Identification of tetramethylarsonium in rice grains with elevated arsenic content†
Helle R.
Hansen
*a,
Andrea
Raab
ab,
Adam H.
Price
a,
Guilan
Duan
c,
Yongguan
Zhu
c,
Gareth J.
Norton
a,
Jörg
Feldmann
b and
Andrew A.
Meharg
a
aInstitute of Biological and Environmental Sciences, University of Aberdeen, Cruickshank Building, St Machar Drive, Aberdeen, UK AB24 3UU. E-mail: h.r.hansen@abdn.ac.uk
bTESLA (Trace Element Speciation Laboratory) and Marine Biodiscovery Laboratory, University of Aberdeen, Aberdeen, Scotland, UK AB24 3U
cResearch Center for Eco-environmental Sciences, Chinese Academy of Sciences, Beijing, 100085, China
First published on 15th November 2010
Abstract
Tetramethylarsonium has for the first time been identified in a commercially grown food product, rice, constituting up to 5.8% of the total arsenic in the rice.
Environmental impactMillions of people are exposed to arsenic through consumption of contaminated drinking water or foods, such as rice. Here we identify tetramethylarsonium in rice grain, a cationic arsenic species normally not associated with a terrestrial food source. We propose that future studies on uptake, transformation and metabolism of arsenic associated with the terrestrial environment will have to include analytical techniques also capable of unequivocally identifying cationic arsenic species, which elutes in the void volume of traditionally applied analytical methods based on anion exchange chromatography. In order to understand the mechanism of arsenic uptake in plants and the correlation between exposure and toxic effect in human, it is essential to obtain the complete picture of the arsenic species actually present at each stage. |
Arsenic speciation in the environment is complex, existing in both inorganic and organic forms, with interconversion between species regulated by biotic and abiotic processes.1Anion exchange chromatography (AEC), coupled with an element specific detector such as inductively coupled plasma mass spectrometry (ICP-MS), is a commonly used method for separating between inorganic As (As(III) and As(V)) and organic As (methylarsonic acid (MA(V)) and dimethylarsinic acid (DMA(V))) present in clinical2 and biological3 samples. When using a strong AEC column, such as the Hamilton PRP-X100 column, with a mobile phase of either ammonium phosphate, nitrate, sulfate, carbonate or a combination of these at pH 6–9,2–4As(III), DMA(V), MA(V) and As(V) can be conveniently separated (Fig. 1). Arsenite (As(III)), however, elutes near the void volume, potentially co-eluting with cationic As species. A slight separation between cationic As species, such as arsenobetaine (AB) and As(III), may be obtained, and the method has been used for separating AB, As(III), DMA(V), MA(V), and As(V) in blood and urine, although AB, As(III), and DMA(V) were not baseline separated.4 To avoid any co-elution, hydrogen peroxide may be added to samples in order to oxidise As(III) to As(V), thus minimizing the As(III) peak to ensure baseline separation. Due to the complexity of biological samples this oxidation is often incomplete and the amount of inorganic As still has to be calculated as the sum of the As(III) and As(V) peaks. Analysis for As species, using AEC-ICP-MS,3 was conducted upon extracts of rice grain‡ from a field site geogenically elevated in As in the Hunan province, China (latitude 26°45′ and longitude 111°52′). The total As concentrations in the rice grains were 0.38–2.0 mg kg−1 dry mass (mean of 0.71 ± 0.23 mg kg−1, n = 142). (CRM NIST 1568a rice powder was analysed throughout as quality control for the total determinations; the certified value of total As was 0.29 ± 0.03 mg kg−1 and the measured was 0.26 ± 0.02 mg kg−1 (n = 25)). It was observed that when increasing the amount of hydrogen peroxide added to the extracts of rice (100/500 µL of sample), and using a new (and thus with high resolution) Hamilton PRP-X100 column (PEEK 250 × 4.6 mm), a significant void volume As peak (Rt 2.2 min) was baseline separated from the As(III) peak (Fig. 1). The peak was present in 123 out of 142 samples analysed, constituting up to 8.1% of the sum of speciation or up to 5.8% of the total As in the rice (Table 1). No correlation was found between the concentration of the front peak and total As concentration or the sum of speciation. As many different genotypes (38) of rice were analysed a lack of correlation was not unexpected. The As speciation results of the CRM NIST 1568a rice powder (organic As: 168 ± 7 µg kg−1, inorganic As: 76 ± 9 µg kg−1 and the sum of species/total As: 85 ± 5.4%, n = 9) are comparable to previously reported values (organic As: 143–185 µg kg−1 and inorganic As: 80–109 µg kg−1)5 (no certified values exists).
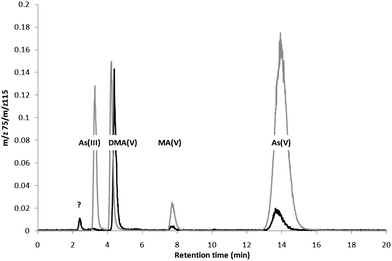 | ||
| Fig. 1 AEC-ICP-MS chromatograms of rice extract (black) and a standard solution mixture (grey). | ||
| Rice variety | Total As in grain µg kg−1 dry mass | Front peak by AEC as % of speciation (sum of species/total) | Tetra by CEC as % of speciation (sum of species/total) |
|---|---|---|---|
| Rathuwee | 599 | 6.7 (87%) | 4.8 (70%) |
| Shirkati | 697 | 4.3 (102%) | 3.2 (68%) |
| Rathuwee | 533 | 8.1 (70%) | 6.8 (65%) |
| Al-chio-hong | 868 | 5.7 (66%) | 5.0 (62%) |
| Paung malaung | 785 | 2.7 (103%) | 2.1 (88%) |
| Kachilon | 1223 | 2.1 (95%) | 1.5 (87%) |
The identity of the unknown As species was investigated in six of the samples with the highest content, by using cation exchange chromatography (CEC)-ICP-MS. An already established CEC method6 using a Varian Ionosphere 5C column (150 × 4.6 mm) was applied for analysis of rice extracts (hydrogen peroxide omitted). With this method a species of As present in the rice extracts co-eluted with the tetramethylarsonium (Tetra) standard (Fig. 2A). The retention times of other cationic As species (such as AB, dimethylarsinoylethanol, trimethylarsine oxide (TMAO) and arsenocholine (AC), the latter two are shown in Fig. 2A) known to be strongly retained on the column were also checked, but these had significantly different retention times from the unknown As species in the rice extracts. When spiking the rice extracts with a standard of Tetra, the co-elution was confirmed (Fig. 2B). A different CEC system,§ with a minor mobile phase gradient change and thus insignificant influence on the As response factor, was used for quantifying Tetra in the rice extracts. Comparable amounts to those determined by AEC were observed (Table 1).
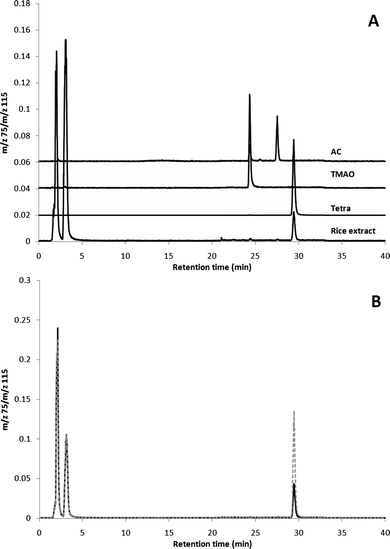 | ||
| Fig. 2 CEC-ICP-MS chromatograms of (A) a rice extract and standards (all offset) of Tetra, trimethylarsine oxide (TMAO) and arsenocholine (AC) and (B) of rice extract (black) and the extract spiked with 5 µg L−1Tetra (grey). | ||
In order to ensure that the occurrence of Tetra in the rice extracts was not due to microbiological activity during the overnight extraction procedure, extractions were repeated without the overnight extraction step and samples were immediately frozen after the microwave assisted extraction. The occurrence of Tetra may also be potentially due to thermal decomposition of tetraalkylated As species during the microwave extraction. There have been numerous reports on As species stability and conversion during thermal treatment,7 and Tetra is a known degradation product of AB, although only in small amounts (0.1–2%) at temperatures >155 °C.8 Nevertheless, to rule out that Tetra in the rice grains was due to thermal decomposition, extraction was also performed by ultrasonication (30 min) in 1% HNO3 or in a methanol/water mixture (9 + 1) (the latter also for excluding microbiological activity), prior to overnight extraction at 4 °C followed by freezing of samples. Tetra was present in all the different extracts.
To confirm the identity of Tetra in the extracts of rice, high-resolution electro spray (ES) mass spectra were obtained of purified samples on an Orbitrap Discovery (Thermo Scientific, UK) at a resolution of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. For purification, fractions of the rice extracts were collected from the CEC system6 at time 28.2–30.3 min, freeze dried, and re-dissolved in water prior analysis.¶ The accurate mass obtained of a Tetra standard was 135.01472 and the unknown in the purified rice extracts was 135.01474,† (mass accuracy of 1.7 and 1.5 ppm error, respectively, theoretical mass of C4H12As+ is 135.01495).
000. For purification, fractions of the rice extracts were collected from the CEC system6 at time 28.2–30.3 min, freeze dried, and re-dissolved in water prior analysis.¶ The accurate mass obtained of a Tetra standard was 135.01472 and the unknown in the purified rice extracts was 135.01474,† (mass accuracy of 1.7 and 1.5 ppm error, respectively, theoretical mass of C4H12As+ is 135.01495).
This is the first report on the presence of Tetra in rice grains (22–35 µg kg−1 dry mass). Mandal et al.9 have previously reported the presence of AB at similar concentrations (8.2–25.8 µg kg−1 dry mass) in extracts of rice from a range of sources (Indian, Japanese, and Thai rice). In that study identification was based on the co-elution between As species in the extracts and standards on an anion exchange and a size exclusion HPLC column. This can only be rated as a tentative identification, since no molecular mass spectrum and no characteristic retention time on a CEC using spiking were attempted, so it can be speculated that Tetra was misidentified as AB. Although Tetra has been mostly associated with marine organisms (mg kg−1 level),10 it has previously been identified at trace levels (low µg kg−1 levels) in several terrestrial plant tissues1 (moss, Cocksfoot grass, Red clover, Ribwort plantain, Green spleenwort, Broad buckler fern, Wild strawberry, Cowberry, and Monkey flower). It is uncertain if the occurrence of Tetra in terrestrial plants originates in soil or is produced in the plants. The main organoarsenic species reported in soil are MA(V), DMA(V),11 and TMAO,12 and their occurrence has been attributed to microorganisms, as both fungi and bacteria are capable of methylating inorganic arsenic.13Tetra, however, seems a less commonly occurring species in the soil environment, but it has been identified at trace level in porewaters of acidic fen soil.14 The origin of Tetra in the Chinese rice analysed in this study can only be speculated at. Tetra may potentially have been present in the paddy soil that the rice grew in, in which case its presence can be attributed to bacteria, fungi (higher fungi are known to produce Tetra),11 or algae, as algae growth is associated with the fields.
Conclusions
The significant presence of Tetra in some rice, and the importance of being able to separate cationic As species from other As species, has been demonstrated in this paper. Unless precaution is taken, Tetra will be counted in the inorganic arsenic fraction when performing As speciation with AEC, as it co-elutes with As(III).Acknowledgements
This work was funded by BBSRC-DFID grant BBF0041841. The authors would like to thank the Red Soil Experimental Station, Chinese Academy of Agricultural Sciences for conducting the field experiment at Qiyang. The rice material was obtained from Dr Susan McCouch, Cornell University, and the plant material was imported into the UK under import license IMP/SOIL/18/2009 issued by Science and Advice for Scottish Agriculture.References
- A. Meharg and J. Hartley-Whitaker, New Phytol., 2002, 154, 29 Search PubMed.
- C. Yuan, X. Lu, N. Oro, Z. Wang, Y. Xia, T. J. Wade, J. Mumford and X. C. Le, Clin. Chem. (Washington, DC, U. S.), 2008, 54, 163 CAS.
- G.-X. Sun, P. N. Williams, Y.-G. Zhu, C. Deacon, A.-M. Carey, A. Raab, J. Feldmann and A. A. Meharg, Environ. Int., 2009, 35, 473 CrossRef.
- T. I. Todorov, J. W. Ejnik, F. G. Mullick and J. A. Centeno, Microchim. Acta, 2005, 151, 263 CrossRef CAS.
- A. Raab, C. Baskaran, J. Feldmann and A. A. Meharg, J. Environ. Monit., 2009, 11, 41–44 RSC.
- C. Newcombe, A. Raab, P. N. Williams, C. Deacon, P. I. Haris, A. A. Meharg and J. Feldmann, J. Environ. Monit., 2010, 12, 832 RSC.
- V. Devesa, D. Vélez and R. Montoro, Food Chem. Toxicol., 2008, 46, 1 CrossRef CAS.
- V. Devesa, A. Martinez, M. A. Súñer, V. Benito, V. Vélez and R. Montoro, J. Agric. Food Chem., 2001, 49, 2267 CrossRef CAS.
- B. K. Mandal, K. Suzuki and K. Anzai, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2007, 42, 1741 Search PubMed.
- A. E. Geiszinger, W. Goessler and K. A. Francesconi, Environ. Sci. Technol., 2002, 36, 2905 CrossRef CAS.
- D. Kühnelt and W. Gössler, Organometallic Compounds in the Environment, Wiley, NY, 2003, pp. 223–275 Search PubMed.
- A. E. Geiszinger, W. Gössler and W. Kosmus, Appl. Organomet. Chem., 2002, 16, 245 CrossRef CAS.
- W. R. Cullen and K. J. Reimer, Chem. Rev., 1989, 89, 713 CrossRef.
- J. H. Huang and E. Matzner, Geochim. Cosmochim. Acta, 2006, 70, 2023 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Accurate mass ES-MS measurements. See DOI: 10.1039/c0em00460j |
| ‡ Sample of dried and milled rice grain (0.2 g) was left in 1% HNO3 (10 mL for AEC and 1 mL for CEC) overnight and subjected to microwave assisted extraction (0–55 °C in 5 min, hold for 5 min, 55–75 °C in 5 min, hold for 5 min, 75–95 °C in 5 min and hold for 30 min). |
| § Zorbax 300-SCX (150 × 4.5 mm) column, mobile phases of milliQ water and 50 mM pyridine formate at pH 2.7 with gradient elution (0–4 min; 0.5 mM, 4–16 min; 0.5–5 mM, 16–20 min; 5 mM, 20–30 min; 0.5 mM) at a flow rate of 1 mL min−1. |
| ¶ LC-MS analysis was performed on a Hamilton PRP-X200 pre-column with 4 mM nitric acid as carrier at a flow rate of 0.2 mL min−1. |
| This journal is © The Royal Society of Chemistry 2011 |
