Edaphic factors affecting the vertical distribution of radionuclides in the different soil types of Belgrade, Serbia
Snežana
Dragović
*a,
Boško
Gajić
b,
Ranko
Dragović
c,
Ljiljana
Janković-Mandić
d,
Latinka
Slavković-Beškoski
de,
Nevena
Mihailović
a,
Milan
Momčilović
a and
Mirjana
Ćujić
a
aUniversity of Belgrade, Institute for the Application of Nuclear Energy, Banatska 31b, 11080, Belgrade, Serbia. E-mail: sdragovic@inep.co.rs
bUniversity of Belgrade, Institute of Land Management, Faculty of Agriculture, Nemanjina 6, 11081, Belgrade, Serbia
cUniversity of Niš, Faculty of Science and Mathematics, Department of Geography, Višegradska 33, 18000, Niš, Serbia
dUniversity of Belgrade, Vinča Institute of Nuclear Sciences, P.O. Box 522, 11001, Belgrade, Serbia
eAnahem Laboratory, Mocartova 10, 11160, Belgrade, Serbia
First published on 10th November 2011
Abstract
The specific activities of natural radionuclides (40K, 226Ra and 232Th) and Chernobyl-derived 137Cs were measured in soil profiles representing typical soil types of Belgrade (Serbia): chernozems, fluvisols, humic gleysols, eutric cambisols, vertisols and gleyic fluvisols. The influence of soil properties and content of stable elements on radionuclide distribution down the soil profiles (at 5 cm intervals up to 50 cm depth) was analysed. Correlation analysis identified associations of 40K, 226Ra and 137Cs with fine-grained soil fractions. Significant positive correlations were found between 137Cs specific activity and both organic matter content and cation exchange capacity. Saturated hydraulic conductivity and specific electrical conductivity were also positively correlated with the specific activity of 137Cs. The strong positive correlations between 226Ra and 232Th specific activities and Fe and Mn indicate an association with oxides of these elements in soil. The correlations observed between 40K and Cr, Ni, Pb and Zn and also between 137Cs and Cd, Cr, Pb and Zn could be attributed to their common affinity for clay minerals. These results provide insight into the main factors that affect radionuclide migration in the soil, which contributes to knowledge about radionuclide behaviour in the environment and factors governing their mobility within terrestrial ecosystems.
Environmental impactThe influence of edaphic factors on radionuclide distribution down soil profiles representing soil types typical for Belgrade, Serbia, and also for the region was examined in this study. Statistically significant correlations between radionuclides and some soil properties and stable element contents were identified. The knowledge gained contributes significantly to understanding the biogeochemical behaviour of radionuclides in the environment and factors governing their mobility within terrestrial ecosystems. |
1. Introduction
Natural radioactivity in the soil arises mainly from primordial radionuclides formed in nucleosynthesis processes in stars before the early stage of the formation of the solar system, i.e.40K and nuclides from the 232Th and 238U series and their decay products.1 Natural radioactivity is of special environmental concern because the majority of the total radiation dose to the world population is from natural sources. Natural environmental radioactivity and the associated external exposure due to gamma radiation depend primarily on the geological and geographical conditions. Distribution of natural radionuclides is governed by weathering, sedimentation, leaching/sorption and precipitation from percolating groundwater or dilution with other materials with different composition, resulting in high variability in their specific activities.2Anthropogenic radionuclides are derived from the radioactive fallout of nuclear fission products during nuclear weapon tests and from the Chernobyl accident in 1986. This accident resulted in significant deposition of 137Cs on surface soils throughout Europe. Due to its relatively long half-life of 30.2 years and chemical behaviour similar to that of potassium, 137Cs is one of the most significant radionuclides in the environment. Measurements of Chernobyl fallout clearly indicate that soil is the main reservoir of 137Cs (ref. 3–5) but its migration behaviour and associated profile distribution are site-specific and depend on soil characteristics and environmental conditions.
Soil acts as a medium for transfer of radionuclides to biological systems and hence it is the basic indicator of radiological contamination of the environment. As part of different soil compounds, radionuclides are subjected to various biogeochemical processes that eventually determine their mobilization and availability for ecological processes. An understanding of the pathways by which radioactivity reaches biota requires an assessment of the soil's physical and chemical properties that affect the abundance and distribution of radionuclides. In recent years much knowledge on the effects of soil properties on biogeochemical mobilization of these radionuclides has been gained. Thus, Navas et al. (2002) showed that depth distribution of radionuclides is affected by soil properties, including pH, carbonates, organic matter and particle size, and soil processes, such as leaching and adsorption.6 An association between 226Ra and 232Th with oxides of iron and manganese was reported by Navas et al. (2005).7 Blanco Rodríguez et al. (2008) found a strong correlation between the finer particles of soil and radionuclide specific activities.8 Buccianti et al. (2009) and Belivermis et al. (2010) confirmed the influence of particle size on radionuclide specific activities in soil.9,10 Nevertheless, there is a scarcity of information on the behaviour of radionuclides in the soils of Serbia.11,12
In this study the depth distribution of the natural radionuclides, 40K, 226Ra and 232Th, and Chernobyl-derived 137Cs, was studied in profiles representing typical soil types of the Belgrade area.13 Although a substantial body of knowledge already exists in this area, in the majority of studies correlations between natural or anthropogenic radionuclides and a limited number of soil parameters, mainly particle size,6–10,14–22 pH, organic matter content,6,10,16–18,20–22carbonate content6,7,16 or selected stable elements,7,20,21 have been investigated. To assess the pedogenic effect on the distribution of radionuclides, the relationships between radionuclide specific activities and a number of soil properties (particle size distribution, pH, organic matter content, carbonate content, cation exchange capacity, bulk density, particle density, saturated hydraulic conductivity and specific electrical conductivity) were analysed. The relationships between radionuclides and major and trace element concentrations in soil (Al, Ca, Cd, Co, Cr, Cu, Fe, K, Li, Mg, Mn, Na, Ni, Sr, Pb, Zn and Ti) were also assessed to gain insight into the biogeochemical behaviour of the analysed radionuclides. An additional contribution of the study is that the analysed soil types are also major ones in the region.13
2. Materials and methods
2.1. Study area and soil sampling
Belgrade is the capital and the largest city of Serbia with a population of about 1.8 million. It lies 117 m above sea level in the northern part of central Serbia (N-44°49′14′′, E-20°27′44′′) at the confluence of the Danube and Sava rivers. The climate is moderate continental with four seasons. The high plasticity of the Belgrade relief, south of both rivers, has allowed the city to spread over many hills. North of the rivers Sava and Danube there are alluvial plains and loessial plateaus, which are divided by a steep section. There are two structural floors in the Belgrade area tectonics. The first is composed of jurassic and cretaceous sediments and magmatic products. Parts of the mesozoic joist on the west consist of flysh sediments of the upper cretaceous series, covered by neogen sediments with horizontal stratification important for the development of pedological cover. The second structural floor is composed of neogen sediments. More than 70% of the territory of Belgrade is covered by quaternary deposits of alluvial lake, alluvial marsh, alluvial, delluvial, delluvial–prolluvial, prolluvial and eolic sediments.23Soil profiles were sampled in May 2008 at six locations where the soil types are representative for the territory of Belgrade (Fig. 1). According to the FAO (2006) the soils are classified as: chernozems (A), fluvisols (B), humic gleysols (C), eutric cambisols (D), vertisols (E) and gleyic fluvisols (F).24 To ensure representativeness, three subsamples were taken from each site. They were mixed to form a composite sample for analysis. Soil samples were collected every 5 cm from the uppermost layer down to 50 cm depth at each site. Samples were collected using a stainless steel spade, cylinders and a plastic scoop. To avoid cross-contamination disposable gloves were used and the equipment was first brushed to eliminate residues from the previous sample and then flushed with soil from the new sampling site. The surface vegetation, visible roots, stones and other debris were removed from the samples. The samples were then dried at room temperature to constant weight, homogenized and passed through a 2 mm sieve for radioactivity measurements and particle size analysis and ground in a mortar for other analyses.
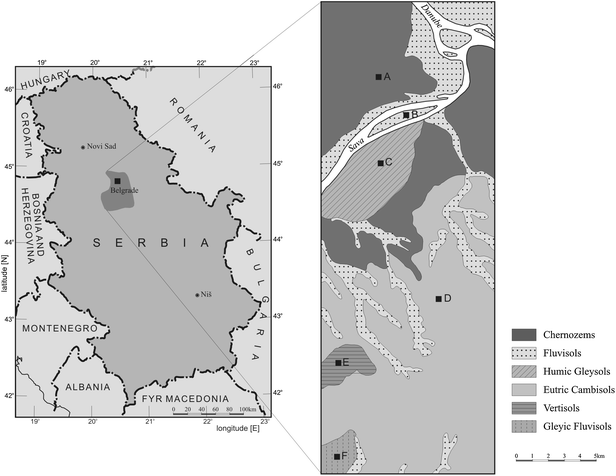 | ||
| Fig. 1 Simplified pedologic map of Belgrade showing the location of sampling sites. | ||
2.2. Analytical methods
Radioactivity measurements were performed using an HPGe gamma-ray spectrometer (ORTEC-AMETEK, 34% relative efficiency and 1.65 keV FWHM for 60Co at 1.33 MeV). The detector was surrounded by a shield consisting of 100 mm low-level lead (less than 50 Bq kg−1 of 210Pb), including an interior lining consisting of 2 mm Cu, 1 mm Cd and 4 mm Perspex. The samples were then kept in hermetically sealed Marinelli beakers of 500 mL volume for about 4 weeks to allow equilibrium between 226Ra and its daughters. The energy calibration and relative efficiency calibration of the gamma spectrometer were carried out using a 500 mL Marinelli calibration source MBSS2 supplied by Czech Metrological Institute and the standard gamma-ray spectrometry reference material IAEA-RGU-1 supplied by International Atomic Energy Agency (IAEA). For the purpose of quality assurance, the calibration was checked using standard reference materials (IAEA-375, EML 596 QAP 9803). Prior to the sample measurement, the environmental background at the laboratory site was determined with an empty Marinelli beaker under identical measurement conditions. Background spectral intensities were later subtracted from corresponding sample intensities. The activity of each sample was measured for 60 ks.The specific activities of 40K and 137Cs were determined from their gamma-ray lines at 1460.8 keV and 661.6 keV, respectively. The specific activity of 226Ra was assessed from the gamma-ray lines of 214Bi (609.3 keV) and 214Pb (295.2 and 352.0 keV). The specific activity of 232Th was evaluated from the gamma-ray lines of 228Ac at 338.4, 911.1 and 968.9 keV, assuming that a state of secular equilibrium exists between 232Th, 228Ra and 228Ac. Gamma Vision 32 MCA emulation software was used to analyse gamma-ray spectra.25 Self-absorption corrections were made for a given geometric setup based on coefficients obtained experimentally through measurements and comparison of the efficiency for samples with different densities.
The 137Cs inventory was calculated using the following equation:26
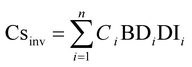 | (1) |
The traditional pipette method was used for particle size analysis.27 Once the organic matter was removed by H2O2, the remaining mineral sample was weighed and subjected to particle size analysis to determine the following fractions: sand (0.05–2 mm), silt (0.002–0.05 mm) and clay (<0.002 mm). Soil pH was measured in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 soil–water suspensions.28 The organic matter content was determined by dichromate digestion based on the Walkley–Black method.29Carbonates were measured volumetrically using Scheibler's calcimeter for CaCO3 content.30 The total cation exchange capacity of the sorptive complex was calculated as the sum of the hydrolytic acidity and total exchange bases, both measured according to Kappen (1929).31 Dry bulk density was determined using undisturbed soil cylinders (100 cm3).32 Particle density was measured with a pycnometer.32 Saturated hydraulic conductivity was measured by the falling-head method according to Klute and Dirksen (1986).33 The specific electrical conductivity was measured in a 1
5 soil–water suspensions.28 The organic matter content was determined by dichromate digestion based on the Walkley–Black method.29Carbonates were measured volumetrically using Scheibler's calcimeter for CaCO3 content.30 The total cation exchange capacity of the sorptive complex was calculated as the sum of the hydrolytic acidity and total exchange bases, both measured according to Kappen (1929).31 Dry bulk density was determined using undisturbed soil cylinders (100 cm3).32 Particle density was measured with a pycnometer.32 Saturated hydraulic conductivity was measured by the falling-head method according to Klute and Dirksen (1986).33 The specific electrical conductivity was measured in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 water suspension using a WTW inoLab ph/Cond 720 instrument.34
5 water suspension using a WTW inoLab ph/Cond 720 instrument.34
For metal concentrations, samples were digested with HNO3 and H2O2 in a microwave oven and then concentrations were determined using a Perkin Elmer PE3100/MHS-1 Atomic Absorption Spectrometer.35 By the applied method all elements that could become environmentally available were dissolved. The standard reference material (SRM 2711) from the National Institute of Standards and Technology was used for quality assurance and quality control. The concentrations obtained were within 10% range of the certified values. Reagent blanks were also included in each batch of analyses to check for any contamination of the soil sample extracts. Average values for two replicates were taken for each determination.
The influence of soil depth and soil type on the distribution of analysed parameters was analysed by one-way ANOVA using the software package SPSS 16.0 for Windows.36 The same software package was also used for evaluation of significant relationships between specific activities of radionuclides and soil physicochemical characteristics and stable element contents by Pearson correlation.
3. Results and discussion
3.1. Distribution of natural radionuclides and 137Cs in soils
The mean values of natural radionuclide specific activities, i.e. 600 Bq kg−1 for 40K, 38.9 Bq kg−1 for 226Ra and 46.6 Bq kg−1 for 232Th (Table 1), fell within the range for Serbian soils and those from the Belgrade area.37,38 The specific activities of 40K, 226Ra and 232Th were found to be influenced by soil type (Table 2, Sig. ≤0.05). The mean specific activity of 137Cs was found to be 18.5 Bq kg−1. The specific activities of radionuclides (Table 1) varied by a factor up to 1.7 for 40K, 2.8 for 226Ra and 232Th and 540 for 137Cs.| Parameter | 40K | 226Ra | 232Th | 137Cs |
|---|---|---|---|---|
| Mean | 600 | 38.9 | 46.6 | 18.5 |
| Median | 600 | 38.1 | 46.7 | 4.35 |
| Mode | 600 | 26.1 | 28.4 | 0.50 |
| Standard deviation | 74.4 | 11.8 | 11.5 | 33.2 |
| Range | 310 | 42.7 | 48.6 | 160 |
| Minimum | 450 | 23.0 | 27.6 | 0.30 |
| Maximum | 760 | 65.7 | 76.2 | 160 |
| Variable | Source of variation | F | Sig. |
|---|---|---|---|
| 40K | Depth | 0.16 | 0.997 |
| Soil type | 37.8 | 0.000 | |
| 226Ra | Depth | 0.04 | 1.000 |
| Soil type | 192 | 0.000 | |
| 232Th | Depth | 0.19 | 0.994 |
| Soil type | 54.6 | 0.000 | |
| 137Cs | Depth | 9.32 | 0.000 |
| Soil type | 1.033 | 0.408 | |
| Al | Depth | 1.30 | 0.265 |
| Soil type | 3.24 | 0.012 | |
| Ca | Depth | 0.06 | 1.000 |
| Soil type | 57.3 | 0.000 | |
| Cd | Depth | 0.40 | 0.936 |
| Soil type | 3.20 | 0.013 | |
| Co | Depth | 0.45 | 0.899 |
| Soil type | 8.57 | 0.000 | |
| Cr | Depth | 0.26 | 0.982 |
| Soil type | 52.3 | 0.000 | |
| Cu | Depth | 0.48 | 0.883 |
| Soil type | 1.80 | 0.127 | |
| Fe | Depth | 0.55 | 0.832 |
| Soil type | 8.27 | 0.000 | |
| K | Depth | 1.08 | 0.397 |
| Soil type | 10.1 | 0.000 | |
| Li | Depth | 1.78 | 0.094 |
| Soil type | 3.40 | 0.010 | |
| Mg | Depth | 0.25 | 0.985 |
| Soil type | 19.3 | 0.000 | |
| Mn | Depth | 0.76 | 0.655 |
| Soil type | 3.95 | 0.004 | |
| Na | Depth | 0.80 | 0.624 |
| Soil type | 1.70 | 0.152 | |
| Ni | Depth | 0.77 | 0.647 |
| Soil type | 3.33 | 0.011 | |
| Sr | Depth | 0.88 | 0.545 |
| Soil type | 3.70 | 0.006 | |
| Pb | Depth | 1.45 | 0.190 |
| Soil type | 7.84 | 0.000 | |
| Zn | Depth | 0.57 | 0.818 |
| Soil type | 8.80 | 0.000 | |
| Ti | Depth | 1.17 | 0.335 |
| Soil type | 2.05 | 0.086 | |
| Sand | Depth | 0.10 | 1.000 |
| Soil type | 50.6 | 0.000 | |
| Silt | Depth | 1.71 | 0.112 |
| Soil type | 8.31 | 0.000 | |
| Clay | Depth | 1.20 | 0.311 |
| Soil type | 9.81 | 0.000 | |
| pH | Depth | 0.26 | 0.983 |
| Soil type | 135 | 0.000 | |
| Organic matter | Depth | 7.53 | 0.000 |
| Soil type | 1.90 | 0.111 | |
| Cation exchange capacity | Depth | 0.02 | 1.000 |
| Soil type | 855 | 0.000 | |
| Carbonates | Depth | 0.08 | 1.000 |
| Soil type | 79.2 | 0.000 | |
| Bulk density | Depth | 8.74 | 0.000 |
| Soil type | 3.15 | 0.014 | |
| Particle density | Depth | 5.98 | 0.000 |
| Soil type | 4.48 | 0.002 | |
| Sat. hydraulic conductivity | Depth | 61.5 | 0.000 |
| Soil type | 0.32 | 0.897 | |
| Spec. electrical conductivity | Depth | 3.78 | 0.001 |
| Soil type | 8.30 | 0.000 |
The distribution of radionuclide specific activities down the depth profiles is presented in Fig. 2. In all soil profiles specific activities of natural radionuclides showed homogeneous distribution (Table 2). Homogeneous distribution of natural radionuclides was also reported by Navas et al. (2005) for soils in the Central Spanish Pyrenees and those from Burullus Lake, Egypt,7,39 while increasing 40K specific activities with depth were reported by Fujiyoshi and Sawamura (2004).40 The differences in 40K depth distribution have generally been attributed to the variability of organic matter and mineral composition of the soil and to soil biological activity including root distribution.40 The specific activities of 137Cs were maximal at 10–15 cm depth, which is in accordance with numerous studies showing that 137Cs deposition is mostly contained within the surface soil layer.41–43 The 137Cs specific activities in the surface soil layers showed a wide range of values (from 8.3 to 160 Bq kg−1 in the 0–5 cm layer and from 8.4 to 120 Bq kg−1 in the 5–10 cm layer), which could be attributed to non-homogeneous surface contamination after the Chernobyl accident. The 137Cs inventories calculated according to eqn (1) were found to vary between 60 Bq m−2 for profile C and 320 Bq m−2 for profile E, which is in accordance with values reported for Belgrade area after the Chernobyl accident.44 The migration of 137Cs in soil is slow because it is adsorbed onto clay minerals, silt and humic substances.3,45,46 The extent of 137Cs binding to soil components, i.e. the amount and distribution of this radionuclide in plant rooting zone, greatly influences its bioavailability.47 The vertical distribution of 137Cs is influenced not only by physical factors, such as the quantity and intensity of precipitation following deposition and the soil physicochemical properties, but also by biological factors, such as absorption and re-deposition by plants and biological characteristics of the soil.3 It has been found that microflora strongly contribute to the immobilization of 137Cs in soils.48 In profile B the specific activities of 137Cs decreased with depth up to 25 cm and then slightly increased down the profile. As seen in Tables 3 and 4 some other physical properties of the soil (pH, organic matter, carbonates, K) showed discontinuity at the 25 cm depth. This soil profile (fluvisols) is collected in the alluvial plain of the Sava River which is flooded periodically. The physical and chemical characteristics of fluvisols depend on the nature and sequence of layers and length of periods of soil formation after or between flood events. The observed trend in the 137Cs specific activities and other soil properties could be attributable to the different mechanical, mineralogical and chemical composition of layers in the studied fluvisols profile.49 The 137Cs depth distribution could be also influenced by bioturbation, which is considered by many authors to be an important cause of vertical movement of 137Cs.40 Following the Chernobyl accident there were a number of studies on migration of 137Cs in soils. An exponential decrease of 137Cs specific activities with soil depth and a strong influence of soil density on the 137Cs profile were reported by Miller et al. (1990).50 The application of a model based on an advection–dispersion equation showed significant regional differences in the apparent soil water velocity and in the dispersion coefficients, which were attributed to real physical differences in the soils.51
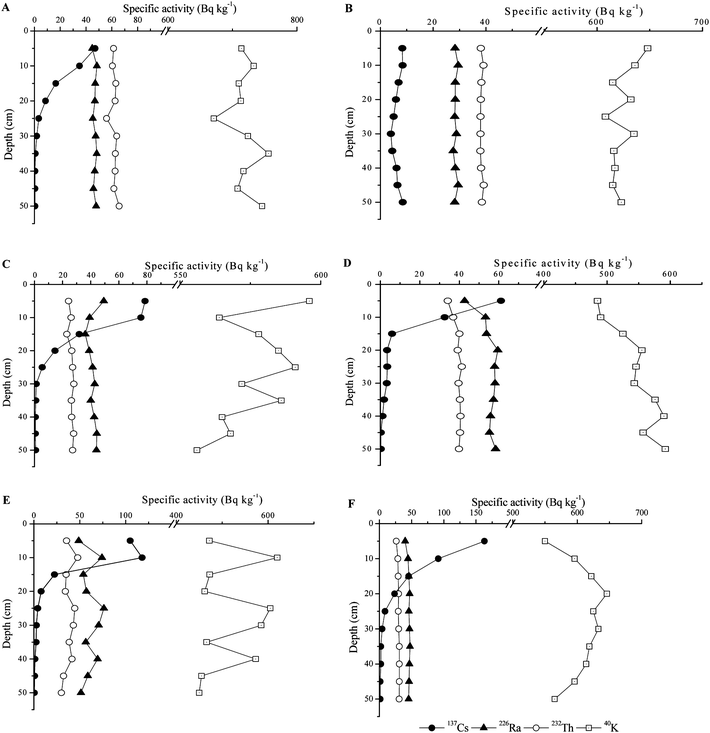 | ||
| Fig. 2 Depth distribution of radionuclides in the 5 cm interval samples of the studied soil profiles (A—chernozems, B—fluvisols, C—humic gleysols, D—eutric cambisols, E—vertisols, and F—gleyic fluvisols). The uncertainties of the radioactivity measurements were 9–10% at the 95% level of confidence. | ||
| Site | Depth/cm | pH | Org. matter (%) | Carbonates (%) | Cation exch. capacity/cmol kg−1 | Bulk density/g cm−3 | Particle density/g cm−3 | Sat. hidr. cond./mm h−1 | Spec. el. cond./μS cm−1 |
|---|---|---|---|---|---|---|---|---|---|
| A | 0–5 | 6.11 | 5.81 | 0.19 | 30.7 | 1.10 | 2.49 | 2370 | 170 |
| 5–10 | 6.27 | 4.17 | 0.18 | 29.9 | 1.32 | 2.49 | 1300 | 85.8 | |
| 10–15 | 6.75 | 3.64 | 0.14 | 28.4 | 1.37 | 2.50 | 640 | 76.8 | |
| 15–20 | 6.51 | 3.26 | 0.00 | 29.6 | 1.28 | 2.52 | 410 | 85.5 | |
| 20–25 | 7.20 | 4.33 | 0.21 | 27.1 | 1.28 | 2.54 | 290 | 82.1 | |
| 25–30 | 6.77 | 5.03 | 0.19 | 29.8 | 1.45 | 2.58 | 210 | 81.3 | |
| 30–35 | 6.99 | 3.47 | 0.12 | 29.8 | 1.54 | 2.61 | 100 | 79.7 | |
| 35–40 | 7.61 | 3.38 | 0.17 | 32.6 | 1.48 | 2.64 | 110 | 76.1 | |
| 40–45 | 7.47 | 3.64 | 0.20 | 29.2 | 1.42 | 2.66 | 82.9 | 79.4 | |
| 45–50 | 7.87 | 4.12 | 0.18 | 30.6 | 1.38 | 2.67 | 59.8 | 84.1 | |
| B | 0–5 | 8.50 | 4.81 | 10.1 | 100 | 1.03 | 2.59 | 3070 | 234 |
| 5–10 | 8.75 | 2.81 | 9.48 | 100 | 1.19 | 2.59 | 2130 | 192 | |
| 10–15 | 8.82 | 2.16 | 9.77 | 100 | 1.14 | 2.58 | 1330 | 180 | |
| 15–20 | 8.83 | 2.26 | 9.10 | 100 | 1.14 | 2.62 | 690 | 177 | |
| 20–25 | 8.69 | 1.83 | 0.10 | 110 | 1.17 | 2.61 | 310 | 174 | |
| 25–30 | 8.87 | 2.09 | 9.65 | 100 | 1.18 | 2.64 | 260 | 176 | |
| 30–35 | 8.89 | 2.10 | 10.5 | 110 | 1.28 | 2.57 | 150 | 177 | |
| 35–40 | 8.94 | 2.17 | 10.1 | 100 | 1.31 | 2.61 | 100 | 178 | |
| 40–45 | 8.83 | 2.34 | 9.91 | 100 | 1.30 | 2.64 | 56.0 | 181 | |
| 45–50 | 8.97 | 3.05 | 9.43 | 100 | 1.30 | 2.67 | 21.1 | 184 | |
| C | 0–5 | 8.02 | 8.38 | 4.29 | 97.3 | 1.10 | 2.34 | 1710 | 304 |
| 5–10 | 8.16 | 7.05 | 4.84 | 110 | 1.30 | 2.39 | 1300 | 254 | |
| 10–15 | 8.39 | 5.90 | 5.75 | 98.1 | 1.35 | 2.39 | 960 | 220 | |
| 15–20 | 8.49 | 6.14 | 5.81 | 100 | 1.36 | 2.39 | 310 | 203 | |
| 20–25 | 8.60 | 3.91 | 6.11 | 99.2 | 1.38 | 2.48 | 120 | 189 | |
| 25–30 | 8.72 | 4.15 | 5.53 | 98.4 | 1.42 | 2.53 | 64.1 | 174 | |
| 30–35 | 8.82 | 4.00 | 5.43 | 97.5 | 1.38 | 2.59 | 34.0 | 167 | |
| 35–40 | 8.66 | 6.12 | 4.53 | 97.6 | 1.41 | 2.59 | 24.3 | 168 | |
| 40–45 | 8.83 | 3.76 | 4.09 | 96.4 | 1.49 | 2.61 | 10.5 | 167 | |
| 45–50 | 8.96 | 3.19 | 3.83 | 97.6 | 1.51 | 2.61 | 10.7 | 167 | |
| D | 0–5 | 5.43 | 12.93 | 0.17 | 20.7 | 1.00 | 2.51 | 2110 | 242 |
| 5–10 | 5.06 | 7.69 | 0.21 | 16.6 | 1.18 | 2.57 | 1640 | 113 | |
| 10–15 | 5.19 | 5.76 | 0.19 | 18.7 | 1.31 | 2.60 | 1060 | 92.4 | |
| 15–20 | 5.20 | 4.78 | 0.19 | 18 | 1.37 | 2.62 | 880 | 91.1 | |
| 20–25 | 5.43 | 3.79 | 0.16 | 14.5 | 1.36 | 2.64 | 600 | 79.9 | |
| 25–30 | 5.74 | 3.03 | 0.17 | 10.4 | 1.36 | 2.66 | 390 | 73.5 | |
| 30–35 | 5.82 | 1.53 | 0.24 | 11.1 | 1.52 | 2.65 | 150 | 54.3 | |
| 35–40 | 5.70 | 1.74 | 0.19 | 12.7 | 1.55 | 2.66 | 99.0 | 53.5 | |
| 40–45 | 5.53 | 1.41 | 0.21 | 13.6 | 1.60 | 2.67 | 40.0 | 52.1 | |
| 45–50 | 5.77 | 1.36 | 0.19 | 13.6 | 1.65 | 2.68 | 9.8 | 52.6 | |
| E | 0–5 | 7.08 | 10.55 | 0.27 | 37.9 | 1.24 | 2.31 | 2520 | 180 |
| 5–10 | 7.13 | 5.64 | 0.35 | 29.1 | 1.36 | 2.33 | 1240 | 109 | |
| 10–15 | 7.28 | 5.91 | 0.29 | 24 | 1.51 | 2.34 | 210 | 87.2 | |
| 15–20 | 7.22 | 5.26 | 0.26 | 26.4 | 1.42 | 2.39 | 160 | 85.5 | |
| 20–25 | 7.35 | 4.72 | 0.27 | 26 | 1.46 | 2.52 | 100 | 85.8 | |
| 25–30 | 7.60 | 5.09 | 0.29 | 24.4 | 1.46 | 2.59 | 54.1 | 81.8 | |
| 30–35 | 7.59 | 5.07 | 0.26 | 29 | 1.45 | 2.61 | 23.2 | 76.2 | |
| 35–40 | 7.74 | 2.86 | 0.30 | 27.6 | 1.47 | 2.64 | 20.5 | 72.4 | |
| 40–45 | 7.84 | 2.02 | 0.32 | 26.5 | 1.44 | 2.65 | 18.4 | 72.1 | |
| 45–50 | 7.85 | 2.07 | 0.30 | 30.4 | 1.47 | 2.68 | 12.4 | 78.9 | |
| F | 0–5 | 6.94 | 10.46 | 0.32 | 47.8 | 0.94 | 2.53 | 2470 | 405 |
| 5–10 | 7.22 | 7.50 | 0.27 | 36.5 | 1.28 | 2.53 | 1580 | 203 | |
| 10–15 | 7.20 | 5.31 | 0.05 | 28.6 | 1.35 | 2.56 | 610 | 145 | |
| 15–20 | 7.30 | 4.03 | 0.16 | 30.1 | 1.33 | 2.59 | 320 | 112 | |
| 20–25 | 7.33 | 3.14 | 0.16 | 31.8 | 1.38 | 2.62 | 220 | 93.6 | |
| 25–30 | 7.20 | 4.02 | 0.17 | 28.2 | 1.43 | 2.64 | 210 | 87.1 | |
| 30–35 | 7.06 | 2.72 | 0.10 | 23.6 | 1.48 | 2.66 | 98.5 | 81.1 | |
| 35–40 | 7.00 | 4.21 | 0.10 | 23.8 | 1.50 | 2.66 | 61.2 | 76.3 | |
| 40–45 | 7.55 | 3.97 | 0.21 | 22.4 | 1.53 | 2.67 | 19.9 | 74.7 | |
| 45–50 | 7.39 | 3.07 | 0.09 | 24.6 | 1.56 | 2.69 | 9.0 | 69.4 |
| Site | Depth/cm | Al | Ca | Cd | Co | Cr | Cu | Fe | K | Li | Mg | Mn | Na | Ni | Sr | Pb | Zn | Ti |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 0–5 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
5550 | 1.88 | 19.3 | 111 | 21.6 | 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 660 660 |
1080 | 14.1 | 5260 | 1040 | 128 | 55.6 | 26.0 | 72.5 | 59.0 | 71.3 |
| 5–10 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 560 560 |
7780 | 2.25 | 20.8 | 122 | 22.4 | 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 340 340 |
1050 | 19.8 | 5330 | 860 | 120 | 56.9 | 28.2 | 62.1 | 71.3 | 62.9 | |
| 10–15 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4950 | 2.20 | 18.7 | 118 | 19.9 | 68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
1070 | 19.8 | 5400 | 980 | 122 | 46.4 | 29.5 | 81.4 | 64.3 | 63.5 | |
| 15–20 | 9690 | 5140 | 1.79 | 19.9 | 128 | 32.0 | 61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 190 190 |
1040 | 25.3 | 5420 | 880 | 129 | 46.3 | 29.1 | 91.0 | 65.4 | 60.5 | |
| 20–25 | 9800 | 6460 | 2.07 | 20.7 | 124 | 25.4 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 010 010 |
1100 | 23.8 | 5640 | 1030 | 104 | 43.6 | 26.7 | 49.9 | 70.4 | 48.4 | |
| 25–30 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
5950 | 2.14 | 18.6 | 128 | 21.5 | 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 970 970 |
1080 | 18.6 | 5750 | 1170 | 117 | 63.1 | 28.7 | 66.7 | 70.9 | 48.7 | |
| 30–35 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 680 680 |
6590 | 1.80 | 20.3 | 108 | 20.3 | 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1140 | 15.9 | 6040 | 1420 | 120 | 64.7 | 26.8 | 80.8 | 66.9 | 59.1 | |
| 35–40 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 630 630 |
9410 | 2.17 | 21.2 | 126 | 22.9 | 61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 320 320 |
1240 | 21.7 | 6450 | 1370 | 122 | 57.5 | 30.3 | 72.0 | 58.4 | 68.3 | |
| 40–45 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 110 110 |
8560 | 2.50 | 20.7 | 132 | 28.3 | 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 550 550 |
1260 | 26.0 | 5980 | 1100 | 114 | 66.5 | 26.1 | 70.4 | 64.9 | 66.8 | |
| 45–50 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 230 230 |
7850 | 2.44 | 19.6 | 114 | 25.4 | 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 850 850 |
1180 | 20.1 | 5550 | 960 | 115 | 64.5 | 25.4 | 90.8 | 58.6 | 66.5 | |
| B | 0–5 | 9140 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 090 090 |
1.48 | 14.0 | 113 | 18.3 | 71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 670 670 |
1280 | 17.2 | 4740 | 850 | 94 | 43.9 | 25.0 | 59.2 | 69.8 | 49.9 |
| 5–10 | 9590 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 520 520 |
1.75 | 14.3 | 136 | 25.5 | 61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 890 890 |
1080 | 20.4 | 5240 | 930 | 103 | 53.1 | 27.9 | 63.2 | 67.7 | 40.9 | |
| 10–15 | 9920 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 060 060 |
1.67 | 16.9 | 153 | 25.5 | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 180 180 |
1160 | 22.0 | 5510 | 1040 | 97.1 | 54.5 | 30.9 | 61.5 | 88.2 | 47.4 | |
| 15–20 | 9020 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.85 | 13.7 | 131 | 22.5 | 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 160 160 |
1240 | 20.4 | 5040 | 910 | 113 | 46.9 | 27.0 | 54.8 | 71.2 | 43.4 | |
| 20–25 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 140 140 |
28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 160 160 |
1.74 | 14.4 | 129 | 28.7 | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 590 590 |
1210 | 24.6 | 4920 | 1200 | 113 | 48.2 | 33.1 | 47.8 | 87.7 | 50.7 | |
| 25–30 | 9430 | 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 370 370 |
1.33 | 11.6 | 139 | 27.0 | 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 030 030 |
920 | 24.7 | 5330 | 1380 | 121 | 41.5 | 29.7 | 45.9 | 77.5 | 62.9 | |
| 30–35 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 430 430 |
33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 330 330 |
2.13 | 15.6 | 151 | 25.7 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 750 750 |
1410 | 16.6 | 4980 | 1420 | 148 | 57.3 | 29.4 | 49.9 | 87.7 | 71.3 | |
| 35–40 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 540 540 |
32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 710 710 |
2.14 | 21.8 | 126 | 27.0 | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 560 560 |
1510 | 15.4 | 5680 | 1370 | 124 | 63.4 | 28.6 | 64.8 | 93.3 | 57.3 | |
| 40–45 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 080 080 |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 040 040 |
2.05 | 19.9 | 124 | 26.9 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 970 970 |
1340 | 18.9 | 5390 | 1460 | 124 | 65.6 | 31.5 | 76.7 | 79.0 | 47.1 | |
| 45–50 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 140 140 |
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 610 610 |
2.20 | 19.2 | 127 | 22.0 | 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 180 180 |
1140 | 20.9 | 4250 | 1390 | 103 | 53.6 | 27.1 | 79.3 | 68.7 | 63.6 | |
| C | 0–5 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 850 850 |
33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
1.54 | 14.9 | 129 | 29.8 | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 550 550 |
900 | 25.2 | 5020 | 950 | 111 | 45.4 | 28.3 | 47.2 | 45.8 | 47.3 |
| 5–10 | 9520 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 270 270 |
1.82 | 18.6 | 141 | 30.0 | 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 840 840 |
910 | 17.3 | 4830 | 850 | 114 | 53.9 | 26.4 | 60.1 | 80.9 | 59.2 | |
| 10–15 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 290 290 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 780 780 |
2.06 | 17.9 | 157 | 31.9 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
970 | 18.5 | 5160 | 920 | 118 | 53.8 | 30.1 | 56.3 | 90.0 | 68.9 | |
| 15–20 | 9680 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 080 080 |
2.02 | 15.0 | 134 | 33.0 | 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1460 | 24.0 | 5000 | 930 | 128 | 55.7 | 29.8 | 55.2 | 89.2 | 68.5 | |
| 20–25 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 260 260 |
22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 690 690 |
2.10 | 18.2 | 137 | 27.0 | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 780 780 |
1660 | 26.8 | 5400 | 1020 | 110 | 49.4 | 33.3 | 64.0 | 85.2 | 64.0 | |
| 25–30 | 8870 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 450 450 |
1.75 | 14.7 | 127 | 25.0 | 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 510 510 |
1160 | 23.5 | 4460 | 670 | 102 | 37.8 | 27.3 | 48.9 | 75.2 | 58.3 | |
| 30–35 | 9250 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 480 480 |
1.91 | 16.2 | 120 | 25.4 | 77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 850 850 |
1380 | 22.9 | 5110 | 870 | 109 | 61.1 | 26.4 | 59.4 | 71.0 | 62.5 | |
| 35–40 | 9780 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.38 | 10.6 | 122 | 28.2 | 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 740 740 |
1300 | 14.9 | 5300 | 910 | 127 | 50.7 | 21.9 | 59.6 | 64.3 | 63.3 | |
| 40–45 | 9820 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 360 360 |
1.37 | 13.8 | 128 | 21.6 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 140 140 |
1430 | 21.9 | 4880 | 800 | 141 | 41.7 | 20.2 | 53.4 | 67.3 | 68.0 | |
| 45–50 | 9700 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1.25 | 15.1 | 128 | 19.4 | 71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 320 320 |
1433 | 23.2 | 5090 | 780 | 107 | 43.6 | 22.6 | 61.3 | 62.7 | 50.4 | |
| D | 0–5 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 960 960 |
3410 | 2.17 | 22.7 | 46.4 | 24.4 | 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 510 510 |
1350 | 22.5 | 3500 | 1380 | 125 | 56.8 | 33.9 | 73.5 | 88.0 | 66.6 |
| 5–10 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 720 720 |
3270 | 1.90 | 18.9 | 52.0 | 21.7 | 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 240 240 |
1360 | 21.6 | 3050 | 1370 | 127 | 44.0 | 31.6 | 56.9 | 36.1 | 57.4 | |
| 10–15 | 9560 | 4260 | 2.04 | 17.6 | 69.2 | 23.5 | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
1320 | 25.0 | 2790 | 940 | 127 | 43.0 | 30.0 | 81.0 | 33.1 | 59.2 | |
| 15–20 | 9680 | 1700 | 1.77 | 18.4 | 68.7 | 23.6 | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 060 060 |
1340 | 21.4 | 2890 | 770 | 116 | 47.3 | 27.5 | 76.1 | 36.9 | 60.8 | |
| 20–25 | 9670 | 1680 | 1.98 | 17.6 | 73.8 | 27.3 | 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 620 620 |
1350 | 20.4 | 2640 | 790 | 96.7 | 58.2 | 29.8 | 53.2 | 36.5 | 63.6 | |
| 25–30 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 520 520 |
3920 | 2.27 | 18.3 | 61.8 | 27.4 | 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 960 960 |
1350 | 25.5 | 2760 | 810 | 119 | 46.0 | 32.5 | 50.2 | 45.9 | 57.1 | |
| 30–35 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 410 410 |
4220 | 2.08 | 19.1 | 70.2 | 28.0 | 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 270 270 |
1490 | 20.8 | 2720 | 860 | 140 | 69.0 | 30.5 | 80.7 | 41.5 | 69.1 | |
| 35–40 | 9480 | 4270 | 1.90 | 17.1 | 76.3 | 25.2 | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 650 |
1430 | 15.7 | 2900 | 760 | 112 | 47.5 | 28.8 | 73.2 | 36.7 | 65.6 | |
| 40–45 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 510 510 |
7370 | 2.39 | 19.1 | 70.3 | 26.7 | 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 010 010 |
1580 | 21.7 | 5120 | 860 | 106 | 58.2 | 28.8 | 71.4 | 44.2 | 57.9 | |
| 45–50 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 040 040 |
7000 | 2.10 | 19.4 | 78.6 | 29.0 | 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 480 480 |
1160 | 26.0 | 5640 | 990 | 121 | 67.9 | 34.1 | 72.2 | 39.2 | 72.2 | |
| E | 0–5 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 860 860 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 010 010 |
1.72 | 18.2 | 96.7 | 21.9 | 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 580 580 |
1070 | 8.06 | 5590 | 670 | 123 | 42.1 | 31.7 | 34.3 | 61.7 | 51.2 |
| 5–10 | 8610 | 5790 | 1.39 | 15.4 | 94.0 | 21.1 | 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
840 | 6.32 | 4810 | 730 | 96.1 | 32.7 | 19.6 | 46.0 | 45.5 | 54.7 | |
| 10–15 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 650 |
4980 | 1.90 | 20.1 | 110 | 24.4 | 61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 580 580 |
980 | 7.78 | 5770 | 820 | 141 | 52.5 | 27.8 | 52.7 | 59.9 | 88.4 | |
| 15–20 | 9380 | 4970 | 2.26 | 14.5 | 96.4 | 24.4 | 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 750 750 |
960 | 16.6 | 4980 | 830 | 108 | 40.9 | 24.8 | 58.5 | 45.1 | 41.8 | |
| 20–25 | 9580 | 5600 | 2.18 | 15.9 | 115 | 22.4 | 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 470 470 |
970 | 17.5 | 5270 | 930 | 110 | 46.1 | 28.6 | 53.2 | 47.2 | 47.2 | |
| 25–30 | 9120 | 4780 | 1.68 | 15.8 | 84.1 | 22.3 | 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 970 970 |
840 | 21.1 | 4850 | 1020 | 121 | 47.2 | 24.0 | 45.5 | 47.4 | 62.2 | |
| 30–35 | 9040 | 5190 | 1.80 | 17.6 | 104 | 18.7 | 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 550 550 |
890 | 20.7 | 4530 | 1200 | 97.2 | 43.6 | 28.3 | 57.0 | 34.3 | 57.3 | |
| 35–40 | 9520 | 5110 | 2.38 | 17.1 | 106 | 26.4 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 570 570 |
890 | 16.8 | 4800 | 1040 | 105 | 40.5 | 27.2 | 62.0 | 44.0 | 52.1 | |
| 40–45 | 9060 | 3740 | 1.92 | 17.6 | 91.3 | 23.7 | 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 520 520 |
930 | 15.5 | 4680 | 1650 | 107 | 42.8 | 24.8 | 46.3 | 43.5 | 43.7 | |
| 45–50 | 9450 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 390 390 |
1.60 | 18.9 | 100 | 28.0 | 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 950 950 |
940 | 19.6 | 4900 | 1130 | 117 | 47.9 | 28.7 | 47.6 | 41.9 | 59.4 | |
| F | 0–5 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 130 130 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 440 440 |
2.51 | 17.4 | 114 | 26.7 | 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 830 830 |
990 | 20.3 | 5570 | 1030 | 93.8 | 56.6 | 21.4 | 73.5 | 39.5 | 49.5 |
| 5–10 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 020 020 |
6280 | 2.02 | 18.1 | 101 | 27.6 | 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 620 620 |
1040 | 18.1 | 5050 | 1140 | 96.3 | 48.3 | 22.5 | 54.7 | 63.1 | 44.2 | |
| 10–15 | 9660 | 6210 | 2.07 | 18.5 | 113 | 25.1 | 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 920 920 |
930 | 25.0 | 4770 | 980 | 96.9 | 47.7 | 25.0 | 75.4 | 54.5 | 46.3 | |
| 15–20 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 290 290 |
8180 | 1.94 | 20.0 | 128 | 24.4 | 59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 840 840 |
1070 | 27.5 | 4490 | 1260 | 98.8 | 44.0 | 24.6 | 79.9 | 65.8 | 66.4 | |
| 20–25 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 290 290 |
8370 | 1.90 | 21.7 | 138 | 21.9 | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 140 140 |
1470 | 26.8 | 5780 | 1450 | 97.5 | 64.1 | 30.1 | 68.1 | 71.5 | 43.4 | |
| 25–30 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 290 290 |
7610 | 1.61 | 19.0 | 134 | 22.1 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 650 |
1100 | 25.3 | 5320 | 1490 | 109 | 57.9 | 28.8 | 72.6 | 71.6 | 50.6 | |
| 30–35 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 880 880 |
3810 | 1.94 | 19.0 | 146 | 24.7 | 122![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 670 670 |
940 | 16.1 | 4820 | 1220 | 114 | 44.5 | 29.6 | 62.7 | 63.0 | 67.2 | |
| 35–40 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 130 130 |
7820 | 2.25 | 20.9 | 133 | 27.0 | 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 780 780 |
1010 | 12.4 | 5040 | 1180 | 127 | 41.0 | 28.0 | 69.8 | 80.9 | 64.7 | |
| 40–45 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 040 040 |
7500 | 1.96 | 19.8 | 112 | 23.7 | 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250 |
1070 | 13.0 | 5240 | 1120 | 104 | 48.3 | 27.8 | 53.1 | 67.8 | 46.2 | |
| 45–50 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
7290 | 1.96 | 20.7 | 111 | 21.4 | 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 410 410 |
1070 | 12.4 | 5250 | 1100 | 119 | 48.7 | 24.6 | 83.2 | 58.7 | 62.1 |
3.2. The edaphic characteristics of analysed soils
The main physicochemical characteristics of the analysed soils are presented in Table 3. Most soils were found to be neutral to strongly alkaline and only those from sampling site D were moderately to strongly acidic. The average organic matter content varied from 2.56% for fluvisols to 5.26% for humic gleysols. For all soils, significant variations (Sig. ≤0.05) of organic matter content (decreasing trend) with soil depth were observed (Table 2). The carbonate content differed among profiles, with the average varying from 0.16% for gleyic fluvisols and chernozems to 8.81% for fluvisols. The cation exchange capacity varied from 15.0 cmol kg−1 for gleyic fluvisols to 103 cmol kg−1 for fluvisols. Soils were found to be similar with respect to density and moisture content. Saturated hydraulic conductivity of the analysed soils varied from 436 mm h−1 for vertisols to 812 mm h−1 for fluvisols. The electric conductivity ranged from 90.1 μS cm−1 for chernozems to 201 μS cm−1 for humic gleysols.The particle size distribution with soil profile depth is shown in Fig. 3. The textural characteristics varied significantly (Sig. ≤0.05) among the soil types (Table 2). According to the USDA (1999), the analysed soil falls into the silty loam, silty clay loam or silt clay textural classes.52 No significant variance was observed for particle size fractions of analysed soils with depth (Table 2).
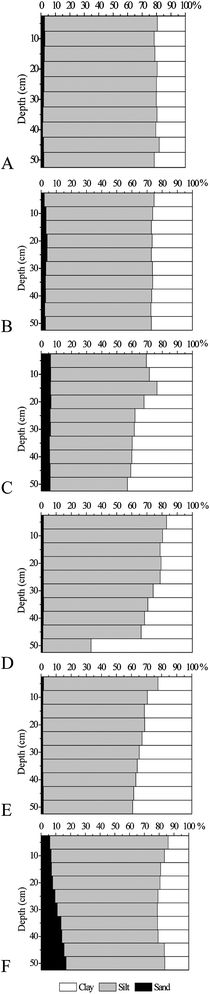 | ||
| Fig. 3 Grain size distribution in the 5 cm interval samples of the studied soil profiles. | ||
The concentrations of all major and trace elements in the soil profiles are shown in Table 4. No significant variation of these elements with soil depth was found (Table 2), while significant variation (Sig. ≤0.05) of all elements, except Cu, Na and Ti, with the soil type was observed.
3.3. Relationships between edaphic factors and radionuclides
The effects of soil physicochemical characteristics and major and trace element contents on radionuclide specific activities were analysed through correlations and the results are summarized in Table 5. Concerning the soil particle size, it is well documented that radionuclides are adsorbed onto clay surfaces or fixed within the lattice structure.14,53,54 In our soils, clay and silt contents were positively correlated with 40K, 226Ra (p < 0.01) and 137Cs (p < 0.05) specific activities. The sand content was negatively correlated (p < 0.01) with 226Ra specific activity. This correlation analysis between radionuclide specific activities and particle size distribution confirmed results obtained world-wide showing that the fine-grained soil fraction has a higher tendency for radionuclide adsorption than coarse-grained soils since the soil particle surface area is larger.15,16Minerals such as smectite, illite, vermiculite, chlorite, allophone and imogolite, as well oxides and hydroxides of silica, aluminium, iron and manganese are the most important for adsorption of radionuclides. This is due to the surface charge of these soil constituents and their three-dimensional structure. Clay minerals carry different kinds of charge, a variable charge, which can be either positive or negative, and a permanent charge which is merely negative.55 The results of the previous study in the Belgrade area have shown that the mineral composition of the clay size mineral fraction, containing illite, smectite, vermiculite, chlorite, kaolinite, quartz and feldspar mix layer silicates, is the main factor of distribution and immobilization of radionuclides in soil.56| 40K | 226Ra | 232Th | 137Cs | |
|---|---|---|---|---|
| a Correlation is significant at the 0.01 level. b Correlation is significant at the 0.05 level. | ||||
| Sand | 0.05 | −0.49a | −0.24 | 0.01 |
| Silt | 0.33a | 0.49a | −0.11 | 0.28b |
| Clay | 0.34a | 0.28a | 0.20 | 0.29b |
| pH | 0.10 | −0.30b | −0.59a | −0.13 |
| Organic matter | −0.18 | −0.19 | 0.09 | 0.73a |
| Cation exch. capacity | −0.27 | −0.27 | −0.25 | 0.41a |
| Carbonates | 0.08 | −0.67a | −0.73a | −0.13 |
| Bulk density | −0.05 | 0.05 | 0.50 | −0.52a |
| Particle density | 0.15 | 0.11 | −0.07 | −0.61a |
| Sat. hydraulic conductivity | −0.04 | −0.03 | −0.19 | 0.68a |
| Spec. electrical conductivity | −0.09 | −0.44a | −0.57a | 0.61a |
| Al | 0.19 | 0.13 | −0.15 | 0.05 |
| Ca | 0.12 | −0.57a | −0.75a | 0.01 |
| Cd | 0.15 | 0.30b | 0.11 | 0.41a |
| Co | 0.18 | 0.32b | 0.12 | 0.02 |
| Cr | 0.45a | −0.09 | 0.60a | 0.68a |
| Cu | −0.06 | −0.24 | −0.16 | 0.06 |
| Fe | 0.31 | 0.48a | 0.51a | −0.22 |
| K | 0.81a | −0.06 | −0.16 | −0.33b |
| Li | 0.17 | −0.04 | −0.18 | −0.26b |
| Mg | 0.14 | 0.21 | −0.28b | 0.06 |
| Mn | 0.12 | 0.54a | 0.37a | −0.16 |
| Na | −0.01 | 0.17 | −0.05 | −0.21 |
| Ni | 0.35a | 0.26b | 0.54a | −0.14 |
| Sr | −0.06 | 0.08 | −0.13 | −0.17 |
| Pb | 0.44a | 0.36a | 0.67a | 0.56a |
| Zn | 0.35a | 0.60a | 0.74a | 0.48a |
| Ti | 0.05 | 0.14 | 0.06 | −0.16 |
The soil pH was found to be unrelated to 40K and 137Cs specific activities, but was negatively correlated with 226Ra (p < 0.05) and 232Th (p < 0.01) specific activities. Negative correlations between pH and radionuclides of the uranium and thorium series were recorded by Tsai et al. (2011).17 Belivermis et al. (2010) reported a negative correlation between pH and 232Th specific activity in soil.10 The lack of correlation between pH and natural radionuclides specific activity reported by Navas et al. (2002) was attributed to a variety of pedogenic processes, such as eluviation and sorption to soil components, which also affect the distribution of radionuclides in soils.6 However, Baeza et al. (1995) did find negative correlations between 137Cs specific activity and pH.18
Positive correlations between 137Cs specific activity and both organic matter content and cation exchange capacity (p < 0.01) were found. Elejalde et al. (1996) also observed that 137Cs specific activities were related to organic matter and cation exchange phenomena.57 Organic matter is a component of great importance because it tends to form soluble or insoluble complexes with radionuclides, which can migrate throughout the profile or be retained in the soil.58,59 No significant correlation was found between the organic matter content and 226Ra and 232Th specific activities which is in accordance with earlier results.16,19,57 The absence of significant correlation between 226Ra and 232Th and organic matter content in analysed soils can be explained by the strong tendency of organic matter content to be inversely correlated with depth while these radionuclides showed homogeneous depth distribution. However, Vandenhove and Van Hees (2007), working with spiked soils, showed that the radium concentrations in soil solution are related to the organic matter or cation exchange capacity of soils.60 Taboada et al. (2006) observed that thorium mobility in soils developed on granitic rocks is related to the presence of organic matter, particularly of simple organic acids and fulvic and humic acids of low molecular weight, which form complexes with thorium in the soil profile.20 Since organic matter is extremely heterogeneous and consists of organic acids, lipids, lignin, fulvic and humic acids, there are a large number of possible reactions and interactions of radionuclides with organic matter. Organic matter contains functional groups that can form complexes with radionuclides. This complexation affects radionuclide mobility, adsorption to soils and bioavailability.55
Negative correlations (p < 0.01) were observed between the carbonate content and 226Ra and 232Th specific activities, which confirms earlier findings.6,7,57 The negative correlation of these radionuclides with carbonates suggests their binding in soils with minerals other than calcite, probably in silicates derived during weathering processes from parent rocks.
Soil density was negatively correlated (p < 0.01) with 137Cs, which is in accordance with the findings of Ligero et al. (2001).19 No correlation between soil density and natural radionuclides was found, which is consistent with the results of Tsai et al. (2011).17 However, in the soils of German forests, the specific activities of 40K were positively correlated with soil density, which indicated that most of the potassium was contained within the mineral components of the soil.40
Positive correlations (p < 0.01) between 137Cs specific activity and both saturated hydraulic conductivity and specific electrical conductivity were observed. Electrical conductivity was negatively correlated with 226Ra and 232Th specific activities. Tsai et al. (2011) found a weak correlation between 40K and electrical conductivity.17
The correlations between radionuclides and major and trace elements (Table 5) revealed that 40K was positively correlated (p < 0.01) with K (as it is present in natural potassium with a constant abundance), Cr, Ni, Pb and Zn, which is in accordance with their common correlations to clay. Strong positive correlations of 40K with Cr and Ni were also reported by Van der Graaf et al. (2007) and those with Pb and Zn by Al-Trabulsy et al. (2010).21,61
Close positive correlations (p < 0.01) were found between 226Ra and 232Th and Fe and Mn, which indicate association of these radionuclides with Fe and Mn oxides (assuming the secular equilibrium between 232Th, 228Ra and 228Ac) or deposition of Fe and Mn oxides on the surfaces of 226Ra and 232Th minerals. This association is supported by strong positive correlations with Ni, Pb and Zn which also appear to be related to the above-mentioned oxides. Similar relationships have been found in other studies.7,62 A positive correlation (p < 0.05) between 226Ra and Co was also observed, which agrees with the findings of Chao and Chuang (2011).62 A negative correlation (p < 0.01) between 226Ra and 232Th and Ca was found, which is in accordance with the negative correlation of these radionuclides with carbonates. A similar relationship was also found by Navas et al. (2005).7 The strong positive correlation (p < 0.01) observed between 232Th and Cr confirms the results of Navas et al. (2005) and Van der Graaf et al. (2007).7,61
The anthropogenic 137Cs was found to be positively correlated (p < 0.01) with Cd, Cr, Pb and Zn, which is in accordance with earlier results for soils from the Zlatibor area, Serbia.22 These correlations could be attributed to the common affinity of these elements for clay minerals.61 The strength of these correlations may be influenced by variations in pH and/or organic substances.
The behaviour of radionuclides depends on elemental properties of the radionuclides, on the mineral and organic inventory of the soil and the chemical reaction milieu.55 Radionuclides and metal ions can be retained in soil by (ad)sorption, precipitation and complexation reactions.63 Their interaction with the soil environment depends on both soil properties and environmental factors. The concentration of competitive elements present in the soil is of particular importance for determining radionuclide distribution between soil and soil solution. Given that the number of sites at which ions may be adsorbed are limited, the adsorption of any particular species decreases as the concentration of competing ions increases. Thus, the adsorption of radium has been shown to be strongly dependent on ionic strength and concentrations of other competing ions.64 The results of Nathwani and Phillips (1979) showed that the addition of competing alkaline earth cations to the soil system can greatly affect radium sorption on the clay minerals.65 Sturchio et al. (2001) observed the decrease in radium sorption with increasing concentrations of Ca2+, Ba2+ and Mg2+in soil which occupy sorption sites available for radium.66 These elements are likely to undergo cation exchange on clay minerals. The concentration of an ion in solution in most soils is determined by cation exchange reactions with the soil matrix which by their nature are competitive but other processes, e.g.co-precipitation, also depend on the concentrations of competing substances in solution.67 For radiocesium, these competitive effects formed the basis of countermeasures at the soil-to-plant level after a nuclear accident.68
Conclusion
In this study the influence of a number of edaphic factors on radionuclide distribution down the depth profile was analysed. The significant variations in radionuclide specific activities and major and trace element concentrations among different soil types were observed. Among radionuclides, different behaviour was distinguished with respect to the soil depth. The distribution of 40K, 226Ra and 232Th was found to be constant down the soil profile depth, while the large variability was observed for 137Cs depth distribution. The correlations between soil properties and radionuclide specific activities found in this study could serve as a basis for determination of radionuclide bioavailability in different ecosystems. Clay and silt contents were positively correlated with 40K, 226Ra and 137Cs. Close positive correlations were found between 226Ra and 232Th and Fe and Mn, which indicates the association of these radionuclides with Fe and Mn oxides, supported also by strong positive correlations with Ni, Pb and Zn. A negative correlation between 226Ra and 232Th and Ca was found, which is in accordance with the negative correlation of these radionuclides with carbonates. The strong positive correlation was observed between 232Th and Cr. The anthropogenic 137Cs was found to be positively correlated with Cd, Cr, Pb and Zn, which confirmed their common affinity for clay minerals. As the large number of parameters which could influence radionuclide migration in soil were analysed, the findings of the study could be useful in the modeling of radionuclide migration in soils. A number of models are already developed to quantify radionuclide mobility of radionuclides in soils but they are not generally applicable because they require many input parameters that are specific for each site. The behaviour of radionuclides in soil is confirmed as a complex phenomenon that depends on a number of physical, chemical and biological properties of the soil. In addition to the soil parameters examined in this study, in further investigations bioturbation should be analysed, by including data on populations and activities of (micro)organisms and root distributions.Acknowledgements
This work was supported by the Ministry of Education and Science of the Republic of Serbia (projects III43009 and 173030).References
- UNSCEAR (United Nations Scientific Committee on the Effect of Atomic Radiation), Sources and Effects of Ionizing Radiation, UNSCEAR 2008 Report, United Nations, New York, 2010 Search PubMed.
- M. Dowdal and J. O'Dea, J. Environ. Radioact., 2002, 59, 91–104 CrossRef.
- P. L. Nimis, Studia Geobot., 1996, 15, 3–49 Search PubMed.
- B. Rafferty, M. Brennan, D. Dawson and D. Dowding, J. Environ. Radioact., 2000, 48, 131–143 CrossRef CAS.
- L. E. C. Ciuffo, M. Belli, A. Pasquale, S. Menegon and H. R. Velasco, Sci. Total Environ., 2002, 295, 69–80 CrossRef CAS.
- A. Navas, J. Soto and J. Machín, Eur. J. Soil Sci., 2002, 53, 629–638 CrossRef.
- A. Navas, J. Machín and J. Soto, Soil Sci., 2005, 170, 743–757 CrossRef CAS.
- P. Blanco Rodríguez, F. Vera Tomé, J. C. Lozano and M. A. Péréz-Fernández, J. Environ. Radioact., 2008, 99, 1247–1254 CrossRef.
- A. Buccianti, C. Apollaro, A. Bloise, R. De Rosa, G. Falcone, F. Scarciglia, A. Tallarico and G. Vecchio, Geoderma, 2009, 152, 145–156 CrossRef CAS.
- M. Belivermis, Ö. Kiliç, Y. Çotuk and S. Topcuoğlu, Environ. Monit. Assess., 2009, 163, 15–26 CrossRef.
- D. Krstić, D. Nikezić, N. Stevanović and M. Jelić, Appl. Radiat. Isot., 2004, 61, 1487–1492 CrossRef.
- S. Nenadović, M. Nenadović, I. Vukanac, A. Djordjević, S. Dragićević and M. Lješević, Nucl. Technol. Radiat. Prot., 2010, 25, 30–36 CrossRef.
- European Soil Bureau Network, Soil Atlas of Europe, European Commission, Office for Official Publications of the European CommunitiesLuxembourg 2005 Search PubMed.
- A. J. VandenBygaart and R. Protz, Can. J. Soil Sci., 1995, 75, 73–84 CrossRef.
- A. Baeza, M. Del Río, A. Jiménez, C. Miró and J. Paniagua, J. Radioanal. Nucl. Chem., 1995, 189, 289–299 CrossRef CAS.
- A. Navas, J. Soto and J. Machín, Appl. Radiat. Isot., 2002, 57, 579–589 CrossRef CAS.
- T. Tsai, C. Liu, C. Chuang, H. Wei and L. Men, J. Radioanal. Nucl. Chem., 2011, 288, 927–936 CrossRef CAS.
- A. Baeza, M. Del Río, A. Jiménez and C. Miró, Radiochim. Acta, 1995, 68, 135–140 CAS.
- R. A. Ligero, I. Ramos-Lerate, M. Barrera and M. Casas-Ruiz, J. Environ. Radioact., 2001, 57, 7–19 CrossRef CAS.
- T. Taboada, A. Martínez Cortizas, C. García and E. García-Rodeja, Sci. Total Environ., 2006, 356, 192–206 CrossRef CAS.
- H. A. M. Al-Trabulsy, A. E. M. Khater and F. I. Habbani, Arabian J. Chem., 2010 DOI:10.1016/j.arabjc.2010.10.001/.
- S. Dragović, N. Mihailović and B. Gajić, Chemosphere, 2008, 72, 491–495 CrossRef.
- M. Komatina and S. M. Komatina, in Groundwater in the Urban Environment. Selected City Profiles, ed. J. Chilton, Balkema, Rotterdam, 1999, pp. 317–322 Search PubMed.
- FAO (Food Agricultural Organization), World Reference Base for Soil Resources. A Framework for International Classification and Communication, World Soil Resources Reports No. 103, Food and Agriculture Organization of the United Nations, Rome, 2006 Search PubMed.
- ORTEC, Gamma Vision 32, Gamma-Ray Spectrum Analysis and MCA Emulation, ORTEC, Oak Ridge, Version 5.3, 2001 Search PubMed.
- R. A. Sutherland, Hydrol. Processes, 1992, 6, 215–225 CrossRef.
- D. L. Rowell, Bodenkunde. Untersuchungsmethoden und ihre Anwendungen, Springer, Berlin, 1997 Search PubMed.
- ISO 10390:2005, Soil Quality—Determination of pH, International Standard Organization, Geneva Search PubMed.
- L. P. Van Reeuwijk, Procedures for Soil Analysis. Technical Paper 9, International Soil Reference and Information Centre (ISRIC), Wageningen, 1986, p. 106 Search PubMed.
- ISO 10693:1995, Soil Quality—Determination of carbonate content–Volumetric Method, International Standard Organization, Geneva Search PubMed.
- H. Kappen, Die Bodenaziditaät, Springer Verlag, Berlin, 1929 Search PubMed.
- G. R. Blake and K. H. Hartge, in Methods of Soil Analysis. Part 1-Physical and Mineralogical Methods, ed. A. Klute, Soil Science Society of America, Madison, WI, 2nd edn, 1986, pp. 363–382 Search PubMed.
- A. Klute and C. Dirksen, in Methods of Soil Analysis. Part 1-Physical and Mineralogical Methods, ed. A. Klute, Soil Science Society of America, Madison, WI, 2nd edn, 1986, pp. 687–734 Search PubMed.
- ISO 11265:1994, Soil Quality—Determination of the Specific Electrical Conductivity, International Standard Organization, Geneva Search PubMed.
- USEPA (United States Environmental Protection Agency), Method 3050B: Acid Digestion of Sediments, Sludges, and Soils, Revision 2, Washington DC, 1996 Search PubMed.
- SPSS (Statistical Package for the Social Sciences) 16.0, Chicago, Illinois, 2007.
- S. Dragović, Lj. Janković, A. Onjia and G. Bačić, Radiat. Meas., 2006, 41, 611–616 CrossRef.
- Lj. Janković-Mandić and S. Dragović, Radiat. Prot. Dosim., 2010, 140, 369–377 CrossRef.
- H. I. El-Reefy, T. Sharshar, R. Zaghloul and H. M. Badran, J. Environ. Radioact., 2006, 87, 148–169 CrossRef CAS.
- R. Fujiyoshi and S. Sawamura, Sci. Total Environ., 2004, 320, 177–188 CrossRef CAS.
- Z. Pietrzak-Flis, I. Radwan, L. Rosiak and E. Wirth, Sci. Total Environ., 1996, 186, 243–250 CrossRef CAS.
- S. Yoshida, Y. Muramatsu, A. M. Dvornik, T. A. Zhuchenko and I. Linkov, J. Environ. Radioact., 2004, 75, 301–313 CrossRef CAS.
- Ö. Karadeniz and G. Yaprak, Radiat. Prot. Dosim., 2008, 131, 346–355 CrossRef.
- Federal Secretariat for Labour, Health, Veterans Affairs and Social Policy, Radioactivity of the Environment of Yugoslavia, Belgrade, 1990 Search PubMed.
- J. P. Absalom, S. D. Young, N. M. J. Crout, A. Sanchez, S. M. Wright, E. Smolders, A. F. Nisbet and A. G. Gillett, J. Environ. Radioact., 2001, 52, 31–43 CrossRef CAS.
- N. Kruyts and B. Delvaux, J. Environ. Radioact., 2002, 58, 175–190 CrossRef CAS.
- J. T. Smith and D. G. Elder, Eur. J. Soil Sci., 1999, 50, 295–307 CrossRef CAS.
- A. Bruckmann and V. Wolters, Sci. Total Environ., 1994, 157, 249–256 CrossRef.
- G. Tóth, L. Montanarella, V. Stolbovoy, F. Máté, K. Bódis, A. Jones, P. Panagos and M. Van Liedekerke, Soils of the European Union, JRC Scientific and Technical Reports, Office for Official Publications of the European Communities, Luxembourg, 2008 Search PubMed.
- K. M. Miller, J. L. Kuiper and I. K. Helfer, J. Environ. Radioact., 1990, 12, 23–47 CrossRef CAS.
- P. Bosew and G. Kirchner, J. Environ. Radioact., 2004, 73, 127–150 CrossRef.
- USDA (United States Department of Agriculture), Soil Taxonomy. A Basic System of Soil Classification for Making and Interpreting Soil Surveys, Handbook No. 436. Soil Survey Staff, Washington DC, 1999 Search PubMed.
- M. Jasinka, T. Niewiadomski and J. Schwbenthan, in Natural Radiation Environment, ed. K. Vohra, U. C. Mishra, K. C. Pillai and S. Sadasivan, Willey, New York, 1982, pp. 206–211 Search PubMed.
- A. Cremers, A. Elsen, P. De Preter and A. Maes, Nature, 1988, 335, 247–249 CrossRef CAS.
- H. Koch-Steindl and G. Pröhl, Radiat. Environ. Biophys., 2001, 40, 93–104 CrossRef CAS.
- Z. P. Tomić, A. R. Đorđević, M. B. Rajković, I. Vukašinović, N. S. Nikolić, V. Pavlović and Č.M. Lačnjevac, Sens. Transducers J., 2011, 125, 115–130 Search PubMed.
- C. Elejalde, M. Herranz, F. Romero and F. Legarda, Water, Air, Soil Pollut., 1996, 89, 23–31 CrossRef CAS.
- C. A. Campbell, in Soil Organic Matter (Developments in Soil Science), ed. M. Schnitzer and S. V. Khan, Elsevier, Amsterdam, 1978, vol. 8, ch. 5, pp. 173–265 Search PubMed.
- F. A. Vega, E. F. Covelo, M. L. Andrade and P. Marcet, Anal. Chim. Acta, 2004, 524, 141–150 CrossRef CAS.
- H. Vandenhove and M. Van Hees, Chemosphere, 2007, 69, 664–674 CrossRef CAS.
- E. R. Van der Graaf, R. L. Koomans, J. Limburg and K. De Vries, Appl. Radiat. Isot., 2007, 65, 619–633 CrossRef CAS.
- J. H. Chao and C. Y. Chuang, Appl. Radiat. Isot., 2011, 69, 261–267 CrossRef CAS.
- E. D. van Hullebusch, P. N. L. Lens and H. H. Tabak, Rev. Environ. Sci. Bio/Technol., 2005, 4, 185–212 CrossRef.
- USEPA (United States Environmental Protection Agency), Understanding Variation in Partition Coefficient, Kd, values, EPA 402-R-04-002C, Washington DC, 2004, vol. 3 Search PubMed.
- J. S. Nathwani and C. R. Phillips, Chemosphere, 1979, 8, 293–299 CrossRef CAS.
- N. C. Sturchio, J. L. Banner, C. M. Binz, L. B. Heraty and M. Musgrove, Appl. Geochem., 2001, 16, 109–122 CrossRef CAS.
- S. Ehlken and G. Kirchner, J. Environ. Radioact., 2002, 58, 97–112 CrossRef CAS.
- B. J. Howard and G. Desmet, Sci. Total Environ., 1993, 137, 1–315 CrossRef.
| This journal is © The Royal Society of Chemistry 2012 |
