F. Djouadi, C. Prip-Buus, S. Gobin, A. Munnich and J. Bastin, Mol. Aspects Med., 2004, 25, 495–520 CrossRef CAS.
- J. A. Menendez, A. Vazquez-Martin, F. J. Ortega and J. M. Fernandez-Real, Clin. Chem., 2009, 55, 425–438 CrossRef CAS.
- S. Smith, FASEB J., 1994, 8, 1248–1259 CAS.
- H. Tilg and A. R. Moschen, Trends Endocrinol. Metab., 2008, 19, 371–379 CrossRef CAS.
- F. A. van de Laar, P. L. Lucassen, R. P. Akkermans, E. H. van de Lisdonk, G. E. Rutten and C. van Weel, Diabetes Care, 2005, 28, 154–163 CrossRef CAS.
- K. Yoshino, Y. Miyauchi, T. Kanetaka, Y. Takagi and K. Koga, Biosci., Biotechnol., Biochem., 2009, 73, 1096–1104 CrossRef CAS.
- T. T. Mai and N. V. Chuyen, Biosci., Biotechnol., Biochem., 2007, 71, 69–76 CrossRef CAS.
- T. Matsui, S. Ebuchi, T. Fujise, K. J. Abesundara, S. Doi, H. Yamada and K. Matsumoto, Biol. Pharm. Bull., 2004, 27, 1797–1803 CrossRef CAS.
- U. J. Jung, N. I. Baek, H. G. Chung, M. H. Bang, J. S. Yoo, T. S. Jeong, K. T. Lee, Y. J. Kang, M. K. Lee, H. J. Kim, J. Y. Yeo and M. S. Choi, Food Chem. Toxicol., 2007, 45, 2022–2029 CrossRef CAS.
- A. Gastaldelli, Y. Miyazaki, M. Pettiti, M. Matsuda, S. Mahankali, E. Santini, R. A. DeFronzo and E. Ferrannini, J. Clin. Endocrinol. Metab., 2002, 87, 5098–5103 CrossRef CAS.
- J. P. Despres, I. Lemieux, J. Bergeron, P. Pibarot, P. Mathieu, E. Larose, J. Rodes-Cabau, O. F. Bertrand and P. Poirier, Arterioscler., Thromb., Vasc. Biol., 2008, 28, 1039–1049 CrossRef CAS.
- G. S. Hotamisligil, Nature, 2006, 444, 860–867 CrossRef CAS.
- E. Bertin, P. Nguyen, M. Guenounou, V. Durlach, G. Potron and M. Leutenegger, Diabetes Metabol., 2000, 26, 178–182 Search PubMed.
- T. You, R. Yang, M. F. Lyles, D. Gong and B. J. Nicklas, Am. J. Physiol., 2005, 288, E741–747 CAS.
- E. Fabbrini, F. Magkos, B. S. Mohammed, T. Pietka, N. A. Abumrad, B. W. Patterson, A. Okunade and S. Klein, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 15430–15435 CrossRef CAS.
- G. R. Herzberg and M. Rogerson, Br. J. Nutr., 1988, 59, 233–241 CrossRef CAS.
- T. S. Kim and H. C. Freake, J. Nutr., 1996, 126, 611–617 CAS.
- U. J. Jung, N. I. Baek, H. G. Chung, T. S. Jeong, K. T. Lee, M. K. Lee and M. S. Choi, J. Med. Food, 2009, 12, 1238–1244 CrossRef.
- S. H. Chang, E. J. Jung, Y. H. Park, D. G. Lim, N. Y. Ko, W. S. Choi, E. Her, S. H. Kim, K. D. Choi, J. H. Bae, S. H. Kim, C. D. Kang, D. J. Han and S. C. Kim, J. Pharm. Pharmacol., 2009, 61, 1043–1050 CrossRef CAS.
- T. A. Geissman, J. Org. Chem., 1966, 31, 2523–2526 CrossRef CAS.
- S. Y. Ryu, M. H. Oak and K. M. Kim, Planta Med., 2000, 66, 171–173 CrossRef CAS.
- K. Umano, Y. Hagi, K. Nakahara, A. Shoji and T. Shibamoto, J. Agric. Food Chem., 2000, 48, 3463–3469 CrossRef CAS.
- C. Buettner, E. D. Muse, A. Cheng, L. Chen, T. Scherer, A. Pocai, K. Su, B. Cheng, X. Li, J. Harvey-White, G. J. Schwartz, G. Kunos, L. Rossetti and C. Buettner, Nat. Med., 2008, 14, 667–675 CrossRef CAS.
- L. Jiang, Q. Wang, Y. Yu, F. Zhao, P. Huang, R. Zeng, R. Z. Qi, W. Li and Y. Liu, PLoS One, 2009, 4, e6884 CrossRef.
- S. Miyazawa, H. Hayashi, M. Hijikata, N. Ishii, S. Furuta, H. Kagamiyama, T. Osumi and T. Hashimoto, J. Biol. Chem., 1987, 262, 8131–8137 CAS.
- R. J. Wanders, P. Vreken, S. Ferdinandusse, G. A. Jansen, H. R. Waterham, C. W. van Roermund and E. G. Van Grunsven, Biochem. Soc. Trans., 2001, 29, 250–267 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
