Supramolecular assembly of biohybrid photoconversion systems†
Mateus B.
Cardoso‡
a,
Dmitriy
Smolensky§
a,
William T.
Heller
a,
Kunlun
Hong
b and
Hugh
O'Neill
*a
aCenter for Structural Molecular Biology, Oak Ridge National Laboratory, Chemical Sciences Division, Oak Ridge, TN 37831, USA. E-mail: oneillhm@ornl.gov; Fax: +1 865-574-8363; Tel: +1 865-574-5004
bCenter for Nanophase Materials Sciences, Oak Ridge National Laboratory, Oak Ridge, TN 37831, USA
First published on 22nd November 2010
Abstract
Self-assembled membrane architectures have great potential for the development of materials for the conversion of solar energy into electricity or fuels. Discovering the design principles that promote self-assembly in natural photosynthetic systems may provide inspiration for the development of synthetic solar conversion systems. We report for the first time that naturally occurring light harvesting antennae can alter the phase behavior of a poly(ethylene oxide)-block-poly(propylene oxide)-block-poly(ethylene oxide) (PEO–PPO–PEO) block copolymer system from micellar to lamellar structures mimicking their role in maintaining the supramolecular architecture of the photosynthetic membrane. Small-angle neutron scattering shows that PEO43–PPO16–PEO43 micelles undergo a phase transition from a micellar state to a lamellar structure with a ∼60 Å spatial repetition in the presence of plant light harvesting complex II (LHCII). In addition, spectrophotometric analysis indicates that the protein self-assembles in the synthetic membrane structure. Photodependent hydrogen production mediated by LHCII embedded in the block copolymer had a maximum rate of 6.4 µmol h−1 per mg chlorophyll. The production of H2 was sustained for greater than 100 hours showing the potential of this approach for the development of self-assembled bioinspired photoconversion systems. Although excited energy transfer is the primary function of LHCII, this work provides evidence that the protein complex can also perform electron transfer, a role not known to occur in vivo. The significance of this work is that it provides a novel approach for developing a new class of membrane-based smart material with a well-controlled architecture that is dependent on the assembly of interacting components, and it could also have important implications in self-repair and control of energy transfer in photoconversion devices.
Broader contextNew systems and design strategies must be pursued to develop materials that allow fuels such as H2, to be photogenerated with photons that match the solar spectrum, in order to satisfy a significant fraction of the global energy demand for clean renewable energy. One approach to achieve this goal is to extract the highly efficient photosynthetic components from plants and incorporate them into robust nanostructured systems to fabricate engineered biomimetic hybrid devices. This paper reports a biohybrid system that combines amphiphilic block copolymers with the natural light harvesting antenna protein, light harvesting complex II (LHCII). A key aspect of the work is that LHCII can alter the phase behavior of the block copolymer system from micellar to lamellar structures mimicking the role the protein plays in maintaining the supramolecular architecture of the photosynthetic membrane. The biohybrid composite structures support photodependent H2 production for extended time periods (>100 h) viaplatinum nanoparticles formed in situ, mediated by photoactivation of LHCII. This work demonstrates a novel approach for developing a new class of smart materials with architectures that are dependent on the assembly of interacting components. These design considerations could have important implications in self-repair and control of energy transfer in photoconversion devices. |
Introduction
The conversion of solar energy to electricity or chemical fuels by artificial photosynthesis is one of the most challenging goals in modern chemistry.1–3 Bioinspired photosynthesis research seeks to develop a system that takes inspiration from natural photosynthesis to synthetically emulate its exquisite architecture and function while simultaneously producing a more robust and controllable system.4 Although current technological approaches have not yet achieved the density of the molecular circuitry and organization that is found in natural photosynthetic systems, there have been significant advances in re-designing the photosynthetic apparatus either by integrating the protein complexes into electrode materials or by de novo synthesis of mimics of the protein complexes.3,5–9Natural photosynthetic systems can also provide inspiration for control and organization of the supramolecular structure of synthetic systems through their natural tendency to self-assemble. Light harvesting complex II (LHCII) is an integral membrane protein in the plant chloroplast thylakoid membrane.10,11 It binds and orients chlorophyll (Chl) molecules such that they direct photons to the photosynthetic reaction centers and it functions in the dissipation of excess excitation energy under high-light conditions. In addition, LHCII plays a major role in imposing a bilayer configuration in the photosynthetic membrane. In its absence, the membrane lipids assume an inverted hexagonal phase and under physiological conditions the presence of LHCII produces the membrane's supramolecular structural organization through its interaction with the lipids (Fig. 1).12
 | ||
| Fig. 1 Schematic illustration of the role of LHCII in the structural organization of the thylakoid membrane of plants. | ||
In recent years, biologically inspired synthetic strategies have emerged in which the molecular building blocks self-assemble in particular patterns to form higher-order supramolecular complexes.13,14 The main challenge is producing the so-called ‘smart materials’ that form homogeneous and well-defined structures that respond to external stimuli in a useful and predictable manner.15 One class of candidate smart materials are block copolymers that can self-assemble into nanostructures with tunable phase morphology that is dependent on their chemical composition.16 Poly(ethylene oxide)–poly(propylene oxide)–poly(ethylene oxide) block copolymers (PEO–PPO–PEO) are amphiphilic triblock copolymers that can aggregate into micelles in aqueous solution.17 They are available commercially (for example, Pluronic®, BASF Corporation) with various ratios of hydrophilic (PEO) to hydrophobic (PPO) blocks. In addition to their uses as detergents, coating agents and emulsifiers, PEO–PPO–PEO block copolymers are biocompatible and have been investigated as drug delivery systems and for gene therapy.18 The structure and shape of the micelles formed depend on the composition and molecular mass of the polymers, concentration, and temperature of the polymer solution. At high concentration, several of PEO–PPO–PEO block copolymers are known to undergo thermo-reversible gelation into lyotropic crystalline phases.17
Here, we report on the interaction of LHCII with PEO–PPO–PEO block copolymers to determine the structural design principles for the discovery of synthetic architectures for solar energy conversion. In aqueous solution, the PEO43–PPO16–PEO43 block copolymer studied forms compact micelles even in the presence of the detergents required to purify LHCII from the native membrane. The addition of LHCII–detergent complex to the block copolymer drives the formation of lamellar structures while the protein retains its spectroscopic signature in the presence of the block copolymer. Further, the LHCII can mediate photodependent hydrogen production catalyzed by platinum nanoparticles in the presence of PEO43–PPO16–PEO43. The results demonstrate that natural proteins from the photosynthetic apparatus can be used in conjunction with polymeric scaffolds to produce functional photosynthetic materials.
Results and discussion
The stability of LHCII in PEO–PPO–PEO solutions
The spinach LHCII protein was isolated and purified as described previously.19 The interaction of LHCII with a range of PEO–PPO–PEO block-copolymers was investigated using circular dichroism (CD) spectroscopy to determine how the molecular mass and relative lengths of the hydrophilic and hydrophobic blocks of the copolymer impact the stability of LHCII. A decrease in the circular dichroism signal relative to a control sample is indicative of unfolding due to the release of chlorophyll from the protein.20PEO–PPO–PEO polymers with masses between 2500 and 6500 g mol−1 and hydrophobic blocks ranging from 900 to 3000 g mol−1 were tested (Table S1†). Fig. 2 shows that LHCII is stable in PEO–PPO–PEO polymers with hydrophobic blocks of less than 2000 g mol−1 at a polymer to protein ratio of 9000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for at least 48 hours. In contrast, for block-copolymers with hydrophobic blocks greater than 2000 g mol−1, the protein loses its activity within 12 hours.
1 for at least 48 hours. In contrast, for block-copolymers with hydrophobic blocks greater than 2000 g mol−1, the protein loses its activity within 12 hours.
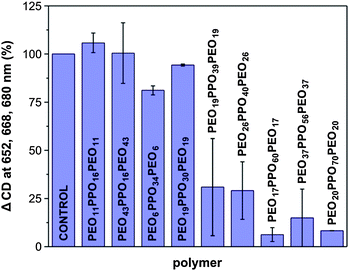 | ||
| Fig. 2 Investigation of the effect of PEO–PPO–PEO block copolymer composition on the stability of LHCII. The mol ratio of block copolymer to LHCII (0.09 mg protein ml−1; 0.03 mg Chl ml−1) was 9000 in 10 mM Tris–HCl pH 7.7 and 0.015% (m/v) dodecyl maltoside. Measurements were recorded after 24 h incubation (n = 2). See Table S1† for further details. | ||
PEO43–PPO16–PEO43, hereafter referred to as F38, was selected for further analysis because it was demonstrated that LHCII was stable in solutions of this polymer over a wide range of concentrations at polymer to protein mol ratios up to 9000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. In order to investigate if LHCII can modulate the phase behavior of F38 we postulated that relatively high concentrations of F38 would be required. Although, there was no prior data published for the phase behavior of F38, phase transitions in other block copolymer systems are generally observed at concentrations greater than ∼15 to 20% (w/w) in an aqueous solution.17 For LHCII stabilized in dodecyl maltoside (DM), the protein
1. In order to investigate if LHCII can modulate the phase behavior of F38 we postulated that relatively high concentrations of F38 would be required. Although, there was no prior data published for the phase behavior of F38, phase transitions in other block copolymer systems are generally observed at concentrations greater than ∼15 to 20% (w/w) in an aqueous solution.17 For LHCII stabilized in dodecyl maltoside (DM), the protein![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) detergent
detergent![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) F38 mol ratio was 1
F38 mol ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 375
375![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2667–4417, depending on the final concentration of block copolymer.
2667–4417, depending on the final concentration of block copolymer.
Circular dichroism analysis
The excitonic couplings between adjacent chlorophylls in LHCII can be observed in its visible CD spectrum which is very sensitive to pigment–pigment interactions and can distinguish between monodisperse and aggregated states of purified LHCII preparations.20–22 The CD spectrum of LHCII solubilized in DM at protein to block copolymer ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3550 and the control spectra in the absence of the block copolymer are shown in Fig. 3A. The spectrum is similar to previously reported spectra for LHCII.23,24 In the blue region, main excitonic bands are at (−) 438, (+) 446, (−) 460, (+) 483 and (−) 492 nm, while in the red region, the main bands are found at (−) 652, (+) 668 and (−) 680 nm.
3550 and the control spectra in the absence of the block copolymer are shown in Fig. 3A. The spectrum is similar to previously reported spectra for LHCII.23,24 In the blue region, main excitonic bands are at (−) 438, (+) 446, (−) 460, (+) 483 and (−) 492 nm, while in the red region, the main bands are found at (−) 652, (+) 668 and (−) 680 nm.
 | ||
Fig. 3 The effect of F38 on the spectroscopic properties of LHCII solubilized in DM. A, B, and C panels are the circular dichroism, UV visible, and linear dichroism spectra, respectively. The dashed lines represent the control samples (no F38), while the solid lines are the spectra of the LHCII solutions in 20% (w/w) F38. The mol ratios of LHCII![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) detergent: detergent:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PEO43–PPO16–PEO43 were 1 PEO43–PPO16–PEO43 were 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 375: 375:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3550 and the concentration of LHCII was 1.42 mg ml−1 (0.46 mg Chl ml−1) and 0.23% (w/v) DM. 3550 and the concentration of LHCII was 1.42 mg ml−1 (0.46 mg Chl ml−1) and 0.23% (w/v) DM. | ||
The (−) 460 nm band has previously been reported to change in response to the aggregation state of LHCII. A shoulder at 460 nm is indicative of monodisperse trimeric LHCII, while a minimum is usually observed for aggregated states of the protein.23,24 In the LHCII–DM control sample there is a pronounced shoulder at 460 nm, indicating that the protein is at least partially solubilized by the detergent.25 The detergent concentration of this sample is 0.23% (w/v) which is approximately 28 times higher than the critical micelle concentration (0.0082% (w/v)). However, due to the relatively high concentration of protein, the chlorophyll to detergent mol ratio is low at approximately 9. It has previously been reported that complete solubilization of LHCII requires a Chl/DM ratio of 286.25
The CD spectrum of LHCII–DM–F38 is very similar to the control in the red region except that the peak at 670 nm is slightly shifted to longer wavelengths. However, the differences in the blue region are more pronounced. The (−) 460 nm band is a minimum and the CD between 460 and 500 nm is shifted to more positive values. These changes are consistent with aggregation of LHCII as reported in previous investigations.23,24 The shape of the spectrum is similar to the loosely stacked lamellar aggregates of LHCII described by Simidjiev et al.24 except for a less negative 680 nm excitonic band. CD spectra were also recorded for the protein to block copolymer ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2667 and 1
2667 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4417 for LHCII–DM and are characteristic of LHCII in an aggregated state (see ESI, Fig. S1†).
4417 for LHCII–DM and are characteristic of LHCII in an aggregated state (see ESI, Fig. S1†).
Linear dichroism analysis
Linear dichroism (LD) refers to the differential absorption of plane-polarized light by an oriented sample and occurs because molecules fixed in space absorb plane-polarized light maximally in well-defined molecular directions.26 Previously, using techniques such as LD and polarized fluorescence it has been demonstrated that, in vivo, chlorophyll tends to be highly oriented in the native thylakoid membrane.27,28 The linear dichroic properties of LHCII are observed when trimers form higher order aggregates upon exposure to a variety of conditions. This gives rise to large changes in spectroscopic properties of bound pigments.The visible absorption (Fig. 3B) and the corresponding LD spectra (Fig. 3C) of LHCII–DM at protein to F38 ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3550 as well as the controls in the absence of the block copolymer are shown. The shape of the LD spectrum of the LHCII–DM–F38 sample is similar to previously reported studies except that the δA of the spectrum is negative. The sign of the LD spectra is related to the method of sample preparation. For instance, a positive LD spectrum can be obtained by entrapping the LHCII in polyacrylamide gels followed by 2-dimensional squeezing perpendicular to the incident light.21 On the other hand, a negative LD spectrum is obtained by using an electric field for sample alignment.29 In this study the samples were compressed between detachable wall quartz cuvettes to form thin-films (100 µm) prior to measurement.
3550 as well as the controls in the absence of the block copolymer are shown. The shape of the LD spectrum of the LHCII–DM–F38 sample is similar to previously reported studies except that the δA of the spectrum is negative. The sign of the LD spectra is related to the method of sample preparation. For instance, a positive LD spectrum can be obtained by entrapping the LHCII in polyacrylamide gels followed by 2-dimensional squeezing perpendicular to the incident light.21 On the other hand, a negative LD spectrum is obtained by using an electric field for sample alignment.29 In this study the samples were compressed between detachable wall quartz cuvettes to form thin-films (100 µm) prior to measurement.
The LD spectrum of the LHCII–DM–F38 displays minima at (−) 680 nm and (−) 505 nm and a broad weak maximum (+) ∼420 nm indicating that A⊥ > A∥ and the proteins are preferentially aligned parallel to the direction of the transmitted light.29 In contrast, the control LHCII–DM sample did not present a discernible signal indicating that there is little or no orientation evident in this sample. The peak at (−) 503 nm has also been observed in lamellar aggregates of LHCII and also in the LD spectra of whole chloroplasts and thylakoids.21,27 Therefore, the presence of these spectral features provides strong evidence for orientation of the LHCII trimers in LHCII–DM–F38 at a protein to block copolymer ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3550.
3550.
LD
spectra were also recorded for protein to block copolymer ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2667 and 1
2667 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4417 for LHCII in DM (see ESI, Fig. S2†). There was no discernible LD signal in the lower F38 concentration (1
4417 for LHCII in DM (see ESI, Fig. S2†). There was no discernible LD signal in the lower F38 concentration (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2667 ratio). At the highest block-copolymer concentration examined (1
2667 ratio). At the highest block-copolymer concentration examined (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4417 ratio), the LD signal was approximately 2 fold lower in the red region and the peak in the blue region was shifted to shorter wavelengths compared to the same LHCII–DM sample at the 1
4417 ratio), the LD signal was approximately 2 fold lower in the red region and the peak in the blue region was shifted to shorter wavelengths compared to the same LHCII–DM sample at the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3550 ratio. This may indicate a disruption of inter-protein interactions between LHCII complexes. A similar shift was reported when lamellar aggregates of LHCII were solubilized in DM at a Chl
3550 ratio. This may indicate a disruption of inter-protein interactions between LHCII complexes. A similar shift was reported when lamellar aggregates of LHCII were solubilized in DM at a Chl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DM ratio of 28625 which could indicate a disruption in the long range associations of the complexes. However, given that the LD spectrum of this sample is also similar to the absorption spectrum, the influence of spectral artifacts such as form or textural dichroism cannot be discounted here.27
DM ratio of 28625 which could indicate a disruption in the long range associations of the complexes. However, given that the LD spectrum of this sample is also similar to the absorption spectrum, the influence of spectral artifacts such as form or textural dichroism cannot be discounted here.27
Small-angle neutron scattering analysis
The small-angle neutron scattering (SANS) profiles of LHCII–DM at protein to block copolymer ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3550 and the control sample in the absence of the block copolymer are shown in Fig. 4A. Although the scattering profiles with and without protein are similar for q values > 0.13 Å−1, the scattering profile in the presence of LHCII has two distinct regions which are not observed in the polymer/detergent mixtures. Firstly, in the mid-q-region, the LHCII–DM–F38 sample has a well-defined peak at q ≈ 0.1 Å−1 which is indicative of the presence of a lamellar phase with repeat spacing of approximately 60 Å (Fig. 4A). In addition, in the low-q-region, the scattering profile of the protein-block copolymer sample differs greatly from the scattering profile of the sample without protein. A power-law fit to the scattering profiles in this region changes from a q∼0 regime in the absence of protein to a q−4 regime when the LHCII is added. A power-law slope of −4 occurs due to surface scattering from large structures and is consistent with scattering from the surfaces of the domains containing the lamellar stacks.
3550 and the control sample in the absence of the block copolymer are shown in Fig. 4A. Although the scattering profiles with and without protein are similar for q values > 0.13 Å−1, the scattering profile in the presence of LHCII has two distinct regions which are not observed in the polymer/detergent mixtures. Firstly, in the mid-q-region, the LHCII–DM–F38 sample has a well-defined peak at q ≈ 0.1 Å−1 which is indicative of the presence of a lamellar phase with repeat spacing of approximately 60 Å (Fig. 4A). In addition, in the low-q-region, the scattering profile of the protein-block copolymer sample differs greatly from the scattering profile of the sample without protein. A power-law fit to the scattering profiles in this region changes from a q∼0 regime in the absence of protein to a q−4 regime when the LHCII is added. A power-law slope of −4 occurs due to surface scattering from large structures and is consistent with scattering from the surfaces of the domains containing the lamellar stacks.
 | ||
Fig. 4
SANS analysis of the interaction of LHCII with F38. Panel A: the SANS profile of LHCII–DM–F38 is shown as squares and the solid line is the SANS profile of F38–DM. The mol ratios of LHCII![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DM DM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) F38 are 1 F38 are 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 375: 375:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3550. The concentration of LHCII was 1.42 mg ml−1 (0.46 mg Chl ml−1) and 0.23% (w/v) DM. The F38 concentration was 20% (w/w). A schematic lamellar structure is represented. Panel B: SANS data of LHCII solubilized and DM (circles) solution. Theoretical scattering profile of LHCII crystal structure (inset) is presented as the dashed line. Straight lines show the scattering decay of each sample as an indication of aggregation degree of the protein. 3550. The concentration of LHCII was 1.42 mg ml−1 (0.46 mg Chl ml−1) and 0.23% (w/v) DM. The F38 concentration was 20% (w/w). A schematic lamellar structure is represented. Panel B: SANS data of LHCII solubilized and DM (circles) solution. Theoretical scattering profile of LHCII crystal structure (inset) is presented as the dashed line. Straight lines show the scattering decay of each sample as an indication of aggregation degree of the protein. | ||
The experimental scattering curves of LHCII–DM in the absence of block copolymer and the simulated scattering profile of LHCII trimers calculated from its crystal structure10 are shown in Fig. 4B. The experimental and the theoretical scattering curves are similar in the mid-q-range but differ significantly at low q. A power-law fit to the theoretical LHCII scattering curve in the low q-region (0.006 < q < 0.020 Å−1) yields an exponent of ∼0, as has been previously demonstrated for monodisperse LHCII trimers in detergent solution.19 Deviation of the exponent to larger negative values, as observed in the scattering curves of the LHCII–DM (q∼−1), is indicative of the presence of larger order aggregates. This agrees well with the CD analysis which showed that the LHCII–DM is partially aggregated but maintains some of the features of a monodisperse system.
A model for the interaction of LHCII with F38 to form a lamellar structure can be proposed where the hydrophobic PPO block interacts with the hydrophobic region of the protein, similar to the interaction of LHCII with the aliphatic chains of lipids, while the hydrophilic PEO block extends into the solvent. The lamellar repetition of 60 Å, calculated based on the position of the peak at ∼0.1 A−1 in the SANS profile, is slightly larger than the thickness of the native thylakoid membrane (50 Å) as measured previously by SANS.30 A fit to the scattering profile of F38 in dilute solution (0.4% (w/w)) showed that it forms spherical micelles similar to other PEO–PPO–PEO block copolymers (see Fig. 5).31,32 The micelles have a dense spherical core (PPO) of radius Rc = 12.4 ± 2.1 Å and Gaussian chains (PEO) attached to the core with radius of gyration RG = 18.2 ± 3.4 Å.31 The average aggregation number (Nagg) is 38 ± 14 molecules per micelle. Fig. 6 shows the lateral view of the model in which the protein is shown side-by-side with a fully extended PEO–PPO–PEO copolymer. The previously calculated PPO size for PEO43–PPO16–PEO43 in the lamellar block-copolymer structure and the Rc value calculated here agree well with the average size of the lateral hydrophobic region of the protein which is around 25 Å. On the other hand, the length of an extended PEO block would be much larger than ∼35 Å necessary for completing the lamellar repetition (60 Å) indicating that PEO chains do not adopt a fully extended conformation in the membrane structure.
 | ||
| Fig. 5 Small-angle X-ray scattering (SAXS) profile of F38 micelles. The SAXS profile of 0.4% (w/w) F38 solution in H2O is presented with its corresponding fit assuming a spherical micelle of dense PPO spherical core with Gaussian PEO chains attached to the core. | ||
 | ||
| Fig. 6 Schematic representation of the interaction of F38 and LHCII in lamellar environment. In the LHCII crystal structure, blue, yellow, and green colors of the polypeptide backbone represent polar charged, polar uncharged, and hydrophobic amino acid residues, respectively. | ||
The scattering profiles recorded for the protein to block copolymer ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2667 and 1
2667 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4417 for LHCII solubilized in DM also exhibit a lamellar peak at ∼0.1 Å−1 (see ESI, Fig. S3A and B†). However, there is no clear correlation between the LD and SANS analysis. Unlike the 1
4417 for LHCII solubilized in DM also exhibit a lamellar peak at ∼0.1 Å−1 (see ESI, Fig. S3A and B†). However, there is no clear correlation between the LD and SANS analysis. Unlike the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3550 protein to copolymer ratio sample, these samples do not have a well-defined linear dichroic spectrum. It indicates that inter-protein interactions between adjacent LHCII molecules are not a prerequisite for the formation of biohybrid lamellar structures. However, the ability to direct inter-protein communication should be considered an advantage from a device architecture perspective.
3550 protein to copolymer ratio sample, these samples do not have a well-defined linear dichroic spectrum. It indicates that inter-protein interactions between adjacent LHCII molecules are not a prerequisite for the formation of biohybrid lamellar structures. However, the ability to direct inter-protein communication should be considered an advantage from a device architecture perspective.
Photodependent hydrogen production
The light-dependent production of molecular hydrogen mediated by the LHCII–DM was used as a measure of the catalytic activity of the biohybrid assembly in the presence of F38. The reaction pathway was similar to previously reported work33–35 except that LHCII was present as the photoactive component instead of photosystem I (PSI). No hydrogen was observed for the first 5 light cycles (Fig. 7) which can be attributed to the formation of the Pt catalyst initiated by the LHCII-catalyzed photoprecipitation of platinum from a sodium hexachloroplatinate solution. Previous investigations with PSI have shown that once sufficient platinum has been deposited (>50 atoms),36 photoevolution of hydrogen proceeds. The maximum rate of hydrogen production was obtained after the 14th light cycle (6.4 µmol H2 h−1 per mg Chl). However, the rate of H2 production remained relatively high for the duration of the experiment and was ∼4.8 µmol h−1 per mg Chl after the 24th light cycle (100 h) when the reaction was terminated. Control reactions carried out in the absence of LHCII produced no hydrogen. | ||
| Fig. 7 Light-dependent evolution of hydrogen mediated by LHCII in F38 solution. The reactions were carried out with LHCII (10 µM Chl), 20% (w/w) F38, 0.5 mM Na2PtCl6, and 20.0 mM sodium ascorbate in 20 mM sodium phosphate pH 7.0 at 25 °C. Light cycles of 2 h on and 2 h off were used at a constant light intensity of 600 µmol m−2 s−1. The arrow indicates the start of the first light cycle (0.9 h). | ||
Photoinduced hydrogen systems containing an electron donor, photosensitizer, an electron relay, and a hydrogen production catalyst have been widely studied for conversion of solar energy to chemical fuels. The maximum rate of hydrogen production is comparable to previously reported studies. In the case of LHCII, an average rate of 9.82 µmol H2 h−1 per mg Chl was estimated based on the yield of H2 reported during a 3 h experiment.37 The reaction pathway employed NADH as an electron donor, methyl viologen as a mediator, and Pt nanoparticles formed ex situ, at 30 °C. The maximum rate of hydrogen production for cyanobacterial PSI was 5.5 µmol h−1 per mg Chl using a reaction pathway similar to the one reported here except an electron relay protein was required to re-reduce the protein and the reaction temperature was 60 °C.35 Direct comparison of hydrogen production rates is difficult due to the differences in the electron transfer pathways to the Pt catalyst and the type and intensity of illumination used in each study. However, in the work reported here, sustainable H2 production for at least 100 h is demonstrated compared to 3 h or less in the other studies.
Excitation energy transfer (EET), the process by which antenna complexes absorb light and funnel the resulting excited state energy rapidly and efficiently to an acceptor moiety, is the primary function of antenna complexes such as LHCII.38 The final step in excitonic diffusion within the antenna system is transfer of energy to a special chlorophyll dimer that is present in photosynthetic reaction centers such as PSI. This represents the first step in the conversion of excitonic to redox or chemical energy where the special chlorophyll dimer in the reaction centers triggers charge separation and a subsequent relay of electron transfer steps. LHCII is only known for its role in EET processes and not for electron transfer. However, the work presented here provides evidence that LHCII can, in the absence of a natural photosynthetic reaction center acceptor, perform electron transfer, which is a role not known to occur in vivo.
A detailed examination of the X-ray crystal structure of LHCII10 has provided insight into the arrangement of chlorophylls within the complex and identified four chlorophyll dimers that are important for exciton diffusion within the trimer, terminating in a red-shifted chlorophyll a pair (Chl 611–612) that is responsible for energy transfer to an adjacent LHCII or reaction center.39 This chlorophyll pair is arranged in a similar manner to the special chlorophyll pairs found in photosynthetic reaction centers. One possible explanation for the ability of LHCII to mediate photoinduced hydrogen production is that in the absence of an adjacent LHCII or reaction center, a charge separation reaction is induced that results in an electron transfer from LHCII to a nearby Pt nanoparticle. The Pt catalyst is formed in situ by photocatalytic reduction of sodium hexachloroplatinate and therefore likely to be in close proximity to LHCII.
This work raises some important mechanistic questions, not least of which is that the photoevent that leads to H2 generation is not predicated on an excited state capable of participating in the two-electron chemistry required for H2 production. There are no redox shuttles present in the reaction to couple the one electron transfer of the photosensitizer to a H2 generating catalyst. This suggests that the charge separated state is stabilized by LHCII long enough that energy wasting recombination reactions do not occur, allowing the formation of H2. The mechanism for electron transfer from LHCII to the Pt catalyst and the stabilization of the charge separated state remain to be determined. The ability of LHCII to act as a mediator for photodependent H2 production in the presence of PEO–PPO–PEO polymers shows great promise for the development of a biohybrid solar fuel system.
Experimental
Isolation of LHCII
Commercially obtained spinach leaves were used as the starting material. All steps were carried out as described earlier with minor modifications.19 The supernatant from the thylakoid membrane solubilization step was subjected to a continuous sucrose gradient (0.1–1.0 M) containing 20 mM Tricine and 0.04% (w/v) Triton X-100 and centrifuged at 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm (Beckman Ti 70 rotor) for 5 h. In the final step, the pellets that contained the LHCII complexes were recovered, washed with 10 mM Tris–HCl buffer pH 7.6 containing 0.01% w/v dodecyl maltoside (DM) and centrifuged at 10
000 rpm (Beckman Ti 70 rotor) for 5 h. In the final step, the pellets that contained the LHCII complexes were recovered, washed with 10 mM Tris–HCl buffer pH 7.6 containing 0.01% w/v dodecyl maltoside (DM) and centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min. The pellets were then dissolved in 10 mM Tris–HCl buffer pH 7.6 containing 1.5% w/v DM and kept at 4 °C for 24 h. The LHCII preparation was centrifuged at 3000 rpm for 2 min to remove aggregated material and stored at 4 °C. The chlorophyll (Chl) content in LHCII was determined in 80% acetone as previously described.40 The protein content of LHCII was based on the known Chl content of LHCII (8 Chl a and 6 Chl b per monomer).
000 rpm for 5 min. The pellets were then dissolved in 10 mM Tris–HCl buffer pH 7.6 containing 1.5% w/v DM and kept at 4 °C for 24 h. The LHCII preparation was centrifuged at 3000 rpm for 2 min to remove aggregated material and stored at 4 °C. The chlorophyll (Chl) content in LHCII was determined in 80% acetone as previously described.40 The protein content of LHCII was based on the known Chl content of LHCII (8 Chl a and 6 Chl b per monomer).
Preparation of LHCII–PEO–PPO–PEO mixtures
Poly(ethylene oxide)–poly(propylene oxide)–poly(ethylene oxide) (PEO–PPO–PEO) block copolymers marketed by BASF Corporation under the trade name Pluronic® were received as a gift and used without further purification. For SANS experiments, a 40% (w/w) solution of F38 (PEO43–PPO16–PEO43) was prepared by dissolving the polymer in 10 mM Tris–DCl pD 7.7 in D2O and stirring for 24 h to ensure the complete dissolution of the polymer. The as-prepared polymer solutions were mixed with the detergent solubilized in LHCII to the desired concentration for SANS experiments. The final concentration of DM used in the experiments was chosen in order to maximize interactions between LHCII and the block copolymer. To investigate the stability of LHCII in the presence of PEO–PPO–PEO block copolymers, the protein was dissolved in block copolymers solutions that were prepared in 10 mM Tris–HCl pH 7.7 (see ESI, Table S1†).Spectrophotometry
The circular dichroism and linear dichroism spectra of the samples were recorded using a Jasco 810 spectropolarimeter at 25 °C. All samples were allowed to equilibrate at room temperature for 24 h prior to measurement. For the LHCII/PEO–PPO–PEO stability studies, the loss of CD signal was calculated based on the average change in CD intensity at 652, 668, and 680 nm, compared to the control sample. A detailed explanation is provided in the ESI†.Small-angle scattering
The SANS experiments were carried out on the Bio-SANS instrument at the High Flux Isotope Reactor of Oak Ridge National Laboratory.41SANS measurements were carried out at room temperature in 1.0 mm path quartz cuvettes. The appropriate buffer solutions were also collected for use in the data reduction. Scattering data and the associated backgrounds were recorded using two detector distances, specifically 1.1 m and 6.8 m, using a 1 m × 1 m position sensitive He3-detector. The wavelength was set to 6 Å with a wavelength spread, Δλ/λ, of 0.15. The detector settings provide scattering vectors (q) 0.0065 < q < 0.7 Å−1 (q = (4π/λ)sin (θ/2), λ is the neutron wavelength and θ is the scattering angle), which allowed sufficient angular coverage to enable background subtraction. Data reduction followed standard procedures to correct for dark current, detector response and transmission and solvent background prior to azimuthal averaging to produce the 1-dimensional scattering profile. The simulated scattering curve of LHCII was calculated from the LHCII trimetric crystal structure10 using the program ORNL_SAS.42SAXS measurements were performed on the 5-ID beamline at the Advanced Photon Source (APS-Argonne). The incident X-ray monochromatic beam (λ = 0.729 Å) was detected on a marCCD 165 detector (4 × 4 binning) placed 5084 mm away from the sample, covering a scattering vector q ranging from 0.008 to 0.25 Å−1. Polymer samples were flowed through a capillary tube while the collimated X-ray beam was passed horizontally through a chamber containing the sample. The measurements were performed at room temperature and each SAXS pattern was collected for 10 seconds. Five frames were recorded for each sample in order to check any polymer degradation. No evidence of polymer degradation was observed within 5 frames. Silver behenate powder was used as standard to calibrate the sample-to-detector distance, the detector tilt and the direct beam position. Transmission, dark current and capillary corrections were performed on the 2D image before further data processing. The isotropic scattering patterns were radially averaged. Finally, the scattering pattern obtained from the polymer was subtracted from the scattering pattern of water.
Photodependent hydrogen production
The 15 ml reaction contained the LHCII–DM protein preparation (9 µg chlorophyll ml−1; 10 µM), sodium hexachloroplatinate (0.5 mM), sodium ascorbate (20 mM), and 0.02 M Na phosphate pH 7.0. F38 was present in the reaction at a final concentration of 20% (w/w). Sodium ascorbate acted as a sacrificial electron donor to LHCII. The experimental apparatus for continuous measurement of hydrogen was described previously.33 After assembly of the reaction apparatus and equilibration of the reagents, the reaction was illuminated for 2 h on/off cycles using 660 nm light-emitting diodes (QBEAM 2001; Quantum Devices, Barneveld, WI) with an intensity of 600 µE m−2 s−1, as measured by a Licor Model LI-189 Quantum flux meter. The reaction was carried out at 25 °C.Conclusions
Discovering the design principles that promote self-assembly in natural photosynthetic systems may provide inspiration for the development of synthetic solar conversion systems. The ability to exert control over structural organization across multiple length scales is critical for the fabrication of functionally integrated supramolecular systems and can help to address the challenges in coupling light harvesting, photoredox, and catalytic components for the conversion of light into electricity or fuels. In this study, we show for the first time that LHCII can induce a phase transition in PEO–PPO–PEO block copolymer solutions from the micellar state to a lamellar state demonstrating that LHCII can play a role analogous to its role in photosynthesis in modulating the phase behavior of synthetic block-copolymers. Spectrophotometric characterization of LHCII provides evidence for self-assembly of the protein molecules embedded in the block copolymer membrane. In addition, LHCII mediated photocatalytic hydrogen production viaPt that is formed in situ in the presence of F38 demonstrates the potential of this approach for the development of self-assembled bioinspired photoconversion systems.The significance of this work is that it provides a novel approach for the development of a new class of membrane-based smart materials inspired by natural photosynthetic membranes. Membrane-based systems are attractive for the development of photoconversion systems because their architectures can be optimized for the assembly of interacting components, and also offer the potential for compartmentalization of oxidation and reduction processes. In addition, the ability to modulate the phase transition of such as system could be used to control redox processes where removal of the active component causes a phase shift decreasing the photoconversion rate, and also has important implications in self-repair where degradation of an active component causes a phase shift signaling that repair is required.
Acknowledgements
This research was sponsored by the Laboratory Directed Research and Development Program of Oak Ridge National Laboratory (ORNL), managed by UT-Battelle, LLC, for the US Department of Energy (DOE) under contract no. DE-AC05-00OR22725. The authors also acknowledge ORNL's Center for Structural Molecular Biology (Project ERKP291) supported by the Office of Biological and Environmental Research, US DOE. D.S. was supported by a DOE Science Undergraduate Laboratory Internship and Higher Education Research Experience internship managed by Oak Ridge Institute of Science and Education. Part of this work was performed at the DuPont-Northwestern-Dow Collaborative Access Team (DND-CAT) located at Sector 5 of the Advanced Photon Source (APS). DND-CAT is supported by E.I. DuPont de Nemours & Co., The Dow Chemical Company and the State of Illinois. Use of the APS was supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, under contract no. DE-AC02-06CH11357. M.B.C. thanks Capes-Brazil for the support.References
- N. Armaroli and V. Balzani, The future of energy supply: challenges and opportunities, Angew. Chem., Int. Ed., 2007, 46, 52–66 CrossRef CAS.
- G. W. Crabtree and N. S. Lewis, Solar energy conversion, Phys. Today, 2007, 60, 37–42 CrossRef CAS.
- V. Balzani, A. Credi and M. Venturi, Photochemical conversion of solar energy, ChemSusChem, 2008, 1, 26–58 CrossRef CAS.
- D. Gust, T. A. Moore and A. L. Moore, Mimicking photosynthetic solar energy transduction, Acc. Chem. Res., 2001, 34, 40–48 CrossRef CAS.
- S. X. Ye, et al., Amphiphilic four-helix bundle peptides designed for light-induced electron transfer across a soft interface, Nano Lett., 2005, 5, 1658–1667 CrossRef CAS.
- B. M. Discher, et al., Design of amphiphilic protein maquettes: controlling assembly, membrane insertion, and cofactor interactions, Biochemistry, 2005, 44, 12329–12343 CrossRef CAS.
- T. S. Balaban, Tailoring porphyrins and chlorins for self-assembly in biomimetic artificial antenna systems, Acc. Chem. Res., 2005, 38, 612–623 CrossRef CAS.
- S. M. Kaniber, M. Brandstetter, F. C. Simmel, I. Carmeli and A. W. Holleitner, On-chip functionalization of carbon nanotubes with photosystem I, J. Am. Chem. Soc., 2010, 132, 2872–2873 CrossRef CAS.
- T. Nagase and H. Naito, Percolative behavior of transient photoconductivity in metal-free phthalocyanine nanocrystals, Thin Solid Films, 2008, 516, 2558–2561 CrossRef CAS.
- Z. F. Liu, et al., Crystal structure of spinach major light-harvesting complex at 2.72 angstrom resolution, Nature, 2004, 428, 287–292 CrossRef CAS.
- R. Standfuss, A. C. T. van Scheltinga, M. Lamborghini and W. Kuhlbrandt, Mechanisms of photoprotection and nonphotochemical quenching in pea light-harvesting complex at 2.5A resolution, EMBO J., 2005, 24, 919–928 CrossRef CAS.
- I. Simidjiev, et al., Self-assembly of large, ordered lamellae from non-bilayer lipids and integral membrane proteins in vitro, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 1473–1476 CrossRef CAS.
- G. M. Whitesides and B. Grzybowski, Self-assembly at all scales, Science, 2002, 295, 2418–2421 CrossRef CAS.
- J. M. Lehn, Toward self-organization and complex matter, Science, 2002, 295, 2400–2403 CrossRef CAS.
- D. G. Anderson, J. A. Burdick and R. Langer, Materials science—Smart biomaterials, Science, 2004, 305, 1923–1924 CrossRef CAS.
- I. W. Hamley, Nanostructure fabrication using block copolymers, Nanotechnology, 2003, 14, R39–R54 CrossRef CAS.
- P. Alexandridis and T. A. Hatton, Poly(ethylene oxide)–poly(propylene oxide)–poly(ethylene oxide) block-copolymer surfactants in aqueous-solutions and at interfaces—thermodynamics, structure, dynamics, and modeling, Colloids Surf., A, 1995, 96, 1–46 CrossRef CAS.
- X. Y. Xiong, K. C. Tam and L. H. Gan, Polymeric nanostructures for drug delivery applications based on pluronic copolymer systems, J. Nanosci. Nanotechnol., 2006, 6, 2638–2650 CrossRef CAS.
- M. B. Cardoso, D. Smolensky, W. T. Heller and H. O'Neill, Insight into the structure of light-harvesting complex II and its stabilization in detergent solution, J. Phys. Chem. B, 2009, 113, 16377–16383 CrossRef CAS.
- J. P. Ide, D. R. Klug, W. Kuhlbrandt, L. B. Giorgi and G. Porter, The state of detergent solubilized light-harvesting chlorophyll-a/b protein complex as monitored by picosecond time-resolved fluorescence and circular-dichroism, Biochim. Biophys. Acta, Bioenerg., 1987, 893, 349–364 CrossRef CAS.
- A. V. Ruban, et al., Characterisation of LHC II in the aggregated state by linear and circular dichroism spectroscopy, Biochim. Biophys. Acta, Bioenerg., 1997, 1321, 61–70 CrossRef CAS.
- V. Barzda, L. Mustardy and G. Garab, Size dependency of circular-dichroism in macroaggregates of photosynthetic pigment-protein complexes, Biochemistry, 1994, 33, 10837–10841 CrossRef CAS.
- I. Simidjiev, V. Barzda, L. Mustardy and G. Garab, Isolation of lamellar aggregates of the light-harvesting chlorophyll a/b protein complex of photosystem II with long-range chiral order and structural flexibility, Anal. Biochem., 1997, 250, 169–175 CrossRef CAS.
- I. Simidjiev, V. Barzda, L. Mustardy and G. Garab, Role of thylakoid lipids in the structural flexibility of lamellar aggregates of the isolated light-harvesting chlorophyll a/b complex of photosystem II., Biochemistry, 1998, 37, 4169–4173 CrossRef CAS.
- P. H. Lambrev, et al., Importance of trimer–trimer interactions for the native state of the plant light-harvesting complex II., Biochim. Biophys. Acta, Bioenerg., 2007, 1767, 847–853 CrossRef CAS.
- T. R. Dafforn and A. Rodger, Linear dichroism of biomolecules: which way is up?, Curr. Opin. Struct. Biol., 2004, 14, 541–546 CrossRef CAS.
- J. Breton, M. Michelvi and G. Pailloti, Orientation of pigments and structural proteins in photosynthetic membrane of spinach-chloroplasts—linear dichroism study, Biochim. Biophys. Acta, Bioenerg., 1973, 314, 42–56 CrossRef CAS.
- G. I. Garab and J. Breton, Polarized-light spectroscopy on oriented spinach-chloroplasts fluorescence emission at low-temperature, Biochem. Biophys. Res. Commun., 1976, 71, 1095–1102 CrossRef CAS.
- A. G. Gagliano, N. E. Geacintov and J. Breton, Orientation and linear dichroism of chloroplasts and sub-chloroplast fragments oriented in an electric-field, Biochim. Biophys. Acta, Bioenerg., 1977, 461, 460–474 CrossRef CAS.
- V. I. Gordeliy, V. G. Cherezov, A. D. TuganBaranovskaya and L. S. Yagujinskiy, Investigation of the structure of thylakoid membranes (spinach) by means of small-angle neutron scattering, Biochem. Mol. Biol. Int., 1996, 38, 485–491 CAS.
- K. Mortensen and J. S. Pedersen, Structural study on the micelle formation of poly(ethylene oxide)–poly(propylene oxide)–poly(ethylene oxide) triblock copolymer in aqueous-solution, Macromolecules, 1993, 26, 805–812 CrossRef CAS.
- J. S. Pedersen and M. C. Gerstenberg, Scattering form factor of block copolymer micelles, Macromolecules, 1996, 29, 1363–1365 CrossRef CAS.
- J. F. Millsaps, B. D. Bruce, J. W. Lee and E. Greenbaum, Nanoscale photosynthesis: photocatalytic production of hydrogen by platinized photosystem I reaction centers, Photochem. Photobiol., 2001, 73, 630–635 CrossRef CAS.
- H. O'Neill and E. Greenbaum, Spectroscopy and photochemistry of spinach Photosystem I entrapped and stabilized in a hybrid organosilicate glass, Chem. Mater., 2005, 17, 2654–2661 CrossRef CAS.
- I. J. Iwuchukwu, et al., Self-organized photosynthetic nanoparticle for cell-free hydrogen production, Nat. Nanotechnol., 2010, 5, 73–79 CrossRef CAS.
- E. Greenbaum, Interfacial photoreactions at the photosynthetic membrane interface—an upper limit for the number of platinum atoms required to form a hydrogen-evolving platinum metal catalyst, J. Phys. Chem., 1988, 92, 4571–4574 CrossRef CAS.
- S. Ishigure, et al., Photoinduced hydrogen production with a platinum nanoparticle and light-harvesting chlorophyll a/b-protein complex of photosystem II (LHCII) from spinach system, Bull. Chem. Soc. Jpn., 2009, 82, 93–95 CrossRef CAS.
- R. E. Blankenship, Molecular Mechanisms of Photosynthesis, Blackwell Science, Oxford, UK, 2002 Search PubMed.
- T. S. Balaban, Relevance of the diastereotopic ligation of magnesium atoms of chlorophylls in the major light-harvesting complex II (LHC II) of green plants, Photosynth. Res., 2005, 86, 251–262 CrossRef CAS.
- R. J. Porra, W. A. Thompson and P. E. Kriedemann, Determination of accurate extinction coefficients and simultaneous-equations for assaying chlorophyll-a and chlorophyll-b extracted with 4 different solvents—verification of the concentration of chlorophyll standards by atomic-absorption spectroscopy, Biochim. Biophys. Acta, Bioenerg., 1989, 975, 384–394 CrossRef CAS.
- G. W. Lynn, et al., Bio-SANS—A dedicated facility for neutron structural biology at oak ridge national laboratory, Phys. B, 2006, 385–86, 880–882 CrossRef.
- E. Tjioe and W. T. Heller, ORNL_SAS: software for calculation of small-angle scattering intensities of proteins and protein complexes, J. Appl. Crystallogr., 2007, 40, 782–785 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details for analysis of the stability of LHCII in PEO-PPO-PEO solutions; additional circular dichroism and linear dichroism spectra investigating the effect of F38 on LHCII solubilized in DM detergent; small-angle neutron scattering analysis of LHCII in F38 solutions. See DOI: 10.1039/c0ee00369g |
| ‡ Present address: LNLS—Laboratório Nacional de Luz Síncrotron, CEP 13083-970, Caixa Postal 6192, Campinas, SP, Brazil. |
| § Present address: UT-ORNL Graduate School of Genome Science and Technology, 545 Oak Ridge Turnpike, Oak Ridge, TN 37930, USA. |
| This journal is © The Royal Society of Chemistry 2011 |
