Red wine: A source of potent ligands for peroxisome proliferator-activated receptor γ
Alfred
Zoechling
ab,
Falk
Liebner
c and
Alois
Jungbauer
*ab
aChristian Doppler Laboratory for Receptor Biotechnology, Muthgasse 18, A-1190, Vienna, Austria. E-mail: alois.jungbauer@boku.ac.at; Fax: +43 1 3697615; Tel: +43 1 476546226
bUniversity of Natural Resources and Life Sciences, Department of Biotechnology, Muthgasse 18, A-1190, Vienna, Austria
cUniversity of Natural Resources and Life Sciences, Department of Chemistry, Muthgasse 18, A-1190, Vienna, Austria
First published on 19th November 2010
Abstract
Moderate red wine consumption has been correlated with lower incidences of cardiovascular diseases, inflammation, and metabolic diseases such as type 2 diabetes, obesity, and high blood pressure. We studied binding of ligands from different wines to the peroxisome proliferator-activated receptor γ (PPARγ), a key factor in glucose and lipid metabolism. Ellagic acid and epicatechin gallate (ECG) were identified by gas chromatography and mass spectroscopy in the most active wine fractions. They had an affinity to PPARγ similar to that of the standard pharmaceutical agent rosiglitazone, which is used for the treatment of type 2 diabetes. The IC50 values of ellagic acid and ECG were 5.7 × 10−7 M and 5.9 × 10−7 M, respectively. All of the red wines had affinities for PPARγ equivalent to concentrations of rosiglitazone ranging from 52–521 μM. One hundred milliliters of the tested red wines was equivalent to approximately 1.8–18 mg of rosiglitazone. This volume contained an activity equivalent of at least a quarter of (and up to four times) the daily dose of this potent anti-diabetes drug. The ameliorating effects of red wine on metabolic diseases may be partially explained by the presence of PPARγ ligands.
Introduction
Epidemiological studies have suggested that consuming moderate amounts of red wine has certain health benefits. The J-shaped relationship between the amount of alcohol consumed and the risk of cardiovascular disease was first reported by Klatsky et al.1 A correlation between dietary habits, red wine consumption, and the risk of cardiovascular disease in France was found by Renauld and De Lorgeril,2 and it is known as the “French Paradox” A recent epidemiological study performed in Australia on alcohol intake, type of beverage, and risk of type 2 diabetes showed a strong correlation between moderate wine consumption and lower diabetes risk for men and women.3 Weight reduction and lower occurrence of metabolic syndrome was also shown by the SWAN study (Study of Women Across the Nation) and other studies.4–14 These effects have been only observed for moderate wine consumption. On the basis of literature data, moderate consumption leading to beneficial effects on metabolic syndrome and/or related patho-physiological conditions will correspond to about 10–20 g of alcohol per day for women and to 20–30 g of alcohol per day for men.6 However, it is difficult to convey the term “moderate” to the general public, so it is often defined as a glass per day, which may vary from 100 mL to 200 mL. For instance, in the MOLI-SANI project a beneficial health effect has been observed for a consumption of 35 g ethanol per day for men and about 10 g ethanol for women.15 Excessive wine consumption has a clear detrimental effect on health.5–14Animal studies have also suggested that certain wine components exercise a protective role in chronic pathologies. Effects are described for cardiovascular heart diseases and atherosclerosis, hypertension, metabolic diseases (diabetes and metabolic syndrome), and neurodegenerative diseases, as well as cancer.16 However, the molecular modes of action and the different pathways involved are not yet fully understood for most of the active compounds present in red wines. One well-known mechanism is the activation of estrogen receptors through isoflavones. This can lead to activation of endothelial nitric oxide synthase (eNOS) and to release of nitric oxide.17–19 A similar effect on eNOS activation and relaxation of the artery vessels resulting in a decrease in blood pressure has been also described for wine polyphenols.20,21 Another key receptor target in the dietary prevention of metabolic diseases is the peroxisome proliferator-activated receptor γ (PPARγ). Metabolic syndrome is correlated with reduced insulin sensitivity, hypertension, and hence with a higher risk for development of type 2 diabetes and cardiovascular diseases. The transcription factor PPARγ is expressed in many tissues such as adipose, heart, muscle, colon, kidney and liver, and is mainly responsible for adipocyte differentiation and energy storage. It may also play a critical role in the pathogenesis of atherosclerosis by influencing levels of circulating lipids and glucose, or, more directly, by modulating macrophage functions.22–24PPARγ antagonists induce expression of adiponectin in monocytes/macrophages, and consequently monocyte adhesion is substantially reduced. It was recently discovered that human platelets also contain PPARγ. The role of PPAR ligands in preventing unwanted platelet activation, reverse cholesterol transport and chronic inflammatory diseases has been recently shown in several papers.24–28 Both PPARγ and retinoid X receptor (RXR) are released from activated human platelets.29 Furthermore, PPARγ and LXR α are key regulators of the macrophage cholesterol homeostasis, and initiate the process of reverse cholesterol transport (RCT). In this process the excess peripheral cholesterol is taken up by tissue macrophages, and in turn cholesterol is transported in the liver by HDL and then excreted.27,30 RCT is impaired by inflammation.28
Natural ligands for PPARγ are polyunsaturated fatty acids and eicosanoic acid.31 Furthermore, there is evidence that certain polyphenolic compounds (such as the resveratrol in red wine) might have a strong affinity for PPARγ. Cholesterol accumulation could be prevented in macrophagesvia a PPARγ activation of resveratrol.25
Through wine technology, the concentration of polyphenols, which are thought to have potential health effects, can be controlled. As grape skins contain a wide variety of polyphenols in considerable amounts, red wines are usually rich in these compounds. Gallic acid, as well as catechin and epicatechin gallates, are released during fermentation from gallotannins that originate from the grape skin and seeds.32 Substantial amounts of polyphenolic compounds are also leached from oak barrels. Ellagic acid, for example, is a well-characterized compound that is typically found in barrique aged wines. This compound is derived from the ellagitannins originating from oak barrels during wine maturation.33 The chemical structures of some wine polyphenols and rosiglitazone, a pharmaceutical agent that is used in the treatment of type 2 diabetes, are shown in Fig. 1.
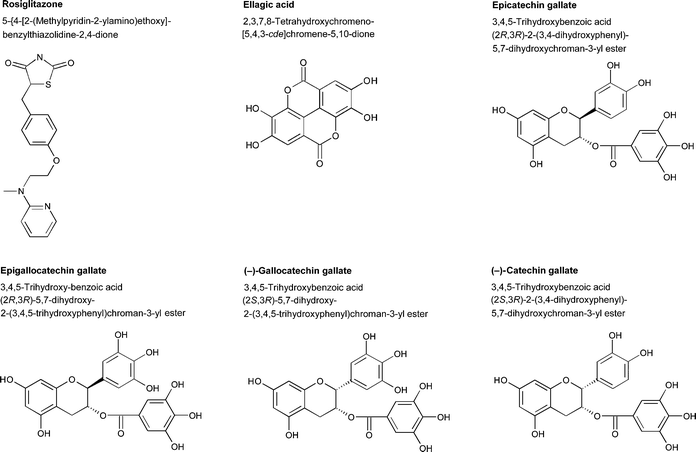 | ||
| Fig. 1 Chemical structures of epicatechin gallate, epigallocatechin gallate, ellagic acid, gallocatechin gallate, catechin gallate, and rosiglitazone. | ||
Some of the biological effects of wine and its constituents are known from epidemiological and animal studies.34–38 To elucidate the blood-glucose-regulating activity of red and white wines, we determined their binding affinities to human PPARγ and compared them with isolated compounds. We selected 12 different Austrian wines. One very potent wine was further investigated for the individual compounds it contained, namely twenty-six polyphenols. By combining the results of wine analysis and biological assays of ligand binding, we were able to identify the compounds that are responsible for most of the PPARγ-binding activity of wines.
Materials and methods
Materials
For ligand-binding assays, we used the selected wines without further purification. Twelve wine varieties were tested for the ability to bind to PPARγ: two Austrian white wines (Neuburger and Rotgipfler) and ten Austrian red wines (one Pinot noir, two St. Laurent, three Zweigelt, and four Blaufränkisch). Of these, we then used one very potent red wine for detailed analysis of its composition and isolation of active compounds. All twelve wines were characterized regarding their concentrations of a total of 24 polyphenols by means of a high-performance liquid chromatography (HPLC) system. Standard compounds were purchased from Sigma (Germany), Indofine (Hillsborough, USA), and Extrasynthese (Genay, France). The grape extract Tannin Grape (Erbslöh, Geisenheim, Germany) and the oak extract Tannin Q (Sulfometa, Krems, Austria) were purchased in a local wine supplement shop.Fourier transform infrared spectroscopy (FTIR), total polyphenol content and antioxidative potential (AOP)
Total acidity, volatile acid, total sugar content, and the amounts of ethanol, glucose, fructose, tartaric acid, malic acid, lactic acid, citric acid and glycerol were analyzed by FTIR spectroscopy as described by Moreira and Santos.39 The antioxidative capacity was estimated using the principle of the TEAC-method (trolox equivalent antioxidative capacity). This method consists of the formation of the green-colored radical cation ABTS˙ from the reaction of ABTS with peroxidase and H2O2 followed by photometric determination at 600 nm. Extinction was measured 3 min after H2O2 addition. The delay in color development is a measure of the antioxidative capacity of the sample and was correlated to the standard.The total phenol content was measured spectrophotometrically, as described by Zoecklin et al.40 using the Folin–Ciocalteu reagent.
Analysis of hydroxycinnamic acids and flavonoids
Hydroxycinnamic acids and flavan-3-ol aglycons were separated by reversed-phase HPLC using two narrow-bore HP-ODS hypersil RP 18.5 μm columns (200 × 2.1 mm and 100 × 2.1 mm) connected in series. For quercetin, naringenin, apigenin, kaempferol and myricetin, acid hydrolysis according to the method of Tsanova-Savova and Ribarova41 was used to determine the total amount of each compound. 10 μL of 0.45 μm membrane-filtered samples were injected, and a linear gradient of 0.5% formic acid pH 2.3 (solvent A) and methanol (solvent B) was used. The column temperature was set to 40 °C, and detection was performed at 320 nm using neat compounds and external calibration for identifying and quantifying individual compounds.Analytical HPLC system
All HPLC runs were performed on an Agilent 1100 HPLC system (Waldbronn, Germany). Fractionation: C18 reversed-phase Luna 3 μm column (Phenomenex, Torrance, CA, USA), flow rate 0.5 mL min−1. Eluent A: deionized water, 5% acetonitrile, 0.1% triflouroacetic acid (TFA). Eluent B: acetonitrile, 0.1% TFA. Gradient: linear from 0 to 17.5% eluent B in 20 min, 17.5 to 50% eluent B in 25 min, and a 10 min hold at 50% eluent B. Regeneration: Step gradient up to 90% eluent B.Preparative HPLC system
Isolation and identification of compounds was achieved by means of a preparative Agilent 1200 HPLC system. 10-mL wine samples were injected and separated into an 80-mL reversed phase-HPLC column C18, 250 × 20.2 mm, 15 μm particle diameter (Phenomenex). Buffer and gradient conditions were the same as for the analytical separation. Flow rate: 26.5 mL min−1. Fractions were taken every 2 min, from 20–40 min. The fractions were evaporated, dissolved in ethanol, and used for measurement of the relative binding affinity (RBA) and for gas chromatography–mass spectroscopy (GC–MS) analysis.Competitive ligand-binding assay
The commercially available PolarScreen PPAR competitor assay green kit was used to measure ligand binding to the human PPARγ ligand-binding domain (PPAR-LBD) (Invitrogen, Carlsbad, CA, USA). Briefly, PPAR-LBD was added to a fluorescent PPAR ligand (Fluormone PPAR Green) to form a PPAR-LBD–Fluormone PPAR Green complex resulting in a high polarization value. Competitors displaced the fluorescent Fluormone PPAR Green ligand from the ligand-binding domain, resulting in a low polarization value. Noncompetitors will not displace the fluorescent ligand from the complex, so the polarization value remains high. The differences in the polarization values prior to and after adding the test compounds were used to determine their relative affinity to the PPAR-LBD. The RBA was calculated using rosiglitazone as the reference compound.GC–MS analysis
The wine (100 mL) was extracted three times with ethyl acetate (30 mL). After drying the combined organic phases over magnesium sulfate, the solvent was largely removed by evaporation. The remaining concentrated sample was subjected to per-silylation with N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) at 70 °C for 12 h. Pure phenol carboxylic acids and flavonoid compounds were silylated according to the same protocol in order to obtain compound-specific retention times and to study the fragmentation behavior of these compounds. GC–MS was performed using a GC 6890N/MSD 5973B instrument (Agilent Technologies). Chromatographic conditions: Column HP-5MS (30 m column length, 0.25 mm diameter, 25 μm film thickness), 0.9 mL min−1helium. Inlet: 280 °C, split (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Oven program: 100 °C (5 min), then 10 °C min−1 to 280 °C (20 min). Auxiliary temperature program: 240 °C (18 min), then 10 °C min−1 to 280 °C (14 min). Ionization: EI mode, 70 eV, 230 °C, 8.5 × 10−6 Torr. Data acquisition and processing: MSDChem software package (Agilent Technologies), NIST 2002 mass spectral library (National Institute of Standards and Technology, USA).
1). Oven program: 100 °C (5 min), then 10 °C min−1 to 280 °C (20 min). Auxiliary temperature program: 240 °C (18 min), then 10 °C min−1 to 280 °C (14 min). Ionization: EI mode, 70 eV, 230 °C, 8.5 × 10−6 Torr. Data acquisition and processing: MSDChem software package (Agilent Technologies), NIST 2002 mass spectral library (National Institute of Standards and Technology, USA).
Data analysis
Data from the competitive ligand-binding assay were fitted using a logistic dose-response model, described as: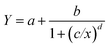 | (1) |
Calculation of the equivalent rosiglitazone concentration
The equivalent concentrations (ECs) of the wine samples were calculated by dividing the potency of rosiglitazone in μM by the potency of the wine samples in L/L: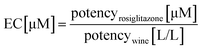 | (2) |
Results
Twelve different Austrian wines were selected for this study according to different grape varieties, sun exposure of the grapes, maceration time, and oak wood contact (Table 1). Prior to biochemical analysis, the polyphenol compositions of the wines were determined by means of HPLC (Table 1). As expected, the content of phenolic compounds and polyphenols was lower in white wines.| (A) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| W1 | W2 | W3 | W4 | W5 | W6 | W7 | W8 | W9 | W10 | W11 | W12 | |
| Variety | Neuburger | Rotgipfler | St. Laurent | Pinot noir | St. Laurent | Zweigelt | BF | BF | Zweigelt | BF | BF | Zweigelt |
| Vintage | 2005 | 2005 | 2003 | 2004 | 2003 | 2004 | 2003 | 2004 | 2005 | 2004 | 2005 | 2004 |
| Sun exposure | − | − | + | + | + | + | + | + | + | + | + | + |
| Skin contact | 1 h | 3 h | 21 d | 18 d | 10 d | 7 d | >14 d | >14 d | 12–14 d | 8 d | 10 d | 10 d |
| Oak contact | − | − | Barrique | Barrique | Barrique | New oak cask | Oak cask | Oak cask | Oak cask | Barrique | − | − |
| (B) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| W1 | W2 | W3 | W4 | W5 | W6 | W7 | W8 | W9 | W10 | W11 | W12 | |
| Density | 0.9916 | 0.9930 | 0.9936 | 0.9921 | 0.9927 | 0.9919 | 0.9927 | 0.9916 | 0.9924 | 0.9915 | 0.9924 | 0.9915 |
| Ethanol (% vol) | 13.2 | 13.8 | 13.1 | 14.1 | 13.2 | 13.5 | 13.4 | 13.6 | 13.5 | 13.3 | 13.5 | 13.3 |
| Sugar (g L−1) | 3.3 | 5.3 | 2.0 | 1.8 | 0.3 | 1.4 | 0.8 | 1.7 | 1.8 | 1.3 | 1.8 | 1.3 |
| Fructose (g L−1) | 4.2 | 6.1 | 0.5 | 0.5 | n.d. | 0.1 | n.d. | 0.1 | 0.1 | n.d. | 0.1 | n.d. |
| Glucose (g L−1) | 1.3 | 1.4 | 1.3 | 1.0 | n.d. | 0.9 | 0.2 | 0.8 | 0.7 | 1.0 | 0.7 | 1.0 |
| Acidity (g L−1) | 5.3 | 5.83 | 4.74 | 4.75 | 4.74 | 4.05 | 5.05 | 4.89 | 5.28 | 4.99 | 5.28 | 4.99 |
| pH value | 3.7 | 3.8 | 3.6 | 3.7 | 3.6 | 3.6 | 3.6 | 3.6 | 3.5 | 3.5 | 3.5 | 3.5 |
| Volatile acids (g L−1) | 0.5 | 0.7 | 0.7 | 0.8 | 0.8 | 0.5 | 0.8 | 0.6 | 0.6 | 0.4 | 0.6 | 0.4 |
| Tartaric acid (g L−1) | 1.2 | 1.1 | 0.8 | 1.0 | 0.5 | 1.1 | 1.1 | 1.7 | 2.2 | 2.5 | 2.2 | 2.5 |
| Malic acid (g L−1) | 2.2 | 3.2 | 0.2 | n.d. | n.d. | n.d. | n.d. | 0.3 | 0.1 | 0.2 | 0.1 | 0.2 |
| Lactic acid (g L−1) | 1.0 | 1.1 | 1.9 | 2.5 | 3.1 | 1.8 | 2.7 | 1.9 | 1.8 | 1.5 | 1.8 | 1.5 |
| Citric acid (g L−1) | 0.2 | 0.2 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Glycerol (g L−1) | 6.5 | 6.9 | 10.8 | 11.3 | 11.0 | 10.7 | 10.7 | 10.2 | 10.5 | 10.7 | 10.5 | 10.7 |
| Gallic acid (mg L−1) | 2.5 | 3.9 | 50.1 | 40.8 | 22.5 | 71.9 | 46.7 | 40.7 | 25.7 | 49.8 | 35.7 | 25.5 |
| Caftaric acid (mg L−1) | 32.1 | 48.1 | 61.5 | 81.6 | 13.7 | 31.4 | 127.0 | 109.3 | 91.9 | 87.1 | 135.4 | 57.5 |
| Tyrosol (mg L−1) | 7.1 | 7.0 | 34.0 | 19.0 | 31.5 | 22 | 32.3 | 45.2 | 28.1 | 20.5 | 24.9 | 23.1 |
| cis-Cutaric acid (mg L−1) | 3.2 | 4.4 | 2.7 | 5.4 | 1.2 | 1.3 | 2.1 | 3.2 | 2.9 | 3.0 | 3.3 | 1.7 |
| trans-Cutaric acid (mg L−1) | 4.6 | 6.8 | 15.7 | 23.2 | 3.9 | 6.3 | 16.8 | 16.7 | 18.5 | 14.8 | 20.5 | 12.3 |
| Catechin (mg L−1) | 22.0 | 25.4 | 94.5 | 141.7 | 85.0 | 85.3 | 72.2 | 68.6 | 79.9 | 78.6 | 61.2 | 26.7 |
| Caffeic acid (mg L−1) | 4.5 | 4.9 | 3.6 | 5.1 | 9.8 | 15.4 | 5.7 | 7.7 | 15.8 | 5.7 | 4.6 | 3.5 |
| Fertaric acid (mg L−1) | 4.2 | 5.7 | 2.5 | 2.6 | 1.6 | 2.1 | 4.8 | 3.7 | 3.4 | 3.7 | 4.5 | 2.0 |
| p-Coumaric acid (mg L−1) | 1.3 | 1.3 | 2.9 | 1.5 | 6.5 | 3.7 | 1.4 | 2.9 | 4.5 | 3.5 | 1.2 | 6.6 |
| Epicatechin (mg L−1) | 8.3 | 7.5 | 43.7 | 94.5 | 32.6 | 77.9 | 42.5 | 45.7 | 52.6 | 61.1 | 40.9 | 17.0 |
| Ferulic acid (mg L−1) | 0.3 | 0.9 | 0.9 | 0.9 | 1.0 | 0.8 | 0.5 | 0.4 | 0.8 | 0.5 | 0.6 | 0.6 |
| Mono-anthocyanins (mg L−1) | n.d. | n.d. | 107 | 88 | 56 | 127 | 33 | 62 | 215 | 83 | 146 | 95 |
| Total polyphenols (g L−1) | 0.08 | 0.09 | 1.46 | 1.33 | 1.51 | 1.65 | 1.71 | 1.05 | 1.55 | 1.2 | 1.26 | 0.80 |
| AOP (mM) | 2.0 | 2.3 | 19.8 | 24.9 | 30.5 | 34.8 | 47.0 | 21.8 | 26.8 | 22.8 | 29.7 | 19.4 |
The main purpose of our study was to determine the PPARγ-binding capacity of selected red wines and to isolate potent ligands with anti-diabetic effects. PPARγ ligand-binding activity was determined by a competitive ligand-binding assay based on a human LBD-derived construct. In order to compare the wines, we defined an equivalent concentration (EC) using rosiglitazone as a reference compound. Rosiglitazone is used for treating type 2 diabetes,42–44 and has a relatively high binding affinity for PPARγ. This enabled us to quantify the RBA (relative binding affinity) of any given wine without knowing its exact chemical composition or the concentration of individual compounds binding to the PPARγ receptor. The RBAs for all 12 of the wines that we studied are provided in Fig. 2. By approximating the RBA values with a logistic dose-response curve, we determined the potency of each wine and related it to the potency of rosiglitazone(eqn 2). This allowed us to compare the EC of each wine (Table 2). The two white wines showed negligibly low binding to the receptor, which can be explained by the low polyphenol content of these wines. All of the red wines contained remarkably high EC of rosiglitazone, ranging from 52 to 521 μM.
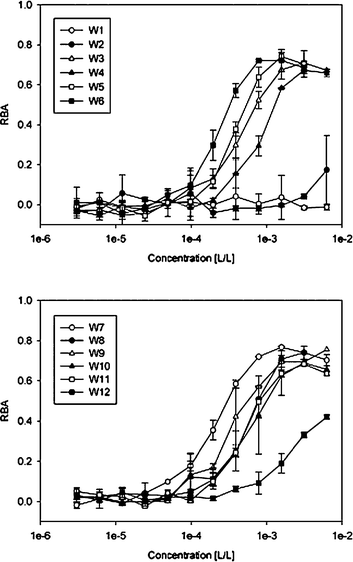 | ||
| Fig. 2 Relative binding affinities (RBAs) of 12 different wines. Dilutions of the wine samples were used for the ligand-binding assay. Each wine was tested in duplicate at minimum. | ||
| Wine | Potency [L/L]a | Equivalent concentration of rosiglitazone [μM]b | Equivalent daily dose contained in 100 mL wine |
|---|---|---|---|
| a Calculated by eqn (1). b calculated by eqn (2). | |||
| W1 | n.d. | n.d. | — |
| W2 | n.d. | n.d. | — |
| W3 | 5.0 × 10−4 | 240.0 | 2.1 |
| W4 | 8.6 × 10−4 | 139.5 | 1.2 |
| W5 | 3.8 × 10−4 | 315.8 | 2.8 |
| W6 | 2.4 × 10−4 | 500.0 | 4.5 |
| W7 | 2.3 × 10−4 | 521.7 | 4.7 |
| W8 | 6.0 × 10−4 | 200.0 | 1.8 |
| W9 | 2.9 × 10−4 | 413.8 | 3.7 |
| W10 | 8.5 × 10−4 | 141.2 | 1.3 |
| W11 | 4.8 × 10−4 | 250.0 | 2.2 |
| W12 | 2.3 × 10−3 | Active | — |
Since the peroxisome proliferator-activated receptor gamma (PPARγ) is a key factor in glucose and lipid metabolism, we studied the binding of potential PPARγ ligands from different Austrian wines. We evaluated the binding affinity of: (a) known wine compounds with hormone receptor–binding affinity such as resveratrol, kaempferol, myricetin and quercetin; (b) compounds that are present in wines in high concentrations such as anthocyanins and catechins; and (c) oak-wood-derived compounds such as ellagic acid. None of the low molecular weight phenolic acids that we tested, such as gallic acid, ferulic acid and syringic acid, but also anthocyanidins, catechin, epicatechin and ethyl gallate, showed a response in our test system. trans-Resveratrol, a well-documented hormone receptor ligand, showed only very low binding affinity. Myricetin, quercetin, kaempferol, apigenin, piceatannol, naringenin, malvidin and cyanidin were ligands with medium IC50 values (in the μM range). Ellagic acid, catechin gallate (CG), gallocatechin gallate (GCG), epicatechin gallate (ECG), and epigallocatechin gallate (EGCG) were identified as highly potent PPARγ ligands, with at least one order of magnitude higher affinity (Table 3). The logistic dose-response curves of these compounds, with rosiglitazone as the reference compound, are shown in Fig. 3. The estimated IC50 values of the four gallates CG, GCG, ECG and EGCG were similar to the IC50 of 2.1 × 10−7 M of rosiglitazone. Wine W6, with an EC of 500 μM, was subjected to a detailed analysis of individual compounds, and subsequent fractionation by means of RP-HPLC. The fractions were then analyzed for their PPARγ activity, and certain compounds were identified as the most potent PPARγ ligands. The fractions were additionally analyzed for their RBA after solvent exchange with DMSO for standardized assay conditions. To minimize the false-negative response of highly colored fractions, all of the samples were diluted prior to the measurement using the two-solvent gradient described in the Materials and methods sectiono: the most potent compounds were eluted at 15–30% acetonitrile (fractions 11–20 in Fig. 4). From the elution behavior, we concluded that these compounds were weakly to moderately polar. As the amount of phenolic compounds in the fractions was too small for GC–MS analysis, we also performed semi preparative RP-HPLC. The scale-up of the column was performed to keep the retention time constant. The most abundant compounds of each fraction could be identified by means of GC–MS analysis (Table 4). We identified epicatechin gallate in fraction 14, ellagic acid in fraction 15, and quercetin in fraction 16. These were found to be very potent ligands, and were responsible for the high activity of these fractions. The very high abundance of catechin, epicatechin and ethyl gallate (fractions 11–13, respectively) could explain the high RBA affinity of these fractions, although the IC50 of the neat compounds was higher than 10−4 M. The physiological relevance of such low-potency compounds in vivo is questionable.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)
2)
| Compound | CAS number | IC50 [mol L−1] |
|---|---|---|
| Rosiglitazone | 122320-73-4 | 2.1 × 10−7 |
| Gallic acid | 149-91-7 | n.d. |
| Caffeic acid | 331-39-5 | n.d. |
| Syringic acid | 530-57-4 | n.d. |
| Ferulic acid | 1135-24-6 | n.d. |
| o-Coumaric acid | 614-60-8 | n.d. |
| p-Coumaric acid | 7400-08-0 | n.d. |
| trans-Resveratrol | 501-36-0 | Active |
| Kaempferol | 520-18-3 | 3.0 × 10−5 |
| Quercetin | 6151-25-3 | 5.7 × 10−6 |
| Apigenin | 520-36-5 | 1.6 × 10−5 |
| Myricetin | 529-44-2 | 2.0 × 10−6 |
| Naringenin | 67604-48-2 | 3.3 × 10−5 |
| Cyanidin | 528-58-5 | 1.4 × 10−6 |
| Malvidin | 643-84-5 | Active |
| Oenin | 7228-78-6 | n.d. |
| Kuromanin | 7084-24-4 | n.d. |
| Piceatannol | 10083-24-6 | 8.2 × 10−5 |
| Catechin | 154-23-4 | n.d. |
| Epicatechin | 490-46-0 | n.d. |
| Ethyl gallate | 831-61-8 | n.d. |
| Epigallocatechin | 970-74-1 | n.d. |
| Epicatechin gallate | 1257-08-5 | 5.9 × 10−7 |
| Epigallocatechin gallate | 989-51-5 | 2.1 × 10−7 |
| Ellagic acid | 476-66-4 | 5.7 × 10−7 |
| Gallocatechin gallate | 4233-96-9 | 2.5 × 10−7 |
| Catechin gallate | 130405-40-2 | 9.1 × 10−7 |
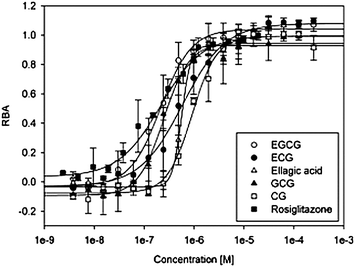 | ||
| Fig. 3 Logistic dose-response curves of ellagic acid, catechin gallate, gallocatechin gallate, epigallocatechin gallate, epicatechin gallate and the synthetic PPARγ ligand rosiglitazone used for calculating inhibitory concentrations (IC50 values). Each compound was tested in duplicate at minimum. | ||
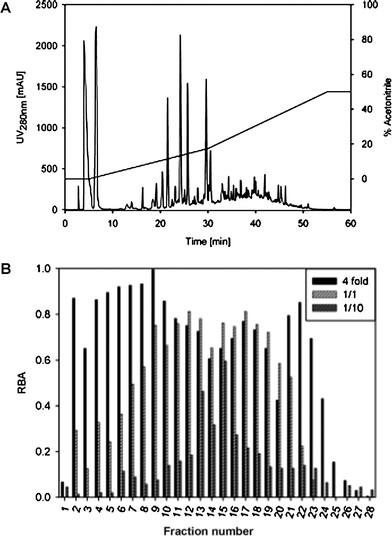 | ||
| Fig. 4 Fractionation of wine W6: (A) HPLC run and (B) ligand-binding assay of the fractions. | ||
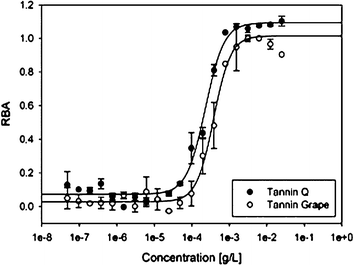
Grape tannin and oak tannin supplements are often used in wine technology as antioxidants, and are added to the mash or the fermented must. These extracts are rich in polyphenols and may also be a potent source of PPARγ ligands.45 Two commercially available extracts were assayed for RBA (Fig. 5). The results clearly show that both extracts have very high binding affinities, with IC50 values of 350 and 270 ng mL−1, respectively.
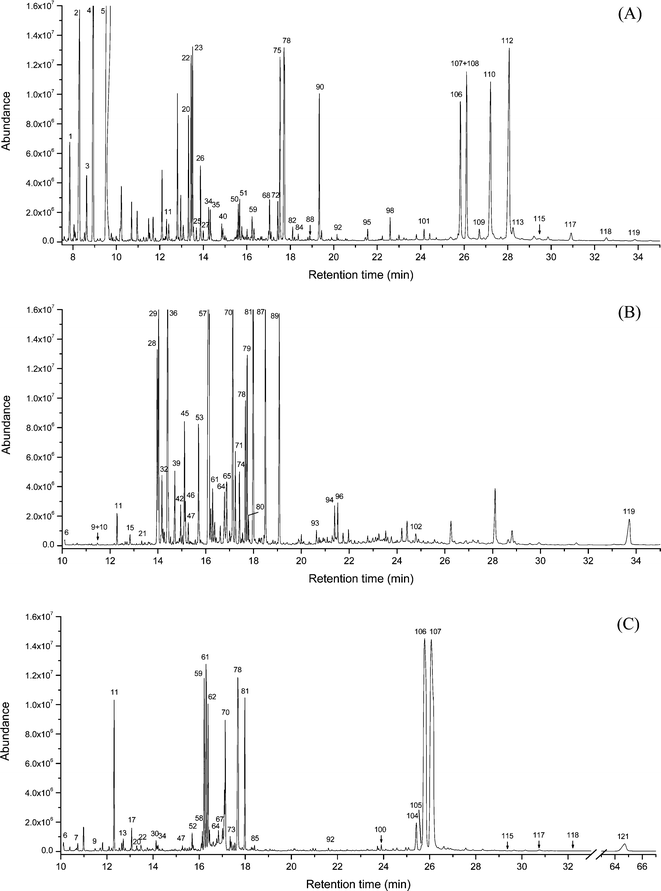
The grape tannin extract, the oak tannin extract, and an extract prepared by liquid–liquid extraction of wine W6 were further characterized by means of GC–MS (Fig. 6) to identify individual compounds as well as to determine the abundance of highly potent PPARγ ligands in red wines. The results revealed that oak tannin extract in particular contained a large amount of ellagic acid. The grape extract was also found to be a rich source of epicatechin gallate. Furthermore, both extracts contained a large quantity of catechin and epicatechin. The compounds identified in the three extracts are listed in Table 5. A quantitative analysis of the individual compounds was not yet performed, mostly because of a lack of the required corresponding neat per-trimethylsilylated compounds.
| Cpd no. | Retention time [min] | Compound | Wine | Grape | Oak |
|---|---|---|---|---|---|
| 1 | 7.85 | Phenylethanol (1 tms) | ✓ | ||
| 2 | 8.53 | Succinic acid ethyl ester (1 tms) | ✓ | ||
| 3 | 8.64 | α-Hydroxyvaleric acid (2 tms) | ✓ | ||
| 4 | 8.94 | Glycerol (3 tms) | ✓ | ✓ | ✓ |
| 5 | 9.61 | Succinic acid (2 tms) | ✓ | ✓ | ✓ |
| 6 | 10.13 | Maleic acid (2 tms) | ✓ | ✓ | |
| 7 | 10.61 | Dihydrodihydroxy-2(3H)furanone (2 tms) | ✓ | ✓ | |
| 8 | 11.17 | Erythrose (3 tms) | ✓ | ||
| 9 | 11.46 | Dihydrodihydroxy-2(3H)furanone (2 tms) | ✓ | ✓ | |
| 10 | 11.49 | Dihydroxybutyric acid (3 tms) | ✓ | ||
| 11 | 12.32 | Malic acid (3 tms) | ✓ | ✓ | ✓ |
| 12 | 12.53 | Deoxyribose (3 tms) | ✓ | ✓ | |
| 13 | 12.64 | Deoxyribose (3 tms) | ✓ | ✓ | |
| 14 | 12.71 | Pyroglutamic acid (2 tms) | ✓ | ||
| 15 | 12.82 | Hydroxymalonic acid (3 tms) | ✓ | ||
| 16 | 13.00 | 1,2,3-Trihydroxybenzene (3 tms) | ✓ | ✓ | |
| 17 | 13.07 | Desoxypentitol (4 tms) | ✓ | ||
| 18 | 13.11 | 2,3,4-Trihydroxybutyric acid (4 tms) | ✓ | ||
| 19 | 13.13 | Desoxypentitol (4 tms) | ✓ | ||
| 20 | 13.29 | Hydroxyphenylethanol (2 tms) | ✓ | ✓ | |
| 21 | 13.32 | Tetronic acid (4 tms) | ✓ | ✓ | |
| 22 | 13.40 | α-Hydroxyglutaric acid (3 tms) | ✓ | ✓ | |
| 23 | 13.51 | Tricarboxylic acid (3 tms) | ✓ | ||
| 24 | 13.55 | Phenyllactic acid (2 tms) | ✓ | ✓ | |
| 25 | 13.65 | Ribose (4 tms) | ✓ | ✓ | ✓ |
| 26 | 13.87 | Ethyl tartrate (3 tms) | ✓ | ||
| 27 | 13.97 | 4-Hydroxybenzoic acid (2 tms) | ✓ | ✓ | |
| 28 | 14.02 | Ribose (4 tms) | ✓ | ✓ | |
| 29 | 14.04 | Arabinose (4 tms) | ✓ | ||
| 30 | 14.12 | Xylonic acid (3 tms) | ✓ | ||
| 31 | 14.17 | Arabinonic acid δ-lactone (3 tms) | ✓ | ||
| 32 | 14.17 | Deoxymannose (4 tms) | ✓ | ||
| 33 | 14.22 | Ribose (4 tms) | ✓ | ||
| 34 | 14.25 | 1,3,5-Trihydroxybenzene (3 tms) | ✓ | ✓ | |
| 35 | 14.33 | Tartaric acid (4 tms) | ✓ | ✓ | |
| 36 | 14.40 | Arabinose (4 tms) | ✓ | ✓ | |
| 37 | 14.44 | Lyxose (4 tms) | ✓ | ||
| 38 | 14.68 | Fucose (4 tms) | ✓ | ||
| 39 | 14.73 | Arabinose (4 tms) | ✓ | ||
| 40 | 14.85 | o-Phthalic acid (2 tms) | ✓ | ✓ | |
| 41 | 14.90 | Syringaaldehyde (1 tms) | ✓ | ||
| 42 | 14.95 | Rhamnose (4 tms) | ✓ | ✓ | |
| 43 | 15.00 | 4-Hydroxy-3-methoxyphenylethanol (2 tms) | ✓ | ||
| 44 | 15.03 | Fucose (4 tms) | ✓ | ||
| 45 | 15.11 | Xylose (4 tms) | ✓ | ||
| 46 | 15.15 | Mannose (5 tms) | ✓ | ||
| 47 | 15.27 | Xylitol (5 tms) | ✓ | ✓ | |
| 48 | 15.38 | Xylonic acid (3 tms) | ✓ | ||
| 49 | 15.55 | 4-Hydroxy-hydrocoumaric acid (2 tms) | ✓ | ||
| 50 | 15.57 | Vanillic acid (2 tms) | ✓ | ✓ | ✓ |
| 51 | 15.67 | 3,4-Dihydroxyphenylethanol (3 tms) | ✓ | ||
| 52 | 15.69 | Glycerophosphoric acid (4 tms) | ✓ | ||
| 53 | 15.70 | Xylose (4 tms) | ✓ | ✓ | |
| 54 | 15.78 | Gentisic acid (3 tms) | ✓ | ||
| 55 | 15.87 | 4-Coumaric acid (2 tms) | ✓ | ||
| 56 | 15.95 | Citric acid ethyl ester (3 tms) | ✓ | ||
| 57 | 16.10 | Myoinositol (5 tms) | ✓ | ✓ | |
| 58 | 16.14 | 3,4,5-Trihydroxycyclohex-1-en-1-carboxylic acid (4 tms) | ✓ | ||
| 59 | 16.22 | D-Fructose (5 tms) | ✓ | ✓ | |
| 60 | 16.24 | Protocatechuic acid (3 tms) | ✓ | ✓ | |
| 61 | 16.29 | Fructose (5 tms) | ✓ | ✓ | |
| 62 | 16.38 | Fructose (5 tms) | ✓ | ✓ | |
| 63 | 16.61 | Mannose (5 tms) | ✓ | ✓ | |
| 64 | 16.78 | Galactose (5 tms) | ✓ | ✓ | |
| 65 | 16.89 | Deoxymyoinositol (5 tms) | ✓ | ||
| 66 | 17.00 | Resorcylic acid (3 tms) | ✓ | ||
| 67 | 17.00 | Gluconic acid lactone (4 tms) | ✓ | ✓ | |
| 68 | 17.00 | Syringic acid (2 tms) | ✓ | ✓ | |
| 69 | 17.12 | (4-Hydroxyphenyl)lactic acid (3 tms) | ✓ | ||
| 70 | 17.14 | D-Glucose (5 tms) | ✓ | ✓ | |
| 71 | 17.24 | Mannose (5 tms) | ✓ | ✓ | |
| 72 | 17.42 | p-Coumaric acid (2 tms) | ✓ | ✓ | |
| 73 | 17.34 | Idonic acid lactone (4 tms) | ✓ | ||
| 74 | 17.41 | Inositol (6 tms) | ✓ | ||
| 75 | 17.47 | Gallic acid ethyl ester (3 tms) | ✓ | ✓ | |
| 76 | 17.55 | D-Mannitol (6 tms) | ✓ | ✓ | |
| 77 | 17.62 | Glucitol (6 tms) | ✓ | ||
| 78 | 17.73 | Gallic acid (4 tms) | ✓ | ✓ | ✓ |
| 79 | 17.74 | Myoinositol (6 tms) | ✓ | ||
| 80 | 17.79 | Mucoinositol (6 tms) | ✓ | ||
| 81 | 17.98 | Glucose (5 tms) | ✓ | ✓ | |
| 82 | 18.11 | 3,4-Dihydroxymandelic acid (3 tms) | ✓ | ✓ | |
| 83 | 18.27 | Gluconic acid (6 tms) | ✓ | ||
| 84 | 18.37 | Palmitic acid (1 tms) | ✓ | ✓ | |
| 85 | 18.40 | Galactonic acid (6 tms) | ✓ | ||
| 86 | 18.44 | Galacturonic acid (5 tms) | ✓ | ||
| 87 | 18.50 | Quercinitol (6 tms) | ✓ | ||
| 88 | 18.90 | Ferulic acid (2 tms) | ✓ | ✓ | |
| 89 | 19,08 | Myoinositol (6 tms) | ✓ | ✓ | |
| 90 | 19.34 | Caffeic acid (3 tms) | ✓ | ||
| 91 | 19.90 | Linoleic acid (1 tms) | |||
| 92 | 20.15 | Stearic acid (1 tms) | ✓ | ✓ | ✓ |
| 93 | 20.63 | Disaccharide derivative | ✓ | ||
| 94 | 21.39 | Arabinofuranoside | ✓ | ||
| 95 | 21.56 | Resveratrol (3 tms) | ✓ | ||
| 96 | 21.52 | Glucuronic acid derivative | ✓ | ||
| 97 | 21.75 | Eicosanoic acid (1 tms) | ✓ | ||
| 98 | 22.60 | A flavanoid (m/z = 484 [M+, 100%], 427, 233) | ✓ | ||
| 99 | 23.28 | Docosanoic acid (1 tms) | ✓ | ||
| 100 | 23.79 | Sucrose (8 tms) | ✓ | ||
| 101 | 24.15 | Resveratrol (3 tms) | ✓ | ✓ | |
| 102 | 24.78 | Maltose (8 tms) | ✓ | ||
| 103 | 25.08 | Tetracosanoic acid (1 tms) | ✓ | ||
| 104 | 25.40 | Sucrose (8 tms) | ✓ | ||
| 105 | 25.72 | Naringenin (3 tms) | ✓ | ✓ | |
| 106 | 25.83 | Epicatechin (5 tms) | ✓ | ✓ | |
| 107 | 26.11 | Catechin (5 tms) | ✓ | ✓ | |
| 108 | 26.20 | Apigenin (3 tms) | ✓ | ✓ | |
| 109 | 26.69 | m/z = 738 [M+], 648, 456 [100%] | ✓ | ✓ | |
| 110 | 27.22 | Catechin (4 tms) | ✓ | ||
| 111 | 27.37 | m/z = 578 [M+], 368 [100%], 283 | ✓ | ||
| 112 | 28.07 | Epicatechin (4 tms) | ✓ | ||
| 113 | 28.25 | m/z = 666 [M+], 384, 355, 283 [100%] | ✓ | ||
| 114 | 29.21 | m/z = 666 [M+], 384, 355, 283 [100%] | ✓ | ||
| 115 | 29.44 | Kaempferol (4 tms) | ✓ | ✓ | |
| 116 | 29.84 | m/z = 652 [M+], 382, 253 [100%] | ✓ | ||
| 117 | 30.92 | Quercetin (5 tms) | ✓ | ✓ | |
| 118 | 32.54 | Myrecetin (5 tms) | ✓ | ✓ | |
| 119 | 33.84 | Ellagic acid (4 tms) | ✓ | ✓ | |
| 120 | 34.58 | β-Sitosterol (1 tms) | ✓ | ||
| 121 | 64.70 | Epicatechin gallate (7 tms) | ✓ |
Discussion
Grape variety, sun exposure, maceration time and oak wood contact are considered to be the key factors affecting the polyphenol content in wines, and dictate the amounts of these compounds that can be extracted from grape skin and oak wood.33,45,46 The studied Austrian white wines contained only about 10% of the amount of phenolic compounds found in the selected Austrian red wines; this is mainly due to the short extraction time of the mash. Blaufränkisch and Zweigelt are two grape varieties with a thick skin, and St. Laurent and Pinot noir grapes have skins of medium thickness. Greater sun exposure causes more polyphenols to be formed in the grape skin for sun protection.47 Wine technology has also an impact on the total amount and variety of polyphenols. Especially during fermentation and maturation in small barrique barrels, the total amounts of phenolic compounds, as well as the percentages of each individual one, are modulated to a larger extent. Red wine W12 had exceptionally low catechin and epicatechin concentrations, and a total polyphenol content of only 800 mg L−1. This could be due to the larger berry size (smaller skin-to-juice ratio) of this grape variety clone, or perhaps due to a lower fermentation temperature and cold-soak period before beginning fermentation; either would result in reduced extraction of polyphenols from the seeds.32 However, these parameters were not recorded, and a direct influence of wine technology on polyphenol composition could not be derived from the available parameters. For this purpose, a more detailed study will be required, and modification of red wine technology with the goal of optimizing polyphenol content and composition with respect to health benefits will be the subject of further investigations.From the ligand-binding studies, using rosiglitazone as reference compound, we could calculate the relative binding affinity of each wine. 4–8 mg is the recommended daily dose for treating type 2 diabetes using rosiglitazone.44 100 mL of the tested red wines contained EC equal to approximately 1.8–18 mg of rosiglitazone. Hence, this volume corresponded to between one-quarter and up to four times the daily dose of rosiglitazone. The anti-diabetic activity of red wines has been discussed before. The results of the present study reveal that at least a portion of this specific biological activity can be attributed to polyphenolic compounds with a high PPARγ-binding affinity. The wines were found to be a rich source of potent PPARγ ligands compared with other plant extracts. A drawback to red wine consumption, which must be taken into account for type 2 diabetes and obesity patients, is their comparatively high sugar and alcohol content. This has been frequently overlooked because moderate wine consumption also correlates with lower body weight compared with non-wine consumers. The four-year SWAN study clearly showed that consuming a glass of wine a day reduces metabolic syndrome.4 A glass of wine approximately corresponds to 10 g alcohol. Reduced waist circumference, higher HDL levels, and lower triglyceride levels have been correlated with moderate wine consumption.48,49 These clinical parameters may also correlate with the intake of PPARγ ligands. We hypothesize that the observed body weight reduction, as well as alterations in the lipid profile, can be attributed at least in part to the high content of PPARγ ligands in red wine. Red wine contains a large variety of different wine polyphenols and other compounds including ethanol. It is still a subject of discussion to what extent the ethanol in during wine fermentation helps to leach compounds from the grape skin.
Two-month intervention studies have also shown that moderate wine consumption leads to statistically significant weight loss.50 The epidemiological and intervention studies are also corroborated by in vivo animal experiments. Rats with chemically induced type 2 diabetes showed reduced hyperglycemia with intake of red wine extracts. In the same study, grape seed procyanidins normalized plasma lipid levels and insulin resistance in fructose-fed animals.51
Ellagitannins are extracted from the oak barrels and oak supplements during wine production and can be hydrolyzed subsequently by acids.33 As the most plentiful compounds in black tea, they are known for their anti-diabetic effects in vivo.52 Ellagic acid, which is also present in pomegranates in high amounts, was first identified as a strong PPARγ binder by us, although an indirect mechanism has been recently suggested by Khateeb et al.53 They found a moderate induction of paroxonase 1 gene (a PPARγ-controlled gene) by ellagic acid. The anti-diabetic effects of pomegranates are well known,54 but to our knowledge, ellagic acid had not been identified as a PPARγ ligand until the present study. ECG and ellagic acid are the most potent ligands in wine for the PPARγ receptor; they are mainly derived from the grape seeds and skin and from oak wood.
The anti-diabetic effects of grape seed extracts and the lower incidence of cardiovascular disease (CVD) due to red wine consumption are well documented.37,55,56 In addition, the recent discovery of PPARγ in platelets and macrophages57 provides evidence for a possible connection between PPARγ ligands and the prevention of inflammatory diseases.30,31 In this study, we have identified ellagic acid and some monomeric gallates derived from procyanidins. The former strongly bind to the PPARγ receptor, and induced transactivation and/or repression appear to be responsible for their potential health benefits. Finally, although the in vivo effects of grape seed procyanidins and tea catechins have been described,37,51,52 further investigations of single compounds with respect to their modes of action at the PPARγ binding site are required to shed more light on the molecular mechanisms.
Acknowledgements
We would like to thank Reinhard Eder from the Federal Collage and Research Institute for Viticulture and Pomology (Klosterneuburg, Austria) for FTIR analysis of the wines.References
- A. L. Klatsky, G. D. Friedman and A. B. Siegelaub, Ann. Intern. Med., 1974, 81, 294–301 CAS.
- S. Renaud and M. De Lorgeril, Lancet, 1992, 339, 1523–1526 CrossRef CAS.
- A. M. Hodge, D. R. English, K. O'Dea and G. G. Giles, Diabetic Med., 2006, 23, 690–697 CrossRef CAS.
- I. Janssen, L. H. Powell and D. Wesely, Winehealth – 3rd International Congress on Wine and Health, Bordeaux, France, 2007 Search PubMed.
- L. Djousse, D. K. Arnett, J. H. Eckfeldt, M. A. Province, M. R. Singer and R. C. Ellison, Obesity, 2004, 12, 1375–1385 CrossRef.
- S. G. Wannamethee, A. E. Field, G. A. Colditz and E. B. Rimm, Obesity, 2004, 12, 1386–1396 CrossRef.
- S. G. Wannamethee, C. A. Camargo Jr, J. E. Manson, W. C. Willett and E. B. Rimm, Arch. Intern. Med., 2003, 163, 1329–1336 CrossRef.
- J. B. Dixon, M. E. Dixon and P. E. O'Brien, Obesity, 2002, 10, 245–252 CrossRef.
- W. H. L. Kao, I. B. Puddey, L. L. Boland, R. L. Watson and F. L. Brancati, Am. J. Epidemiol., 2001, 154, 748–757 CrossRef CAS.
- A. Ceriello, N. Bortolotti, E. Motz, S. Lizzio, B. Catone, R. Assaloni, L. Tonutti and C. Taboga, Eur. J. Clin. Invest., 2001, 31, 322–328 CrossRef CAS.
- S. Carlsson, N. Hammar, S. Efendic, P. G. Persson, C. G. Östenson and V. Grill, Diabetic Med., 2000, 17, 776–781 CrossRef CAS.
- H. Gin, V. Rigalleau, O. Caubet, J. Masquelier and J. Aubertin, Metab.: Clin. Exp., 1999, 48, 1179–1183 CrossRef CAS.
- C. Christiansen, C. Thomsen, O. Rasmussen, M. Balle, C. Hauerslev, C. Hansen and K. Hermansen, Diabetic Med., 1993, 10, 958–961 CrossRef CAS.
- M. C. Chellingsworth, E. Henderson, C. Gavey and H. Connor, Pract. Diabetes Int., 1990, 7, 70–71 Search PubMed.
- F. Centritto, L. Iacoviello, R. di Giuseppe, A. De Curtis, S. Costanzo, F. Zito, S. Grioni, S. Sieri, M. B. Donati, G. de Gaetano and A. Di Castelnuovo, Nutr., Metab. Cardiovasc. Dis., 2009, 19, 697–706 Search PubMed.
- P. M. Aron and J. A. Kennedy, Mol. Nutr. Food Res., 2008, 52, 79–104 CrossRef CAS.
- R. C. M. Siow, F. Y. L. Li, D. J. Rowlands, P. de Winter and G. E. Mann, Free Radical Biol. Med., 2007, 42, 909–925 CrossRef CAS.
- S. Loibl, J. Bratengeier, V. Farines, G. von Minckwitz, B. Spankuch, V. Schini-Kerth, F. Nepveu, K. Strebhardt and M. Kaufmann, Pathol., Res. Prac., 2006, 202, 1–7 Search PubMed.
- E. Anselm, M. Chataigneau, M. Ndiaye, T. Chataigneau and V. B. Schini-Kerth, Cardiovasc. Res., 2007, 73, 404–413 CrossRef CAS.
- T. R. Rathel, R. Samtleben, A. M. Vollmar and V. M. Dirsch, J. Hypertens., 2007, 25, 541–549 CrossRef.
- F. Leighton, S. Miranda-Rottmann and I. Urquiaga, Cell Biochem. Funct., 2006, 24, 291–298 CrossRef CAS.
- J. J. O'Brien, D. M. Ray, S. L. Spinelli, N. Blumberg, M. B. Taubman, C. W. Francis, S. D. Wittlin and R. P. Phipps, Prostaglandins Other Lipid Mediators, 2007, 82, 68–76 CrossRef CAS.
- D. M. Ray, S. L. Spinelli, J. J. O'Brien, N. Blumberg and R. P. Phipps, BioDrugs, 2006, 20, 231–241 CrossRef CAS.
- E. Scotti and P. Tontonoz, Mol. Cell. Biol., 2010, 30, 2076–2077 CrossRef CAS.
- Y. Zhang, Z. Luo, L. Ma, Q. Xu, Q. Yang and L. Si, Int. J. Mol. Med., 2010, 25, 729–734 Search PubMed.
- J. S. Tsai, C. Y. Chen, Y. L. Chen and L. M. Chuang, J. Cell. Biochem., 2010, 110, 1410–1419 CrossRef CAS.
- A. Chawla, Circ. Res., 2010, 106, 1559–1569 CrossRef CAS.
- F. C. McGillicuddy, M. de la Llera Moya, C. C. Hinkle, M. R. Joshi, E. H. Chiquoine, J. T. Billheimer, G. H. Rothblat and M. P. Reilly, Circulation, 2009, 119, 1135–1145 CrossRef CAS.
- D. M. Ray, S. L. Spinelli, S. J. Pollock, T. I. Murant, J. J. O'Brien, N. Blumberg, C. W. Francis, M. B. Taubman and R. P. Phipps, Thrombosis and Haemostasis, 2008, 99, 86–95 Search PubMed.
- A. Majdalawieh and H. S. Ro, Nuclear Receptor Signaling, 2010, 8, e004 Search PubMed.
- R. M. Touyz and E. L. Schiffrin, Vasc. Pharmacol., 2006, 45, 19–28 CrossRef CAS.
- K. Koyama, N. Goto-Yamamoto and K. Hashizume, Biosci., Biotechnol., Biochem., 2007, 71, 958–965 CrossRef CAS.
- A. M. Jordao, J. M. Ricardo-da-Silva and O. Laureano, S. Afr. J. Enol. Vitic., 2005, 26, 25–31 Search PubMed.
- C. R. Pace-Asciak, O. Rounova, S. E. Hahn, E. P. Diamandis and D. M. Goldberg, Clin. Chim. Acta, 1996, 246, 163–182 CrossRef CAS.
- R. Corder, W. Mullen, N. Q. Khan, S. C. Marks, E. G. Wood, M. J. Carrier and A. Crozier, Nature, 2006, 444, 566 CrossRef CAS.
- R. Liew, M. A. Stagg, K. T. MacLeod and P. Collins, Eur. J. Pharmacol., 2005, 519, 1–8 CrossRef CAS.
- A. T. El-Alfy, A. A. E. Ahmed and A. J. Fatani, Pharmacol. Res., 2005, 52, 264–270 CrossRef CAS.
- M. Pinent, M. Blay, M. C. Blade, M. J. Salvado, L. Arola and A. Ardevol, Endocrinology, 2004, 145, 4985–4990 CrossRef CAS.
- J. L. Moreira and L. Santos, Anal. Bioanal. Chem., 2005, 382, 421–425 CrossRef CAS.
- B. W. Zoecklin, K. C. Fugelsang, B. H. Gump and F. S. Nury, Wine analysis and production, Chapman and Hall, New York, 1995 Search PubMed.
- S. Tsanova-Savova and F. Ribarova, J. Food Compos. Anal., 2002, 15, 639–645 CrossRef CAS.
- G. Derosa, S. A. T. Salvadeo, A. D'Angelo, E. Fogari, P. D. Ragonesi, L. Ciccarelli, M. N. Piccinni, I. Ferrari, A. Gravina, P. Maffioli and A. F. Cicero, Arch. Med. Res., 2008, 39, 412–419 CrossRef CAS.
- B. Richter, E. Bandeira-Echtler, K. Bergerhoff, C. Clar and S. H. Ebrahim, Cochrane Database Syst. Rev., 2007, 3, 3 Search PubMed.
- A. J. Wagstaff and K. L. Goa, Drugs, 2002, 62, 1805–1837 CrossRef CAS.
- A. B. Bautista-Ortin, J. I. Fernandez-Fernandez, J. M. Lopez-Roca and E. Gomez-Plaza, J. Food Compos. Anal., 2007, 20, 546–552 CrossRef CAS.
- D. Fournand, A. Vicens, L. Sidhoum, J. M. Souquet, M. Moutounet and V. Cheynier, J. Agric. Food Chem., 2006, 54, 7331–7338 CrossRef CAS.
- G. Mazza, L. Fukumoto, P. Delaquis, B. Girard and B. Ewert, J. Agric. Food Chem., 1999, 47, 4009–4017 CrossRef CAS.
- D. J. Baer, J. T. Judd, B. A. Clevidence, R. A. Muesing, W. S. Campbell, E. D. Brown and P. R. Taylor, American J. Clin. Nutrit., 2002, 75, 593–599 Search PubMed.
- L. H. Opie and S. Lecour, Eur. Heart J., 2007, 28, 1683–1693 CrossRef CAS.
- D. P. Van Velden, S. van der Merwe, E. Fourie, M. Kidd, D. M. Blackhurst, M. J. Kotze and E. P. G. Mansvelt, Winehealth – 3rd International Congress on Wine and Health, Bordeaux, France, 2007 Search PubMed.
- N. A. Al-Awwadi, A. Bornet, J. Azay, C. Araiz, S. Delbosc, J. P. Cristol, N. Linck, G. Cros and P. L. Teissedre, J. Agric. Food Chem., 2004, 52, 5593–5597 CrossRef CAS.
- R. W. Li, T. D. Douglas, G. K. Maiyoh, K. Adeli and A. G. Theriault, J. Ethnopharmacol., 2006, 104, 24–31 CrossRef CAS.
- J. Khateeb, A. Gantman, A. J. Kreitenberg, M. Aviram and B. Fuhrman, Atherosclerosis, 2010, 208, 119–125 CrossRef CAS.
- T. H. W. Huang, G. Peng, B. P. Kota, G. Q. Li, J. Yamahara, B. D. Roufogalis and Y. Li, Toxicol. Appl. Pharmacol., 2005, 207, 160–169 CrossRef CAS.
- K. Karthikeyan, B. R. S. Bai and S. N. Devaraj, Int. J. Cardiol., 2007, 115, 326–333 CrossRef CAS.
- M. Pinent, C. Blade, M. J. Salvado, M. Blay, G. Pujadas, J. Fernandez-Larrea, L. Arola and A. Ardevol, Crit. Rev. Food Sci. Nutr., 2006, 46, 543–550 CrossRef CAS.
- F. Akbiyik, D. M. Ray, K. F. Gettings, N. Blumberg, C. W. Francis and R. P. Phipps, Blood, 2004, 104, 1361–1368 CrossRef CAS.
- A. Zoechling, E. Reiter, R. Eder, S. Wendelin, F. Liebner and A. Jungbauer, Am. J. Enol. Vitic., 2009, 60, 223–232 Search PubMed.
| This journal is © The Royal Society of Chemistry 2011 |